Introduction
Sepsis is a systemic inflammatory reaction that is
triggered by an infective agent (such as bacteria, viruses, fungi
or parasites) (1). Severe sepsis
may result in systemic inflammation, multi-organ failure and septic
shock (2). It is one of the major
health concerns worldwide and also the predominant reason for
intensive care unit (ICU) admission (3). There are 750,000 cases of severe
sepsis diagnosed every year in the United States, accompanied by
unacceptably high mortality rates (4,5).
Sepsis-induced mortality rates are as high as 30–50% in developed
countries. With the rapidly increasing incidence, high mortality
rates, complex pathophysiology and overall difficulties in its
treatment, sepsis is becoming an important focus for researchers
and clinicians (6–9).
Infections and sepsis are accompanied by clinical
signs such as leukocytosis, changes in body temperature and the
development of tachycardia. However, these classic indicators of
systemic inflammation are neither sensitive nor specific for sepsis
(10). They have only moderate
sensitivity and specificity and are not early markers due to the
time taken to produce a reaction. Therefore, early markers are
useful for the diagnosis and treatment of sepsis and are crucial
for overcoming sepsis-associated mortality.
Cytokine levels are an obvious choice as a marker of
sepsis. The systemic release of inflammatory cytokines occurs
several hours prior to other markers of systemic inflammation, such
as acute phase protein release and leucocytosis, suggesting their
potential importance as diagnostic parameters in systemic
inflammatory response syndrome (SIRS) and sepsis (11,12).
When sepsis occurs, multiple redundant inflammatory cytokines are
released into the blood stream, including tumor necrosis factor-α
(TNF-α), interleukin-6 (IL-6), leptin, C-reactive protein (CRP) and
procalcitonin (PCT) (13,14), which are important for mediating
the inflammatory response.
The hormone leptin (molecular weight of 16-kDa) is
mainly generated by adipocytes and contributes to the regulation of
energy balance by informing the brain of the volume of adipose
tissue in the body, thereby regulating food intake and energy
expenditure (15–17). In addition, previous studies have
indicated that leptin may be classified as a cytokine, and is
involved in cell-mediated immunity and cytokine crosstalk (18–22).
The present study was conducted to determine the role of serum
leptin in the early diagnosis of sepsis and to explore its
correlation with additional biomarkers of sepsis and/or multiple
organ failure. This knowledge is likely to be helpful in
understanding the precise mechanisms of sepsis and also potentially
contributes to new diagnostic approaches.
A large-sample-sized study of septic patients from a
medical ICU was conducted. Serum leptin concentrations were
measured during treatment in the ICU to investigate whether leptin
is activated in critical illness, has diagnostic values for sepsis
and/or multiple organ failure and whether leptin may serve as a
prognostic predictor for survival in the ICU and long term.
Materials and methods
Patients
A retrospective study was conducted and a total of
331 patients (115 males and 216 females) were included in the
study. As shown in Table I, the
median age of the patients was 56 years (range, 6–91 years). The
study included 128 patients with sepsis (47 males and 81 females)
with a median age of 53 years. All patients underwent consistent
blood collection at 6:00 a.m. every morning after fasting. This
study was conducted over a period of 24 months from April 2008 to
July 2011 in the ICU of the General Hospital of Chinese People’s
Liberation Army (Beijing, China). The study was in accordance with
the Helsinki Declaration and approval was obtained from the Ethics
Committee of the Chinese PLA General Hospital (project no.
11KMZ04). The subjects and their families were well informed of the
details and written informed consent was obtained prior to the
study.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| | | Age (years) |
|---|
| | |
|
|---|
| Group | No. | M/F | Mean ± SD | Range | Median |
|---|
| Sepsis | 128 | 47/81 | 54±20 | 10–86 | 53 |
| Non-sepsis | 203 | 68/135 | 56±18 | 6–91 | 58 |
Diagnostic criteria for sepsis
The gold standard for the diagnosis of sepsis is a
positive blood culture. The blood cultures were performed
independently more than twice and the same type of positive
bacteremia was present at the end of testing. Thus, marked signs of
infection had appeared and blood cultures were positive in the
enrolled patients of the sepsis group. Previously described
criteria (23) for sepsis were
considered to be met and at least two of the following four
clinical symptoms of SIRS were present: temperature >38°C or
<36°C; heart rate >90 beats/min; respiratory rate >20
breaths/min or PaCO2 <32 mmHg; and white blood cell
(WBC) count >12,000 or <4,000 cells/mm3, or
>10% immature forms. The patients in the control (non-sepsis)
group showed no signs of infection and less than two of the four
clinical SIRS symptoms were present.
Blood sampling
Blood samples were collected and processed within 2
h. Blood was centrifuged at 1,600 × g for 15 min.
Leptin and CRP determination
Serum leptin and CRP concentrations were determined
by quantitative sandwich enzyme immunoassays (Ray Biotech., Inc.,
Minneapolis, MN, USA) according to the manufacturer’s instructions.
The intensity of the color was measured at 450 nm.
PCT determination
PCT levels were measured using an radioimmunoassay
kit (RIN 6025) that utilized specific polyclonal antibodies and
human 125I-N-procalcitonin with a detection limit of 10
pg/tube (Peninsula Lab., Inc., San Carlos, CA, USA).
Statistical analysis
Basic statistical analyses were performed using SPSS
software, version 18.0 (SPSS, Inc., Chicago, IL, USA). Categorical
data are expressed as total numbers and/or relative frequencies.
Continuous data are expressed as the mean ± standard deviation (or
mean ± standard error of the mean). The binary logistic regression
models were chosen to predict the probability of sepsis. Receiver
operating characteristic (ROC) curves were used to evaluate the
corresponding balance between sensitivity and specificity over a
range of predictive factors (such as leptin only and leptin
combined with temperature, platelet count, WBC count and heart
rate), and the optimum cut-off for sensitivity and specificity
corresponding to maximum area under the ROC (AUC) was determined by
selection of the point at which sensitivity plus specificity was
maximal.
Results
Patient characteristics
A total of 331 patients in the ICU of the General
Hospital of Chinese People’s Liberation Army from April 2008 to
July 2011, with a median age of 56 years, were studied. The sepsis
group consisted of 128 patients from the ICU (47 males and 81
females) with a median age of 53 years (Table I).
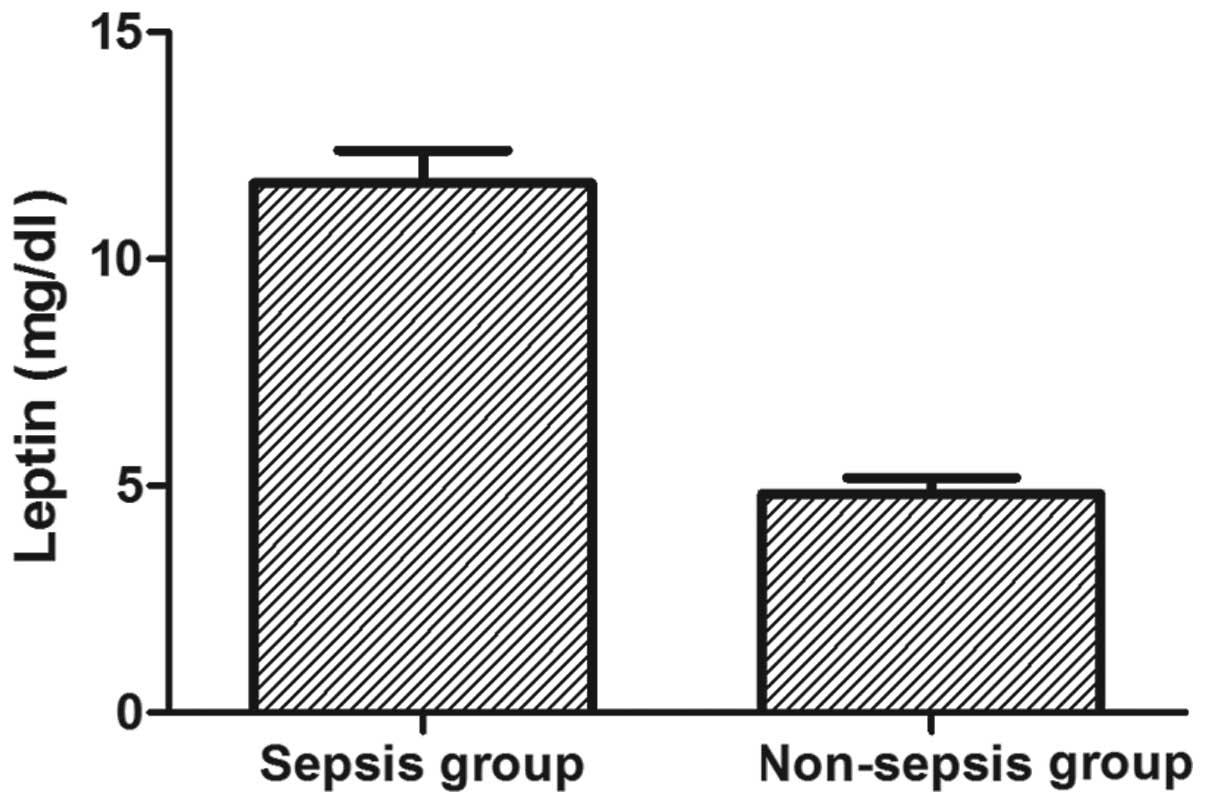
Serum leptin concentrations are elevated
in septic patients
It was first tested whether the serum concentrations
of leptin were elevated in septic patients compared with those in
non-septic controls in the ICU. Septic patients displayed
significantly higher leptin serum concentrations compared with
those of the non-septic controls (mean leptin concentration, 11.67
mg/dl in the sepsis group versus 4.82 mg/dl in the control group;
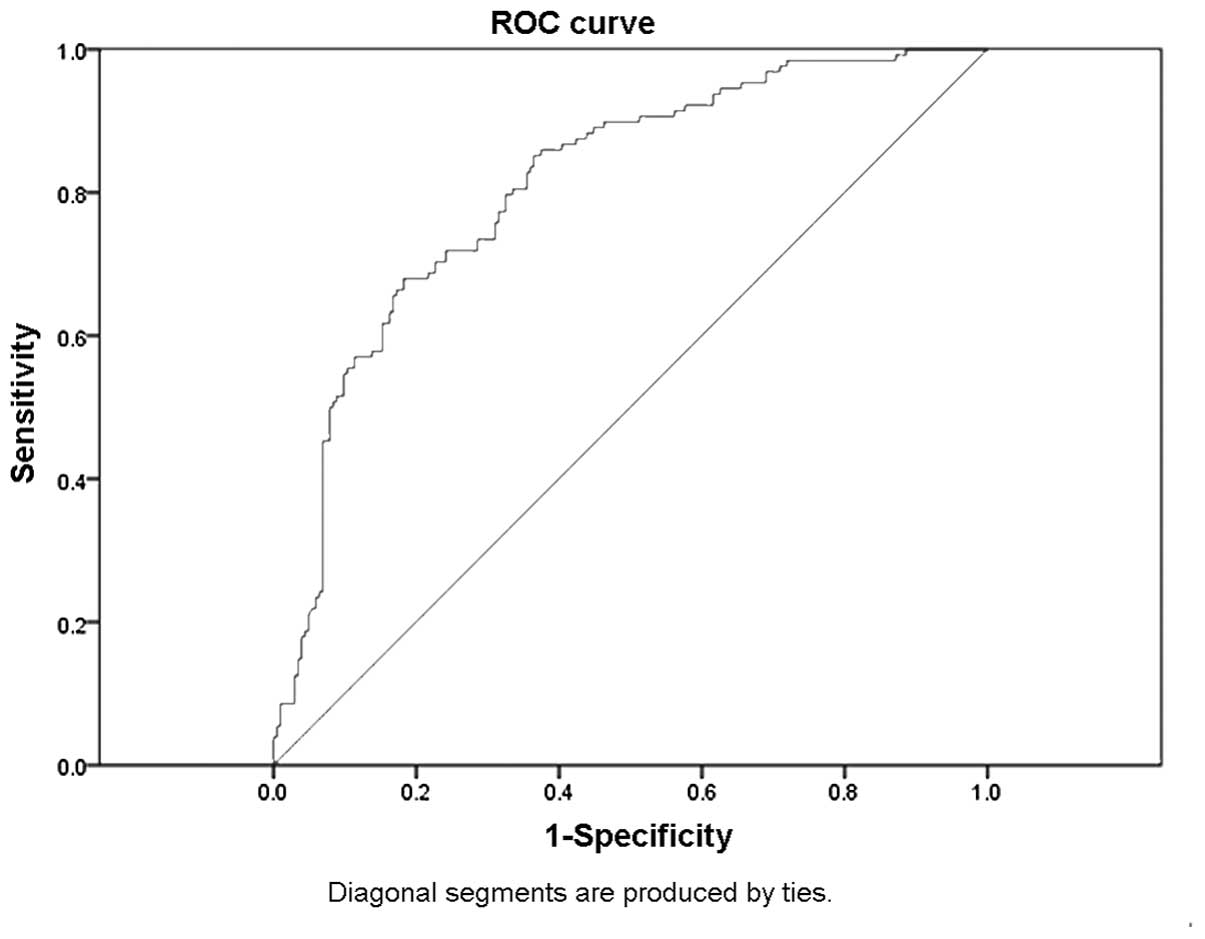
P<0.001; Fig. 1). The data
indicate that leptin is associated with sepsis and a subsequent ROC
curve analysis revealed that leptin, as an independent indicator,
may differentiate septic patients from controls. A cut-off point
set at 5.1 mg/dl leptin showed a sensitivity of 72% and a
specificity of 72%. The accuracy of serum leptin in distinguishing
septic patients from non-septic patients was 76%, and the AUC of
serum leptin was ≤0.8 (Fig.
2).
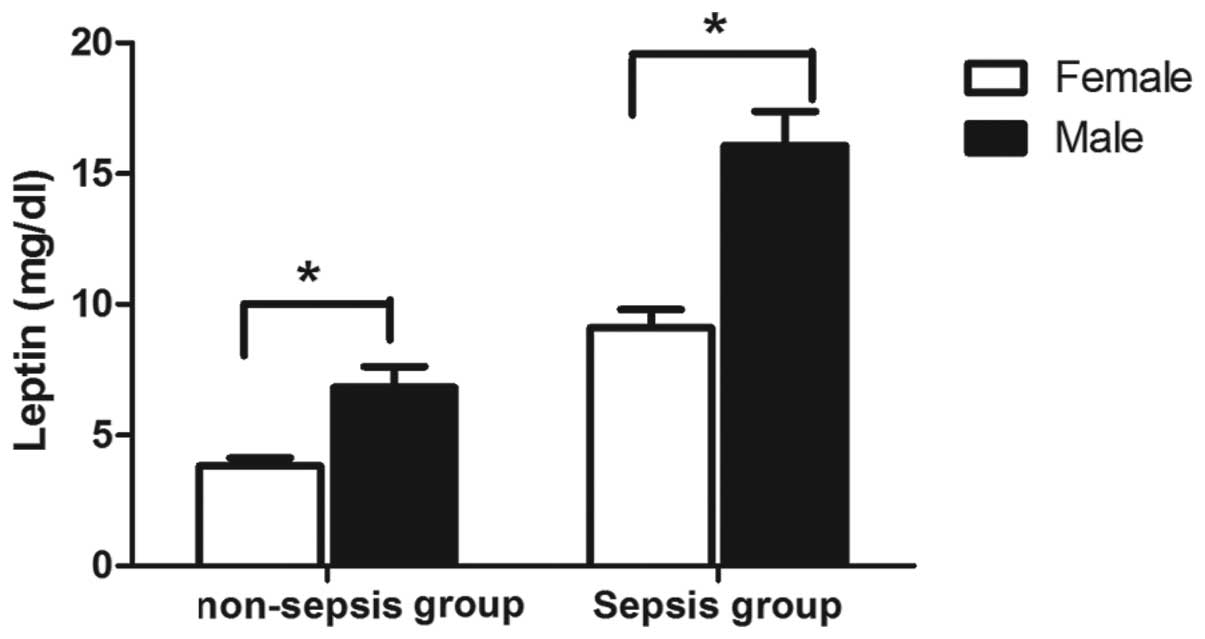
Serum leptin levels in males are higher
than those in females
The differences in leptin concentrations between
male and female patients were evaluated. As shown in Fig. 3, the leptin levels in males were
higher than those in females, regardless of the group. The
elevation of leptin levels was particularly marked in the sepsis
group, in which the mean serum leptin concentrations at admission
were 16.07 μg/l in males and 9.1 μg/l in females (P=1.4E-6)
(Table II).
 | Table IISerum leptin concentrations in male
and female patients with and without sepsis. |
Table II
Serum leptin concentrations in male
and female patients with and without sepsis.
| Gender | Group | Leptin (μg/l) |
|---|
| Female | Non-sepsis | 3.8±0.3 |
| Sepsis | 9.1±0.7 |
| Male | Non-sepsis | 6.8±0.8 |
| Sepsis | 16.1±1.3 |
Comparison of clinical indicators between
the sepsis and non-sepsis groups
Infections and sepsis are accompanied by clinical
and laboratory signs, such as changes in body temperature,
leukocytosis and tachycardia. In the present study, clinical
indicators other than leptin were evaluated. As shown in Table III, the leptin concentrations in
patients with sepsis were significantly higher than those of the
patients in the control group (11.67±0.72 versus 4.82±0.36 mg/dl)
(P<0.001). The mean values of the CRP and PCT concentrations and
the WBC count in the sepsis group were 9.99 mg/dl, 17.86 ng/ml and
15,620 cells/ml, respectively, versus 3.31 mg/dl, 0.34 ng/ml and
8,360 cells/ml, respectively in the non-sepsis group (P<0.001).
Several markers of organ function, such as creatinine levels, the
international normalized ratio (INR) and activated partial
thromboplastin time (aPTT) were also measured. The mean values of
the creatinine concentration and INR in the sepsis group were 99.3
μmol/l and 1.36 respectively, and were significantly higher than
those of the controls (P<0.001; Table III).
 | Table IIIComparison of clinical indicators in
the sepsis and non-sepsis groups. |
Table III
Comparison of clinical indicators in
the sepsis and non-sepsis groups.
| Parameters | Sepsis group (Mean ±
SD) | Non-sepsis group
(Mean ± SD) | P-value |
|---|
| Basic
information |
| No. | 128 | 203 | |
| Age (years) | 54±20 | 56±18 | |
| Male (n) | 47 | 68 | |
| Female (n) | 81 | 135 | |
| Systemic
conditions |
| Body temperature
(°C) | 37.8±0.1 | 36.9±0.0 | <0.001 |
| Heart rate
(beats/min) | 109±2 | 87±1 | <0.001 |
| Markers of
inflammation |
| Leptin
(mg/dl) | 11.67±0.72 | 4.824±0.355 | <0.001 |
| CRP (mg/dl) | 9.99±0.69 | 3.31±1.14 | <0.001 |
| PCT (ng/ml) | 17.86±5.14 | 0.34±0.28 | <0.001 |
| WBCs (×1000
cells/ml) | 15.62±1.17 | 8.36±0.21 | <0.001 |
| Neutrophils
(/%) | 0.853±0.008 | 0.792±0.009 | <0.001 |
| Markers of organ
function |
| Creatinine
(μmol/l) | 99.3±7.6 | 64.7±2.6 | <0.001 |
| INR | 1.36±0.06 | 1.18±0.02 | <0.001 |
| TB (μmol/l) | 42.7±6.5 | 46.1±6.7 | 0.735 |
| aPTT (sec) | 42.9±1.3 | 40.5±1.2 | 0.192 |
| Platelets
(×103/ml) | 169±10 | 198±7 | 0.011 |
Correlation between leptin and other
indicators of sepsis
The correlations between leptin and additional
indicators of sepsis were evaluated. The data revealed no
significant correlations between leptin and other inflammatory
markers, including CRP, PCT and WBCs. The levels of serum leptin
were negatively correlated with platelets and TB (Pearson
correlation coefficient = −0.119 and −0.138; P=0.03 and 0.016,
respectively), and positively correlated with body temperature,
heart rate and creatinine levels (Pearson correlation coefficient =
0.303, 0.111 and 0.286; P=1.9E-8, 0.044 and 4.6E-7, respectively)
(data not shown).
Leptin serum concentrations indicate
sepsis in ICU patients and may be used in a new diagnostic model
for sepsis
Based on the finding that leptin serum
concentrations were higher in the septic group than in the control
group, the ability of serum leptin levels to identify patients with
sepsis in the medical ICU setting was investigated. The diagnostic
accuracy of leptin was compared with classic, routinely used
markers of inflammation and bacterial infection using ROC curve
analyses (24). For leptin, CRP
and PCT levels and body temperature, the AUC statistics were 0.864,
0.925, 0.904 and 0.898, respectively (Fig. 4). These data demonstrate that
leptin is an effective diagnostic marker for sepsis when compared
with classical biomarkers.
Based on the associations between leptin and markers
of organ dysfunction, it was clear that leptin has the potential to
facilitate prognosis and guide the treatment of sepsis. Binary
logistic regression with a likelihood ratio-based forward stepwise
strategy was used to predict the diagnosis of sepsis. The results
revealed that leptin, WBCs, platelets, temperature and heart rate
were all independent predictors in septic patients with odds ratios
of 1.192 [95% confidence interval (CI), 1.119–1.269, P=4E-8), 1.407
(95% CI, 1.251–1.583, P=1.4E-8), 0.992 (95% CI, 0.987–0.996,
P=3.9E-4), 3.187 (95% CI, 1.655–6.139, P=0.001) and 1.063 (95% CI,
1.036–1.092, P=4.7E-6), respectively (Table IV). The logistic regression output
cut-off value (the odds ratio) equaled 0.46, the model was found to
have a sensitivity of 86% and specificity of 93% for sepsis, and
the AUC was 0.953 (P<0.0001). The results revealed that leptin,
combined with temperature, platelet and WBC counts and heart rate
are an effective logistic regression model for the diagnosis of
sepsis (Fig. 5).
 | Table IVAssociations between leptin and other
markers in the sepsis group (the output of the logistic regression
model). |
Table IV
Associations between leptin and other
markers in the sepsis group (the output of the logistic regression
model).
| Odds ratio | 95% CI | P value |
|---|
| Leptin | 1.192 | 1.119–1.269 | 0.000 (4E-8) |
| WBCs | 1.407 | 1.251–1.583 | 0.000 (1.4E-8) |
| Platelets | 0.992 | 0.987–0.996 | 0.000 (3.9E-4) |
| Temperature | 3.187 | 1.655–6.139 | 0.001 |
| Heart rate | 1.063 | 1.036–1.092 | 0.000 (4.7E-6) |
Discussion
Early diagnosis of sepsis may be challenging as
clinical presentations are often nonspecific, bacterial cultures
are time-consuming and laboratory tests lack sensitivity and
specificity. In order to reduce the morbidity and mortality
associated with sepsis, there is an urgent requirement for
effective markers for the diagnosis and monitoring of sepsis. In
the present study, it was observed that the serum leptin
concentrations were significantly higher in the sepsis group than
in the non-sepsis group. Similar results were identified by
Arnalich et al (25), who
also hypothesized that the high leptin levels in survivors with
sepsis may represent host defense mechanisms against bacterial
infection. Notably, the results of the present study indicated that
there were significant differences in leptin concentrations between
males and females. The mean serum leptin concentrations in males
were 2-fold higher than those of females.
Severe sepsis is defined as sepsis associated with
organ dysfunction, hypoperfusion or hypotension, such as serum
creatinine levels of >176.8 μmol/l (2.0 mg/dl), an INR of
>1.5 or an aPTT of >60 sec (26). In the present study, the mean
values of serum creatinine, INR and aPTT were 99.3 μmol/l, 1.36 and
42.9 sec, respectively. Therefore, the patients in the present
study did not have severe sepsis. Furthermore, this was supported
by the positive correlation that was identified between leptin and
creatinine levels, which is an indicator associated with renal
function.
The monitoring of serum leptin levels in septic
patients is important for the early diagnosis of sepsis and the
differentiation between sepsis and SIRS. The results of the present
study showed that the AUC statistics for leptin, CRP and PCT levels
and temperature were 0.864, 0.925, 0.904 and 0.898, respectively.
Thus, leptin may be utilized as a potential diagnostic marker of
sepsis with a similar efficacy to other markers, such as CRP and
PCT (27–30). It is known that PCT and CRP are
useful in the diagnosis of bacterial infection and sepsis, but with
limitations. PCT levels are elevated in septic patients and
correlated with the severity of sepsis; they usually serve to
discriminate between severe sepsis and less severe forms of
infection, but do not correlate with early prognosis (31). Concentrations of CRP have been
monitored in septic patients, but these concentrations fail to
allow immediate diagnosis and prognosis due to the time taken for
the body to produce a reaction and the duration of the increased
serum concentration (32,33). However, leptin is involved in the
network of inflammatory mediators and during SIRS its plasma
concentration is increased by the action of these inflammatory
mediators (34). Moreover, a
significant correlation between leptin and TNF-α has been
identified, which indicates that leptin is a crucial regulator in
the early inflammatory period (35–37).
The exact mechanisms of leptin in sepsis require further study.
As each biomarker has limited sensitivity and
specificity, it is likely that a combined panel of novel
biomarkers, with or without traditional markers of sepsis is
required. Studies using panels of sepsis biomarkers have also
provided encouraging results. The present study demonstrated that
leptin, in combination with other independent factors, such as
temperature, platelet and WBCs and heart rate, may be an effective
diagnostic model of sepsis. The logistic regression output cut-off
value equaled 0.46 and the model was found to have sensitivity of
86% and specificity of 93%.
A useful sepsis marker is required to not only
facilitate the identification of sepsis, but may also be used to
guide therapy. The possible role of leptin in the prognosis of
sepsis requires further study.
In conclusion, leptin is a valuable marker for the
diagnosis of sepsis. A prognostic model has been created that is an
effective logistic regression model for the diagnosis of
sepsis.
Acknowledgements
This study was supported by a grant from the
Foundation of the ‘Twelfth Five-year Plan’ for the Medical Science
Development of People’s Liberation Army (grant no. CWS11J109).
References
|
1
|
Monneret G: Immunology programs must
include sepsis. Science. 328:11062010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arabi Y, Al Shirawi N, Memish Z, Venkatesh
S and Al-Shimemeri A: Assessment of six mortality prediction models
in patients admitted with severe sepsis and septic shock to the
intensive care unit: a prospective cohort study. Crit Care.
7:R116–R122. 2003.PubMed/NCBI
|
|
4
|
Banta JE, Joshi KP, Beeson L and Nguyen
HB: Patient and hospital characteristics associated with inpatient
severe sepsis mortality in California, 2005–2010. Crit Care Med.
40:2960–2966. 2012.PubMed/NCBI
|
|
5
|
Blanco Quirós A, Casado Flores J, Nieto
Moro M, Garrote Adrados JA, Arranz Sanz E and Asensio Antón J:
Meningococcal sepsis in pediatrics. Parameters associated with poor
outcome. An Pediatr (Barc). 61:305–313. 2004.(In Spanish).
|
|
6
|
Hotchkiss RS and Opal S: Immunotherapy for
sepsis - a new approach against an ancient foe. N Engl J Med.
363:87–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen-Wolkowiez M, Benjamin DK Jr and
Capparelli E: Immunotherapy in neonatal sepsis: advances in
treatment and prophylaxis. Curr Opin Pediatr. 21:177–181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O’Callaghan A and Redmond HP: Treatment of
sepsis: current status of clinical immunotherapy. Surgeon.
4:355–361. 2006.
|
|
9
|
Martin GS: Sepsis, severe sepsis and
septic shock: changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fried E, Weissman C and Sprung C:
Postoperative sepsis. Curr Opin Crit Care. 17:396–401. 2011.
View Article : Google Scholar
|
|
11
|
Müller B, Becker KL, Schächinger H, et al:
Calcitonin precursors are reliable markers of sepsis in a medical
intensive care unit. Crit Care Med. 28:977–983. 2000.PubMed/NCBI
|
|
12
|
Andaluz-Ojeda D, Bobillo F, Iglesias V, et
al: A combined score of pro- and anti-inflammatory interleukins
improves mortality prediction in severe sepsis. Cytokine.
57:332–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song R, Kim J, Yu D, Park C and Park J:
Kinetics of IL-6 and TNF-α changes in a canine model of sepsis
induced by endotoxin. Vet Immunol Immunopathol. 146:143–149.
2012.
|
|
14
|
Abdel-Hafez NM, Saleh Hassan Y and
El-Metwally TH: A study on biomarkers, cytokines, and growth
factors in children with burn injuries. Ann Burns Fire Disasters.
20:89–100. 2007.PubMed/NCBI
|
|
15
|
Hoda MR, Theil G, Mohammed N, Fischer K
and Fornara P: The adipocyte-derived hormone leptin has
proliferative actions on androgen-resistant prostate cancer cells
linking obesity to advanced stages of prostate cancer. J Oncol.
2012:2803862012.
|
|
16
|
Miyazaki S, Izawa T, Ogasawara JE, et al:
Effect of exercise training on adipocyte-size-dependent expression
of leptin and adiponectin. Life Sci. 86:691–698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bates SH, Stearns WH, Dundon TA, et al:
STAT3 signalling is required for leptin regulation of energy
balance but not reproduction. Nature. 421:856–859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Behnes M, Brueckmann M, Lang S, et al:
Alterations of leptin in the course of inflammation and severe
sepsis. BMC Infect Dis. 12:2172012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mackey-Lawrence NM and Petri WA Jr: Leptin
and mucosal immunity. Mucosal Immunol. 5:472–479. 2012.PubMed/NCBI
|
|
20
|
Conde J, Scotece M, Gómez R, Gómez-Reino
JJ, Lago F and Gualillo O: At the crossroad between immunity and
metabolism: focus on leptin. Expert Rev Clin Immunol. 6:801–808.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Rosa V, Procaccini C, Cali G, et al: A
key role of leptin in the control of regulatory T cell
proliferation. Immunity. 26:241–255. 2007.PubMed/NCBI
|
|
22
|
Hasenkrug KJ: The leptin connection:
regulatory T cells and autoimmunity. Immunity. 26:143–145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bone RC, Balk RA, Cerra FB, et al:
Definitions for sepsis and organ failure and guidelines for the use
of innovative therapies in sepsis. The ACCP/SCCM Consensus
Conference Committee American College of Chest Physicians/Society
of Critical Care Medicine. Chest. 101:1644–1655. 1992. View Article : Google Scholar
|
|
24
|
Zhang J, She D, Feng D, Jia Y and Xie L:
Dynamic changes of serum soluble triggering receptor expressed on
myeloid cells-1 (sTREM-1) reflect sepsis severity and can predict
prognosis: a prospective study. BMC Infect Dis. 11:532011.
View Article : Google Scholar
|
|
25
|
Arnalich F, López J, Codoceo R, Jim nez M,
Madero R and Montiel C: Relationship of plasma leptin to plasma
cytokines and human survivalin sepsis and septic shock. J Infect
Dis. 180:908–911. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dellinger RP, Levy MM, Rhodes A, et al:
Surviving sepsis campaign: international guidelines for management
of severe sepsis and septic shock: 2012. Crit Care Med. 41:580–637.
2013. View Article : Google Scholar
|
|
27
|
Su L, Han B, Liu C, et al: Value of
soluble TREM-1, procalcitonin, and C-reactive protein serum levels
as biomarkers for detecting bacteremia among sepsis patients with
new fever in intensive care units: a prospective cohort study. BMC
Infect Dis. 12:1572012. View Article : Google Scholar
|
|
28
|
Bele N, Darmon M, Coquet I, et al:
Diagnostic accuracy of procalcitonin in critically ill
immunocompromised patients. BMC Infect Dis. 11:2242011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu Y, Chen J, Cai B, et al: The use of
PCT, CRP, IL-6 and SAA in critically ill patients for an early
distinction between candidemia and Gram positive/negative
bacteremia. J Infect. 64:438–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdollahi A, Shoar S, Nayyeri F and
Shariat M: Diagnostic value of simultaneous measurement of
procalcitonin, interleukin-6 and hs-CRP in prediction of
early-onset neonatal sepsis. Mediterr J Hematol Infect Dis.
4:e20120282012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsangaris I, Plachouras D, Kavatha D, et
al: Diagnostic and prognostic value of procalcitonin among febrile
critically ill patients with prolonged ICU stay. BMC Infect Dis.
9:2132009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Póvoa P, Coelho L, Almeida E, et al: Early
identification of intensive care unit-acquired infections with
daily monitoring of C-reactive protein: a prospective observational
study. Crit Care. 10:R632006.PubMed/NCBI
|
|
33
|
Noor MK, Shahidullah M, Mutanabbi M, Barua
C, Mannan MA and Afroza S: Comparison between CRP and IL-6 as early
markers of neonatal sepsis. Mymensingh Med J. 17(Suppl 2): S72–S76.
2008.PubMed/NCBI
|
|
34
|
Chachkhiani I, Gürlich R, Maruna P, et al:
The postoperative stress response and its reflection in cytokine
network and leptin plasma levels. Physiol Res. 54:279–285.
2005.PubMed/NCBI
|
|
35
|
Cesur S, Irmak H, Eras Z, et al:
Prognostic value of serum TNF-alpha, IL-10, leptin and CRP levels
in newborns with septicemia. Mikrobiyol Bul. 43:607–612. 2009.(In
Turkish).
|
|
36
|
Maruna P, Gürlich R, Frasko R and Haluzík
M: Serum leptin levels in septic men correlate well with C-reactive
protein (CRP) and TNF-alpha but not with BMI. Physiol Res.
50:589–594. 2001.PubMed/NCBI
|
|
37
|
Bracho-Riquelme RL and Reyes-Romero MA:
Leptin in sepsis: a well-suited biomarker in critically ill
patients? Crit Care. 14:1382010. View
Article : Google Scholar : PubMed/NCBI
|