1. Introduction
Salivary α-amylase secretion is influenced by
adrenergic regulation of the sympathetic nervous system and the
hypothalamic-pituitary-adrenal axis (1). Therefore, exercise may affect
salivary α-amylase levels.
Granger et al (1) published a review of biobehavioral
studies of salivary α-amylase in 2007, suggesting that salivary
α-amylase levels markedly increase in response to physical and
psychological stress. Studies by Chatterton et al (2) and Kivlighan and Granger (3) identified that salivary α-amylase
levels increased in response to exercise. Chatterton et al
(2) compared levels of salivary
α-amylase in males prior to and following exercise, a written
examination or rest, and identified that aerobic exercise induced a
three-fold mean increase in α-amylase levels. Kivlighan and Granger
(3) observed that salivary
α-amylase levels increased by an average of 156% in 42 members (21
females) of a collegiate crew team in response to an ergometer
competition.
Following publication of the review by Granger et
al (1), various groups
investigated the correlation between exercise and salivary
α-amylase. A portable system for monitoring salivary α-amylase
activity was launched in Japan at the end of 2005 (4), which stimulated increased interest in
the subject. Certain findings were only published in Japanese. The
present review aims to summarize previous studies concerning the
correlation between exercise and salivary α-amylase levels
published in the English and Japanese literature.
2. Materials and methods
Information was collected from the PubMed
(http://www.ncbi.nlm.nih.gov/pubmed/)
and CiNii (http://ci.nii.ac.jp/) databases. The
latter is a database maintained by the Japanese National Institute
of Informatics (Tokyo, Japan), which comprises literature published
by Japanese authors in academic journals or university memoirs and
is listed in the database of the Japanese National Diet Library
(Tokyo, Japan).
The search terms were ‘saliva’, ‘amylase’ and
‘exercise’. Original studies published after 2006 concerning the
effect of exercise on salivary α-amylase in healthy humans were
selected according to the following exclusion criteria: published
prior to 2006; the article was not original; the participants were
not healthy; the study was intervention-based rather than
exercise-based; salivary α-amylase levels/activity were not
examined; the language was other than English or Japanese.
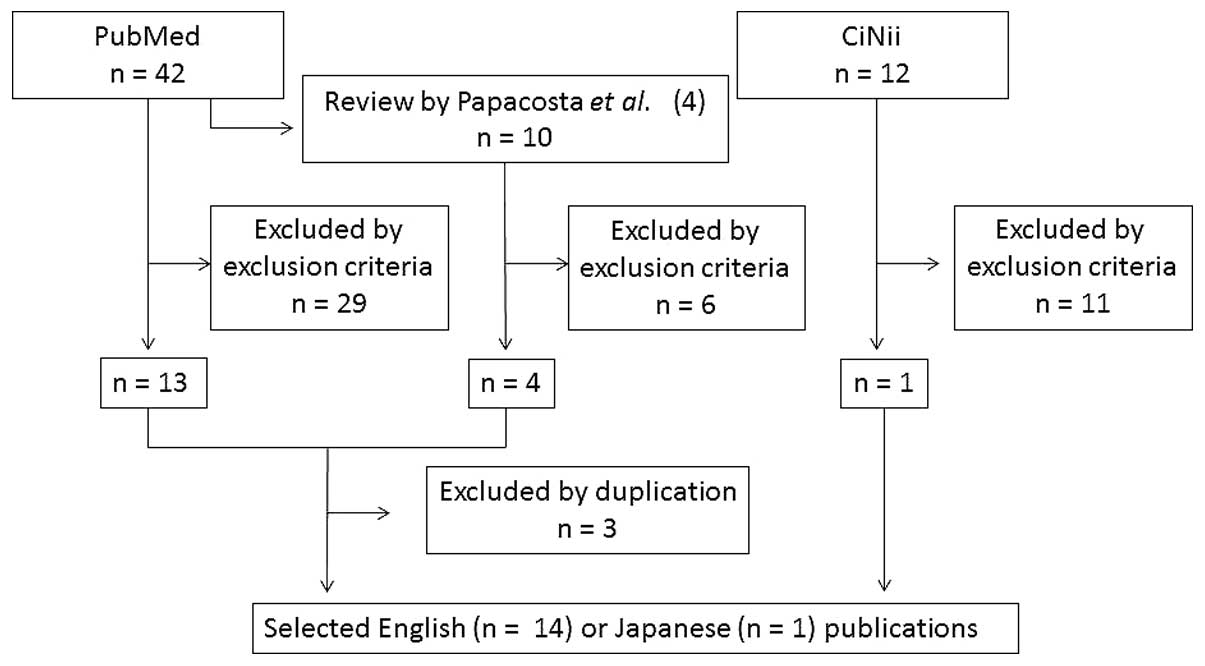
The PubMed search identified 42 studies. Fifteen
reports were excluded as they were published prior to 2006.
Thirteen studies were selected from the titles and abstracts of the
remaining 27 publications, according to the aforementioned
exclusion criteria. The CiNii search identified 12 studies. One was
selected via the same procedure described for the PubMed search.
Among the 42 publications obtained from PubMed, one review article
by Papacosta and Nassis (5) cited
128 publications. According to the exclusion criteria, four
articles were selected from the 10 listed among the references that
described the correlation between salivary α-amylase and exercise
in healthy humans. Three duplicated studies were excluded and the
remaining 15 publications were selected (Fig. 1).
Data are presented as the mean ± SD unless otherwise
specified. P<0.05 was considered to indicate a statistically
significant difference.
3. Results and Discussion
Ten of the 15 publications observed significant
increases in salivary α-amylase activity or levels in response to
exercise, five identified no differences and no studies identified
a reduction (Table I).
 | Table IParameters of primary studies. |
Table I
Parameters of primary studies.
| Subjects | Exercise | Changes in salivary
α-amylase | Trend of change in
salivary α-amylase | Ref. |
|---|
| Endurance-trained
males (n=11); age, 23±1 yearsa | Bicycle
ergometer
70% VO2peak (90 min) | Pre- vs. 45 min
following exercise and post-exercise: 441±81 vs. 1279±248 and
1441±262 U/mla | Increase | 6 |
| Healthy males (n=10);
age, 23±1 yearsa | Bicycle
ergometer
50% or 75% VO2max or repetition of incremental test to
exhaustion (same duration as initial VO2max test) | α-amylase activity,
mean ± SEM (U/ml):
50% VO2max, 450±54→552±77
75% VO2max, 372±65→674±77
Exhaustion, 456±65→710±41 | Increase | 7 |
| Trained male
volunteers with cycling as primary sport (n=24); age, 23±5
years | Bicycle
ergometer
60% (2.5 h) and 75% VO2max to exhaustion | Pre- vs.
post-exercise (to exhaustion), 143±23 vs. 463±22 U/ml | Increase | 8 |
| Healthy males (n=9),
healthy females (n=4); age, 24±5 years | Bicycle
ergometer
55% peak power output at 33°C ≤50%RH up to 3% body weight loss as
sweat | Exercise tended to
increase mean salivary α-amylase activity (NS). Dehydration
decreased secretion rate but, did not influence salivary α-amylase
activity | No change | 9 |
| Healthy
endurance-trained males (n=6); age, 21.8±1.9 years | Treadmill
running
50% and 70% VO2max (30 min each) at 30°C and 40% RH | Pre- vs.
post-exercise, 115±27 vs. 180±29 U/ml | Increase | 10 |
| Elite male wheelchair
athletes (n=23); mean age, 27 years | Treadmill, constant
load: 60% VO2peak (30 min × 2)
Intermittent trial: 20 sets of 2 min at 80% VO2peak and
1 min at 40% VO2peak | Increased following
exercise under constant load and intermittent trial | Increase | 11 |
| Male competitive
endurance runner (n=11); age, 27±7 years | Treadmill
75%VO2max (2 h × 2) | Mean activity
elevated but NS | No change | 12 |
| Male, habitual
exercise ≥3 x/week (n=10); age, 23.5±3.95 yearsa | Treadmill, overnight
fast then 70% VO2peak 1 h after exercise | Mean salivary
α-amylase elevated then leveled marginally but NS | No change | 13 |
| Male national-level
Caucasian cyclists(n=12); age, 22.62±3.51 years | Cycle
ergometer
Initial load of 50 W, increased by 25 W every 2 min to
exhaustion | Elevated α-amylase
concentration in salivary proteins | Increase | 14 |
| Active males (n=21);
age, 24±2 years | Treadmill
3 min warm-up walk at 0.765 m/sec, single exercise test and ≥5
stages of the Bruce protocol following 1.5 min at peak stage;
immediate stop | Pre-exercise vs.
stop-point: 45.9±13.7 vs. 279.3±26.7 U/ml | Increase | 15 |
| Male paraplegic
athletes (n=9); age, 44±2 yearsa | Handcycle
Self-paced time trial (1 h) | Pre- vs.
post-exercise
158±47 vs. 281±72 U/mla | Increase | 16 |
| Healthy elderly males
(n=7) and females (n=13); age, 64.7±8.2 years | Fitness program for
elderly | Pre- vs.
post-exercise (NS)
32.7±34.0 vs. 36.3±34.9 U/ml | No change | 17 |
| Male university
students; n=10; age, 22.2±0.47 years | Walk (20 min) in a
forest or urban environment | Mean activity
increased in the urban environment and was unchanged in the forest
environment (NS) | No change | 18 |
| Black belt taekwondo
athletes; male (n=10), 14±0 yearsa; female (n=6), (13±1 yearsa) | Saliva collected pre-
and post youth competition | Elevated during
competition | Increase | 20 |
| Male professional
swimmers (n=11); age, 21.5±2.16 years | Saliva samples,
collected on the first day of national competition and 2 weeks
after | Increased salivary
α-amylase levels immediately prior to warming up and at 5 min after
competition | Increase | 21 |
A simple comparison or meta-analysis was not
applicable as the type, duration and intensity of exercise, and the
characteristics of the study subjects differed markedly.
Eight studies defined exercise intensity as a ratio
(%) of the maximum or peak oxygen uptake (VO2max and
VO2peak, respectively) or peak power output of the study
participants and four used ergometers (6–9) and
treadmills (10–13) for exercise loading.
Ergometer exercise was consistently demonstrated to
elevate salivary α-amylase activity. Bishop et al (6) noted an increase in salivary α-amylase
activity following exercise at 70% VO2peak for 90 min in
endurance-trained males (age 23±1 years; mean ± SEM). Allgrove
et al (7,8) conducted two studies using a bicycle
ergometer, one of which determined the effect of exercise in ten
active males (age, 23±1 years; mean ± SEM) at intensities of 50%
VO2max, 75% VO2max and at incremental loads
to exhaustion. The duration was matched to the initial
VO2max test. Levels of α-amylase activity increased in
all three trials in response to exercise (7). The other study confirmed these
results in 24 trained male participants (age, 23±5 years) who
cycled for 2.5 h at 60% VO2max followed by 75%
VO2max to exhaustion; the mean salivary α-amylase
activity increased from 143±23 to 463±22 U/ml (8). Fortes et al (9) observed an increase (not significant)
in salivary α-amylase activity during exercise at 55% peak power
output at 33°C, with ≤50% relative humidity; up to 3% of body mass
was lost due to sweat in 13 participants (age 24±5 years). The
control condition, with rehydration to offset fluid loss, was
examined and the kinetics of salivary α-amylase activity were
almost identical. The participants in these four studies of
ergometer exercise were all healthy, with a mean age of ~23–24
years. The intensity of the exercise was low in the study of
dehydration (9). Allgrove et
al (7) showed that α-amylase
activity increased at 50% VO2max and the study by Fortes
et al indicated that the mean α-amylase activity increased
at 55% peak power output, although not significantly (9). Thus, exercise on a bicycle ergometer
at an intensity as low as 55% peak power output may elevate
salivary α-amylase activity.
By contrast, treadmill running generated mixed
results. Fortes and Whitham (10)
observed that α-amylase activity was elevated following running on
a treadmill for 30 min at 50% VO2max followed by 30 min
at 70% VO2max, in six endurance-trained males (age,
21.8±1.9 years). Leicht et al (11) reported that α-amylase activity
increased in 23 wheelchair athletes. However, subsequent
publications did not confirm these results. According to Costa
et al (12), salivary
α-amylase activity increased, although not significantly, in 11
male endurance runners who ran at 75% VO2max for 2 h.
The findings of Rosa et al (13) from a study of 10 active males who
ran on treadmills at 70% VO2max for 1 h supported these
results; the mean salivary α-amylase concentrations were increased
but the increase was not statistically significant. Three of the
four studies, with the exception of the study of wheelchair
athletes, comprised small cohorts, which may account for this
discrepancy.
Five studies demonstrated changes in salivary
α-amylase in response to exercise without specifying the exercise
intensity (14–18). In one of these studies, 12
Caucasian male national-level cyclists underwent a progressive test
on a bicycle ergometer. The initial load was 50 W, which increased
by 25 W every 2 min to exhaustion. The salivary α-amylase
concentration increased in parallel with the increase in load
(14). Galina et al
(15) adopted the Bruce protocol
test using treadmills. Twenty-one active males performed a single
bout of exercise and a minimum of five stages of the Bruce protocol
(19). Salivary α-amylase activity
increased during the exercise and reached the greatest level
following the highest completed stage achieved by each participant
(15). Allgrove et al
(16) examined responses in male
athletes with spinal cord damage. Salivary α-amylase activity
increased from 158±47 to 281±72 U/ml (SEM) following 1 h of
self-paced handcycling time trials in nine physically active male
wheelchair athletes. Ishiguro et al (17) observed changes in α-amylase
activity among healthy elderly individuals (age 64.7±8.2 years)
during a fitness program comprising a 10 min warm up, 30 min of
exercise and a 10 min cool down. The exercise performed was light
aerobic gymnastics with singing developed for the elderly and the
warm up and cool down consisted of stretching. Salivary α-amylase
activity were not affected by the program, as pre-exercise values
compared with post-exercise values were 32.7±34.0 versus 36.3±34.9
U/ml, respectively. Yamaguchi et al (18) identified that levels of salivary
α-amylase activity in 10 male university students (age 22.2±0.5
years) during a 20 min walk, in forest and urban environments, did
not change. With the exception of light gymnastics for the elderly
(17) and relaxed walking
(18), physical exercise appears
to increase salivary α-amylase activity and concentration (14–16).
Chiodo et al (20) and Diaz et al (21) investigated the effect of Taekwondo
and swimming competitions, respectively. Sixteen taekwondo black
belt athletes participated in an official youth competition
consisting of three 2-min rounds with 1-min intervals. Salivary
α-amylase activity was increased by 115% at the end of the
competition compared with the pre-competition values (20). Diaz et al (21) compared the α-amylase concentrations
in saliva during a national swimming competition with those two
weeks following the event (the control day) in 11 professional
swimmers. The α-amylase concentrations immediately prior to warming
up for the race and 5 min after finishing were higher than those at
the same time on the control day. Thus, psychological and physical
stress were considered to contribute to the increase in α-amylase
levels.
In conclusion, exercise has consistently been shown
to increase mean salivary α-amylase activity and concentration in
all studies examined in the present review, including those in
which changes were not significant, with the exception of the
20-min forest walk (18). The
effect tended to be more pronounced at exercise intensities >70%
VO2max in healthy young individuals. Therefore, studies
published following those reviewed by Granger et al
(1) confirm the conclusion that
salivary α-amylase levels markedly increase in response to physical
stress. Therefore, α-amylase levels may be an effective
non-invasive marker of physical stress.
Acknowledgements
This review was supported in part by the Ministry of
Education, Culture, Sports, Science and Technology (MEXT)-Supported
Program for the Strategic Research Foundation at Private
Universities and a grant from the Research Project on Development
of Agricultural Products and Foods with Health-promoting benefits
(NARO), Japan.
References
|
1
|
Granger DA, Kivlighan KT, el-Sheikh M,
Gordis EB and Stroud LR: Salivary alpha-amylase in biobehavioral
research: recent developments and applications. Ann N Y Acad Sci.
1098:122–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chatterton RT Jr, Vogelsong KM, Lu YC,
Ellman AB and Hudgens GA: Salivary alpha-amylase as a measure of
endogenous adrenergic activity. Clin Physiol. 16:433–448. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kivlighan KT and Granger DA: Salivary
alpha-amylase response to competition: relation to gender, previous
experience, and attitudes. Psychoneuroendocrinology. 31:703–714.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamaguchi M, Hanawa N and Yoshida H:
Evaluation of a novel monitor for the sympathetic nervous system
using salivary amylase activity. Seitai-Ikougaku. 45:161–168.
2007.(in Japanese with English abstract).
|
|
5
|
Papacosta E and Nassis GP: Saliva as a
tool for monitoring steroid, peptide and immune markers in sport
and exercise science. J Sci Med Sport. 14:424–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bishop NC, Walker GJ, Scanlon GA, Richards
S and Rogers E: Salivary IgA responses to prolonged intensive
exercise following caffeine ingestion. Med Sci Sports Exerc.
38:513–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allgrove JE, Gomes E, Hough J and Gleeson
M: Effects of exercise intensity on salivary antimicrobial proteins
and markers of stress in active men. J Sports Sci. 26:653–661.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allgrove JE, Oliveira M and Gleeson M:
Stimulating whole saliva affects the response of antimicrobial
proteins to exercise. Scand J Med Sci Sports. March 19–2013.(Epub
ahead of print).
|
|
9
|
Fortes MB, Diment BC, Di Felice U and
Walsh NP: Dehydration decreases saliva antimicrobial proteins
important for mucosal immunity. Appl Physiol Nutr Metab.
37:850–859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fortes MB and Whitham M: Salivary Hsp72
does not track exercise stress and caffeine-stimulated plasma Hsp72
responses in humans. Cell Stress Chaperones. 16:345–352. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leicht CA, Bishop NC and Goosey-Tolfrey
VL: Mucosal immune responses to treadmill exercise in elite
wheelchair athletes. Med Sci Sports Exerc. 43:1414–1421. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costa RJ, Fortes MB, Richardson K, Bilzon
JL and Walsh NP: The effects of postexercise feeding on saliva
antimicrobial proteins. Int J Sport Nutr Exerc Metab. 22:184–191.
2012.PubMed/NCBI
|
|
13
|
Rosa L, Teixeira A, Lira F, Tufik S, Mello
M and Santos R: Moderate acute exercise (70% VO2peak)
induces TGF-β, α-amylase and IgA in saliva during recovery. Oral
Dis. Feb 19–2013.(Epub ahead of print).
|
|
14
|
de Oliveira VN, Bessa A, Lamounier RP, et
al: Changes in the salivary biomarkers induced by an effort test.
Int J Sports Med. 6:377–381. 2010.PubMed/NCBI
|
|
15
|
Gallina S, Di Mauro M, D’Amico MA,
D’Angelo E, Sablone A, Di Fonso A, Bascelli A, Izzicupo P and Di
Baldassarre A: Salivary chromogranin A, but not α-amylase,
correlates with cardiovascular parameters during high-intensity
exercise. Clin Endocrinol (Oxf). 75:747–752. 2011.
|
|
16
|
Allgrove JE, Chapman M, Christides T and
Smith PM: Immunoendocrine responses of male spinal cord injured
athletes to 1-hour self-paced exercise: pilot study. J Rehabil Res
Dev. 49:925–933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishiguro C, Ikuta M, Sugita A, Okada T,
Kozasa Y, Numata Y, Higashino T and Mikouchi N: The effect of the
health education initiative ‘Physical Exercise for Promotion of
Health’ on people in the community. Nihon Sekijuji Toyota Kango
Daigaku Kiyo [Bulletin of Japanese Red Cross Toyota University of
Nursing]. 7:107–119. 2012.(In Japanese).
|
|
18
|
Yamaguchi M, Deguchi M and Miyazaki Y: The
effects of exercise in forest and urban environments on sympathetic
nervous activity of normal young adults. J Int Med Res. 34:152–159.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bruce RA: Exercise testing of patients
with coronary heart disease. Principles and normal standards for
evaluation. Ann Clin Res. 3:323–332. 1971.PubMed/NCBI
|
|
20
|
Chiodo S, Tessitore A, Cortis C, et al:
Stress-related hormonal and psychological changes to official youth
Taekwondo competitions. Scand J Med Sci Sports. 21:111–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diaz MM, Bocanegra OL, Teixeira RR, Soares
SS and Espindola FS: Response of salivary markers of autonomic
activity to elite competition. Int J Sports Med. 33:763–768. 2012.
View Article : Google Scholar : PubMed/NCBI
|