Introduction
Anorexia nervosa (AN) is a mental disorder that is
common worldwide. In 1999, the overall age and gender-adjusted
incidence rate was reported to be 8.3 cases per 100,000
person-years, and a long-term linear increase in the incidence rate
of AN was observed, particularly in adolescent females (1). Approximately 30% of patients with AN
show liver dysfunction (2,3) and histological findings of the liver
indicate similar results to those of patients with nonalcoholic
steatohepatitis (NASH) (4). NASH
is classified into two types: Primary NASH associated with a
lifestyle-related disease, such as metabolic syndrome and secondary
NASH following other diseases (5).
Thus, malnutrition-associated NASH is classified into secondary
NASH (6). Generally, liver
dysfunction complicated by malnutrition improves immediately after
the administration of total parenteral nutrition (TPN) (7–9);
however, it occasionally develops into acute hepatic failure
(4) and no established therapy is
available in such cases.
Primary NASH is an obesity-associated disease and
weight reduction therapy is the only remedy that has been confirmed
to be effective (10). However,
weight reduction therapy has limitations, as a number of patients
with NASH often fail to reduce their weight. Furthermore, weight
reduction therapy is not indicated for patients with secondary NASH
without obesity. Thus, several clinical trials have been conducted
to investigate alternative therapies for NASH. Pioglitazone was the
first drug whose effectiveness for NASH was confirmed in a
randomized control trial (11).
Therefore, pioglitazone may also be a potential therapeutic agent
for patients with secondary NASH.
The present study reports a case of secondary NASH
with AN in a 66-year-old woman, and the effects of pioglitazone on
liver function and histological findings are presented. This study
was approved by the Ethics Committee of Gifu University Graduated
School of Medicine (Gifu, Japan). The patient consented to the
publication of this study.
Case report
A 66-year-old female with AN was referred to the
Division of Internal Medicine at Seki Central Hospital (Gifu,
Japan) in June 2008 for liver dysfunction. The patient neither
consumed alcohol nor was infected with hepatitis. Computed
tomography (CT) and ultrasonography (US) showed normal findings of
the liver. The patient was extremely thin and had a body mass index
(BMI) of only 11.1 kg/m2. Needle biopsy of the liver was
not performed at this time. The patient continued to restrict
dietary intake in spite of counseling in the hospital and her
general condition gradually deteriorated. In January 2009 the
patient felt severe general fatigue and was not able to eat.
Finally, the patient agreed to hospitalization on January 14, 2009
due to severe malnutrition and liver dysfunction.
On admission, the height and weight of the patient
were 150 cm and 25 kg, respectively, corresponding to the same BMI
of 11.1 kg/m2. The body temperature was 37.4°C, blood
pressure was 120/64 mmHg, heart rate was 97 beats/min and
O2 saturation was 98%. The patient’s eyes were neither
anemic nor icteric. Cardiovascular, pulmonary, abdominal and
neurological examinations showed no abnormalities, but the skin was
observed to be dry with decreased turgor. Serum aspartate
aminotransferase (AST) and alanine aminotransferase (ALT) levels
were elevated to 3,665 IU/l and 1,495 IU/l, respectively.
Serological tests for HBsAg and anti-HCVAb were negative. The
prothrombin time was 52%. Elevated levels of serum bilirubin,
creatinine and urea nitrogen were also observed. Thyroid hormone
levels were normal (Table I).
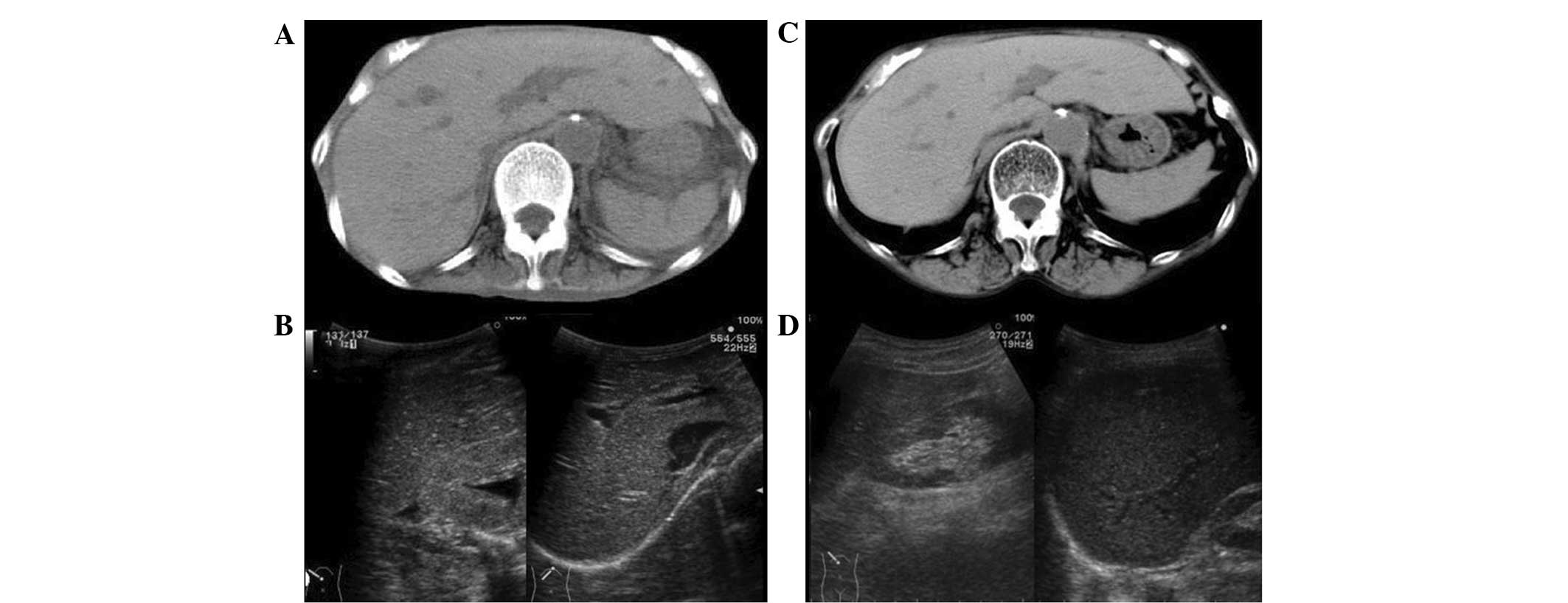
Abdominal CT showed a normal liver and the liver/spleen attenuation
ratio was 1.08 (hepatic CT attenuation was 65.6 Hu). Due to severe
emaciation, subcutaneous or visceral fat was hardly observed
(Fig. 1A). Abdominal US showed a
homogeneous liver pattern and the liver/kidney contrast was not
enhanced (Fig. 1B).
 | Table ILaboratory findings on admission. |
Table I
Laboratory findings on admission.
| Variable | Result |
|---|
| Complete blood
count |
| WBC | 3,100 cells/μl |
| RBC | 374 cells/μl |
| Hb | 12.1 g/dl |
| Ht | 36.4% |
| PLT | 9,6000 cells/μl |
| Neut | 79.3% |
| Lymph | 18.2% |
| Mono | 2.1% |
| Eos | 0.2% |
| Baso | 0.3% |
| Coagulation |
| PT | 52% |
| APTT | 28.5 sec |
| FDP | 71.9 mg/dl |
| Viral markers |
| IgM-anti-HA | (−) |
| HBsAg | (−) |
| Anti-HCV | (−) |
| IgG-anti-VCA | ×320 |
| IgG-anti-EBNA | ×10 |
| IgM-anti-CMV | (−) |
| Serological
tests |
| IgA | 262 mg/dl |
| IgM | 63 mg/dl |
| IgG | 946 mg/dl |
| Autoantibody |
| Anti-microsome | (−) |
|
Anti-tyroglobulin | (−) |
| ANA | 640 fold |
| AMA(M2) | (−) |
| Blood
chemistry |
| AST | 3,665 IU/l |
| ALT | 1,495 IU/l |
| ALP | 1,152 IU/l |
| CHE | 157 IU/l |
| γ-GTP | 237 IU/l |
| LDH | 1,594 IU/l |
| T.Bil | 1.35 mg/dl |
| D-Bil | 0.41 mg/dl |
| Alb | 3.8 g/dl |
| BUN | 94.5 mg/dl |
| Cr | 1.67 mg/dl |
| UA | 8.1 mg/dl |
| CPK | 265 IU/l |
| LDL | 66 mg/dl |
| TG | 23 mg/dl |
| HDL | 137 mg/dl |
| Amy | 288 IU/l |
| Na | 145 mEq/l |
| K | 6.52 mEq/l |
| Cl | 112 mEq/l |
|
NH3 | 35 μg/dl |
| CRP | 0 mg/dl |
| RF | 11.4 IU/ml |
| FBS | 52 mg/dl |
| Hormone |
| Free T3 | <1.0 pg/ml |
| Free T4 | 1.79 ng/dl |
| TSH | 1.85 μU/ml |
| IRI | 0.46 μIU/ml |
| IRG | 110 pg/ml |
| ACTH | 53.6 pg/ml |
| Cortisol | 26.3 μg/dl |
| Others |
| β-D-G | 6.6 pg/ml |
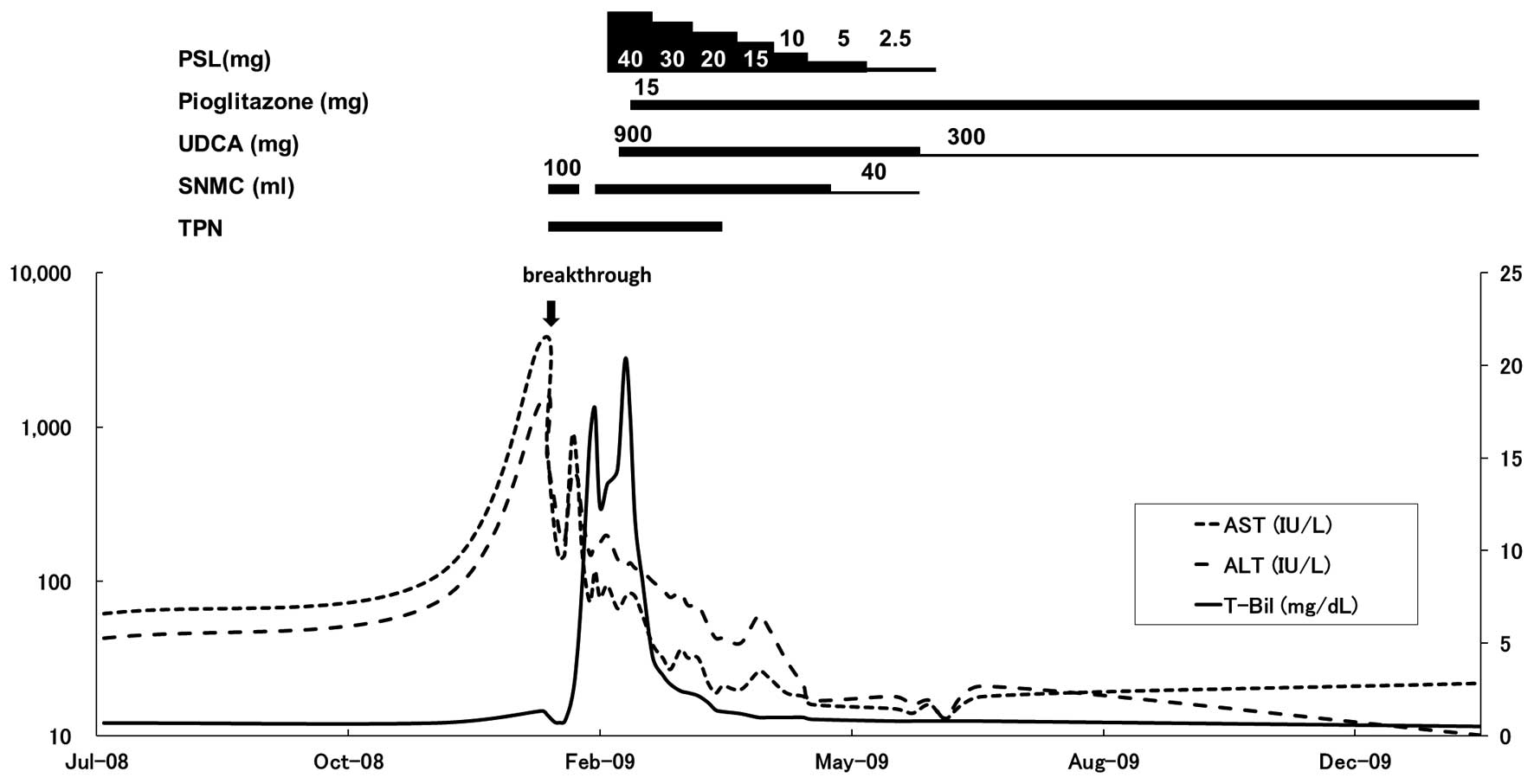
TPN was initiated at 1,000 kcal/day (20 kcal/kg
standard body weight/day or 40 kcal/kg actual body weight/day).
Phosphorus was also added to the TPN to avoid refeeding syndrome.
In addition, 100 ml Stronger Neo-Minophagen C (SNMC; Minophagen
Pharmaceutical Co., Ltd., Tokyo, Japan) was administered every day
to improve the AST and ALT levels, but was discontinued due to
secondary aldosteronism on the eighth day, resulting in increases
in AST and ALT levels (Fig. 2).
SNMC treatment was resumed on the 13th day, and the serum AST and
ALT levels were reduced to 74 and 150 IU/l, respectively. However,
total bilirubin levels increased to 15.9 mg/dl on the 20th day. To
confirm the diagnosis of liver dysfunction and to rule out liver
infection prior to the administration of prednisolone (PSL),
percutaneous needle biopsy of the liver was conducted on the 21st
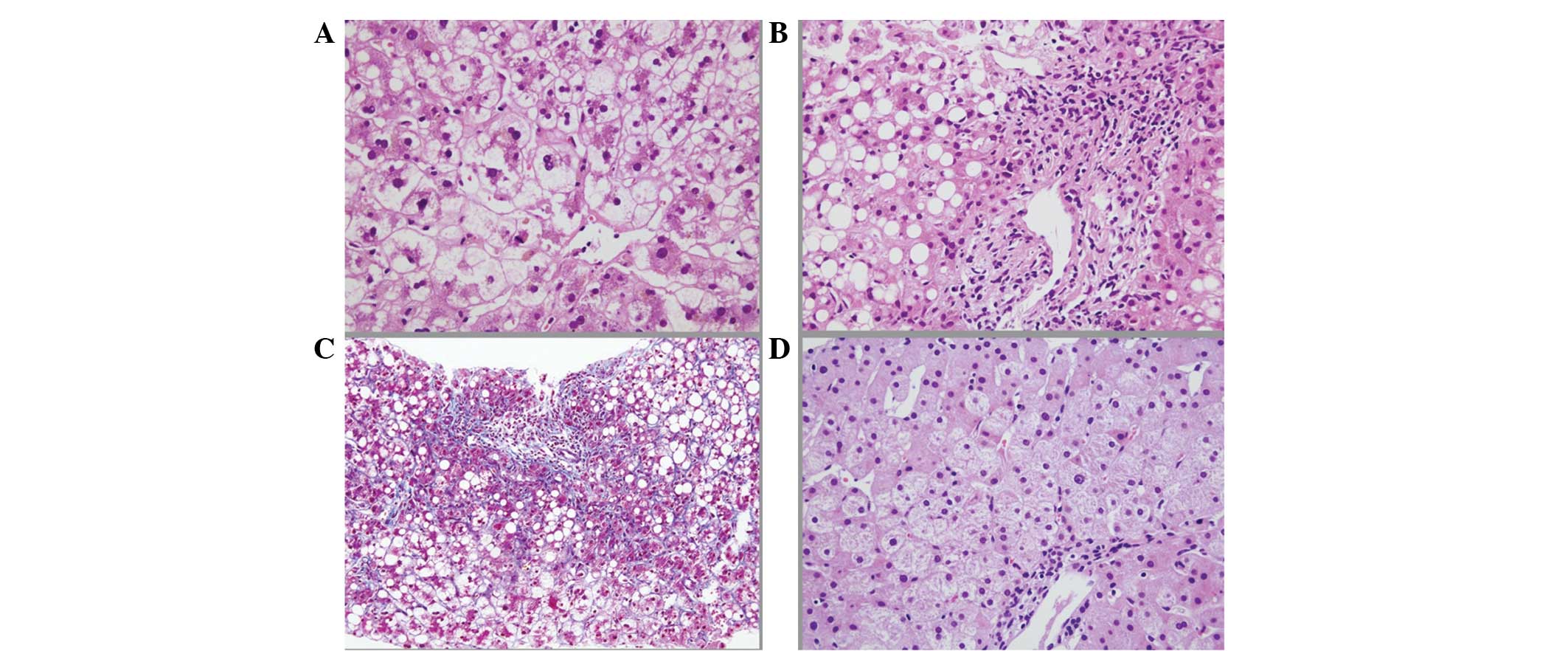
day. The pathological findings revealed: i) Macrovesicular
steatosis up to 50% of the tissue area; ii) zone 3 perisinusoidal
fibrosis with extensive periportal fibrosis; iii) neutrophilic
infiltration; iv) mitochondrial swelling; v) wide spread hepatocyte
ballooning; and vi) intrabiliary canalicular cholestasis. These
histological findings were compatible with NASH (6,12)
and thus led to a diagnosis of severe acute exacerbation of
secondary NASH with AN (Fig.
3A–C). Thereafter, 40 mg PSL was administered daily. However,
the effectiveness of PSL in the treatment of the increasing
jaundice was limited. Therefore, 900 mg ursodeoxycholic acid (UDCA)
was administered (Fig. 2).
Despite the combined treatment with PSL, SNMC, TPN
and UDCA, jaundice continued to increased and the AST and ALT
abnormalities were prolonged (Fig.
2). A previous study showed that the prognosis of patients that
were similar to this case, who did not respond to TPN refeeding
therapy, was extremely poor, mainly due to the late onset of
hepatic failure (4). Therefore,
once the patient’s family were informed of the risks of
pioglitazone for secondary NASH with AN, 15 mg pioglitazone was
administered daily starting on the 31st day. The serum AST and ALT
levels were reduced gradually thereafter and total bilirubin levels
showed a rapid reduction. Liver function was completely normalized
on the 105th day (Fig. 2). The
liver function remained normal after SNMC and PSL treatment were
tapered-off and the UDCA dosage was reduced to 300 mg per day. The
patient became able to walk following physical rehabilitation and
was discharged on the 168th day.
One year later, nutritional assessment, abdominal
CT, abdominal US and liver biopsy tests were performed to evaluate
the effectiveness of pioglitazone. Although the total energy intake
of the patient was 1,000 kcal/day, which showed no change compared
with that prior to treatment, 8 kg of body weight had been gained.
In the CT findings, the liver attenuation was 67.0 Hu and the
liver/spleen attenuation ratio was 1.11 (Fig. 1c), which were almost the same as
the findings on admission. However, the volume of subcutaneous,
visceral and trabecular fat tissues had markedly increased.
Abdominal US (Fig. 1D) also showed
no clear changes compared with that on admission. The histological
findings of the liver needle biopsy specimen (Fig. 3D) showed remarkable improvements in
the fibrosis, steatosis, inflammation and hepatocyte
ballooning.
Discussion
An increase in the number of obese individuals in
the population is of major concern worldwide. NASH is one of the
manifestations of obesity and its associated diseases (13). Simultaneously, the number of
patients with eating disorders (3)
has also increased among young women in developed countries, which
is considered to be associated with cultural transition and
globalization, including modernization, urbanization and
media-exposure promoting the Western beauty-ideal (14). A previous study showed that
elevated transaminase levels were observed in ~30% of patients with
AN (3) and its histological
findings were compatible with secondary NASH (4). Severe weight loss-associated fatty
liver disease has also been observed in protein energy malnutrition
(15) and postoperative
jejunoileal bypass (16).
The mechanisms of acute liver failure in secondary
NASH with AN are considered as follows: i) Circulatory disturbance
of the liver due to dehydration; and ii) starvation-induced
autophagy in the liver (7).
Although the outcome of malnutrition-associated NASH is usually
favorable with a rapid recover following treatment with TPN
(7–9), there have been certain cases with
fatal outcomes regardless of TPN and additional intensive therapy,
including hemodialysis or glucocorticoid administration (4,17,18).
For these patients with secondary NASH a novel therapeutic
treatment is urgently required.
Pioglitazone is an oral antidiabetic agent, which
improves insulin sensitivity through activating the nuclear
peroxisome proliferator activated receptor-γ (PPAR-γ). Clinically,
this mechanism of pioglitazone results in improved glycemic control
and decreased hepatic fat content. Additional studies have shown
that pioglitazone is a promising treatment agent for NASH (11,19).
Furthermore, a previous study showed that pioglitazone was also
effective for alcoholic steatohepatitis in rats without altering
insulin sensitivity (20).
Pioglitazone promotes the differentiation of pre-adipocytes into
adipocytes (21–23) and may result in the redistribution
of triglycerides from the liver into proliferating adipocytes.
Pioglitazone is generally known to cause weight gain as an adverse
effect, which is supported in this case as the patient gained 8 kg
of body weight. From these findings, it appears that pioglitazone
improved liver dysfunction simply through weight gain as its
adverse effect. Thus, pioglitazone may have direct or indirect
effects for patients with secondary NASH with AN without altering
insulin sensitivity.
There are limitations to this case report. There may
be ethical issues regarding the use of pharmaceuticals of this
family in patients with severe liver dysfunction because
idiosyncratic hepatotoxic injury has been reported previously for a
PPAR-γ agonist (24). However,
this was for troglitazone exclusively and, to the best of our
knowledge, pioglitazone has never caused such adverse effects.
Furthermore, several studies of pioglitazone in the treatment of
patients with NASH have confirmed its safety for use in patients
with liver dysfunction (11,19).
It may be questioned whether pioglitazone was effective, since the
serum ALT and AST levels were tending to decrease before the drug
treatment was initiated. Even if pioglitazone was effective, it
remains unclear whether the effects were direct. In the present
case, glucocorticoid, SNMC, TPN and UDCA were also administered.
TPN and glucocorticoids are known to promote hepatic steatosis
(25,26), while the effects of UDCA have yet
to be confirmed on NASH in randomized controlled studies (27). For SNMC, to the best of our
knowledge, there are no previous studies determining its effects on
NASH. In the present study, the histological improvements of the
patient’s liver biopsy specimen and normalization in liver function
after a year are not likely to be due to the agents PSL, TPN, UDCA
or SNMC, as the administration of these agents had already ended or
tapered at the point of histological re-examination. Therefore, it
may be suggested that the improvements were the result of
pioglitazone treatment. These findings support our hypothesis that
pioglitazone had a critical role in both clinical and histological
improvements of the patient’s liver.
Notably, although >50% macrovesicular fatty
changes were observed in the liver biopsy specimen, the
liver-spleen attenuation ratio analyzed from the CT scan was
>0.9, which was greater than that defined for fatty liver.
Additionally, hepato-renal contrast was not observed in abdominal
US. A previous study showed the sensitivity of US for detecting
steatosis in patients with nonalcoholic fatty liver disease was
100%, but was reduced to 77.8% in patients with advanced
histological fibrosis, while the sensitivity of CT scanning was
69.8 and 48.9% respectively, suggesting that the advanced fibrosis
may have interfered with the detection of steatosis by such imaging
modalities (28). This report may
explain the occult liver findings in the CT and US results in the
present case who exhibited moderate fibrosis in the liver.
In conclusion, it is noteworthy that the results of
the present case indicated the effectiveness of pioglitazone on
secondary NASH with AN in both serological and histological
findings. In addition, critical adverse events of pioglitazone were
not observed in the present case. Therefore, the administration of
pioglitazone for acute exacerbation of secondary NASH with AN may
be considered when conventional therapies are not effective as
their outcomes are very poor.
Abbreviations:
|
ALT
|
alanine aminotransferase
|
|
AN
|
anorexia nervosa
|
|
AST
|
aspartate aminotransferase
|
|
ASH
|
alcoholic steatohepatitis
|
|
BMI
|
body mass index
|
|
CT
|
computed tomography
|
|
NASH
|
nonalcoholic steatohepatitis
|
|
PPARγ
|
peroxisome proliferator activated
receptor-γ
|
|
PSL
|
prednisolone
|
|
PT
|
prothrombin time
|
|
SNMC
|
Stronger Neo-Minophagen C
|
|
TPN
|
total parenteral nutrition
|
|
UDCA
|
ursodeoxycholic acid
|
|
US
|
ultrasonography
|
References
|
1
|
Lucas AR, Crowson CS, O’Fallon WM and
Melton LJ III: The ups and downs of anorexia nervosa. Int J Eat
Disord. 26:397–405. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomita K, Haga H, Ishii G, et al: Clinical
manifestations of liver injury in patients with anorexia nervosa.
Hepatol Res. July 11–2013.(Epub ahead of print).
|
|
3
|
Kuboki T, Nomura S, Ide M, Suematsu H and
Araki S: Epidemiological data on anorexia nervosa in Japan.
Psychiatry Res. 62:11–16. 1996. View Article : Google Scholar
|
|
4
|
Sakada M, Tanaka A, Ohta D, et al: Severe
steatosis resulted from anorexia nervosa leading to fatal hepatic
failure. J Gastroenterol. 41:714–715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ludwig J, Viggiano TR, McGill DB and Oh
BJ: Nonalcoholic steatohepatitis: Mayo Clinic experiences with a
hitherto unnamed disease. Mayo Clin Proc. 55:434–438.
1980.PubMed/NCBI
|
|
6
|
Sanyal AJ; American Gastroenterological
Association. AGA technical review on nonalcoholic fatty liver
disease. Gastroenterology. 123:1705–1725. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rautou PE, Cazals-Hatem D, Moreau R, et
al: Acute liver cell damage in patients with anorexia nervosa: a
possible role of starvation-induced hepatocyte autophagy.
Gastroenterology. 135:840–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Narayanan V, Gaudiani JL, Harris RH and
Mehler PS: Liver function test abnormalities in anorexia nervosa -
cause or effect. Int J Eat Disord. 43:378–381. 2010.PubMed/NCBI
|
|
9
|
Dowman J, Arulraj R and Chesner I:
Recurrent acute hepatic dysfunction in severe anorexia nervosa. Int
J Eat Disord. 43:770–772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Promrat K, Kleiner DE, Niemeier HM, et al:
Randomized controlled trial testing the effects of weight loss on
nonalcoholic steatohepatitis. Hepatology. 51:121–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belfort R, Harrison SA, Brown K, et al: A
placebo-controlled trial of pioglitazone in subjects with
nonalcoholic steatohepatitis. N Engl J Med. 355:2297–2307. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matteoni CA, Younossi ZM, Gramlich T,
Boparai N, Liu YC and McCullough AJ: Nonalcoholic fatty liver
disease: a spectrum of clinical and pathological severity.
Gastroenterology. 116:1413–1419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seidell JC: Obesity, insulin resistance
and diabetes - a worldwide epidemic. Br J Nutr. 83(Suppl 1): S5–S8.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smink FR, van Hoeken D and Hoek HW:
Epidemiology of eating disorders: incidence, prevalence and
mortality rates. Curr Psychiatry Rep. 14:406–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osifo BO and Bolodeoku JO: Serum aspartate
and alanine aminotransferase activities in protein energy
malnutrition. Enzyme. 28:300–304. 1982.PubMed/NCBI
|
|
16
|
Meinhardt NG, Souto KE, Ulbrich-Kulczynski
JM and Stein AT: Hepatic outcomes after jejunoileal bypass: is
there a publication bias? Obes Surg. 16:1171–1178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzaki A, Miura H, Takazoe M, Hamada T and
Kitamura S: Nonalcoholic steatohepatitis due to Crohn disease: a
lethal case report. Nihon Shokakibyo Gakkai Zasshi. 100:1212–1218.
2003.(In Japanese).
|
|
18
|
Furuta S, Ozawa Y, Maejima K, et al:
Anorexia nervosa with severe liver dysfunction and subsequent
critical complications. Intern Med. 38:575–579. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aithal GP, Thomas JA, Kaye PV, et al:
Randomized, placebo-controlled trial of pioglitazone in nondiabetic
subjects with nonalcoholic steatohepatitis. Gastroenterology.
135:1176–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomita K, Azuma T, Kitamura N, et al:
Pioglitazone prevents alcohol-induced fatty liver in rats through
up-regulation of c-Met. Gastroenterology. 126:873–885. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hallakou S, Doaré L, Foufelle F, et al:
Pioglitazone induces in vivo adipocyte differentiation in the obese
Zucker fa/fa rat. Diabetes. 46:1393–1399. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kletzien RF, Clarke SD and Ulrich RG:
Enhancement of adipocyte differentiation by an insulin-sensitizing
agent. Mol Pharmacol. 41:393–398. 1992.PubMed/NCBI
|
|
23
|
Laplante M, Festuccia WT, Soucy G, et al:
Mechanisms of the depot specificity of peroxisome
proliferator-activated receptor gamma action on adipose tissue
metabolism. Diabetes. 55:2771–2778. 2006. View Article : Google Scholar
|
|
24
|
Watkins PB: Idiosyncratic liver injury:
challenges and approaches. Toxicol Pathol. 33:1–5. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Khaoustov VI, Krishnan B, et al:
Total parenteral nutrition induces liver steatosis and apoptosis in
neonatal piglets. J Nutr. 136:2547–2552. 2006.PubMed/NCBI
|
|
26
|
Matsumoto T, Yamasaki S, Arakawa A, et al:
Exposure to a high total dosage of glucocorticoids produces
non-alcoholic steatohepatits. Pathol Int. 57:388–389. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leuschner UF, Lindenthal B, Herrmann G, et
al: High-dose ursodeoxycholic acid therapy for nonalcoholic
steatohepatitis: a double-blind, randomized, placebo-controlled
trial. Hepatology. 52:472–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tobari M, Hashimoto E, Yatsuji S, Torii N
and Shiratori K: Imaging of nonalcoholic steatohepatitis:
advantages and pitfalls of ultrasonography and computed tomography.
Intern Med. 48:739–746. 2009. View Article : Google Scholar : PubMed/NCBI
|