Introduction
End-stage renal disease seriously affects human
health and maintenance hemodialysis is the primary therapy for this
condition. Although the technology for maintenance hemodialysis is
continually being developed, malnutrition and inflammation
significantly affect patient prognosis (1). Previous studies have shown that
tryptophan (Trp) is an important regulator of immune homeostasis
and inflammation (2). Trp is one
of the essential amino acids in the human body and has a critical
role in effective T cell function. Depletion of Trp activates
effector T lymphocytes, thereby mediating immune tolerance.
Furthermore, a metabolite of Trp, kynurenine (Kyn), promotes the
generation of regulatory T lymphocytes, which further promote
immune tolerance (3). Indoleamine
2,3-dioxygenase (IDO) is an intercellular rate-limiting enzyme that
catalyzes the conversion of Trp to Kyn and plays a key role in
immune homeostasis in uremic patients (2,4–6). IDO
is also an immunoregulatory signal transduction molecule (7). Therefore, Kyn functions as a new
potential marker in patients with end-stage renal disease.
IDO activity can be estimated by the concentration
ratio of Kyn to Trp (5,8). The Kyn/Trp ratio correlates with
inflammatory markers, including high-sensitivity C-reactive protein
and soluble tumor necrosis factor-receptor-1, and has been
recognized as a promising biomarker for evaluating immune
homeostasis and inflammation (4,5).
High-pressure liquid chromatography (HPLC) is a traditional method
for measuring plasma amino acids levels. However, simultaneous
measurement of Trp and Kyn is difficult using HPLC (9). Schefold et al reported a
costly electrospray-tandem mass spectrometry assay for measuring
Trp and Kyn simultaneously (5).
With regard to clinical practice, several previous approaches to
simultaneously measuring Trp and Kyn using HPLC have been reported
(10,11). However, these methods were limited
to isolating Kyn in uremic patients due to an interference of
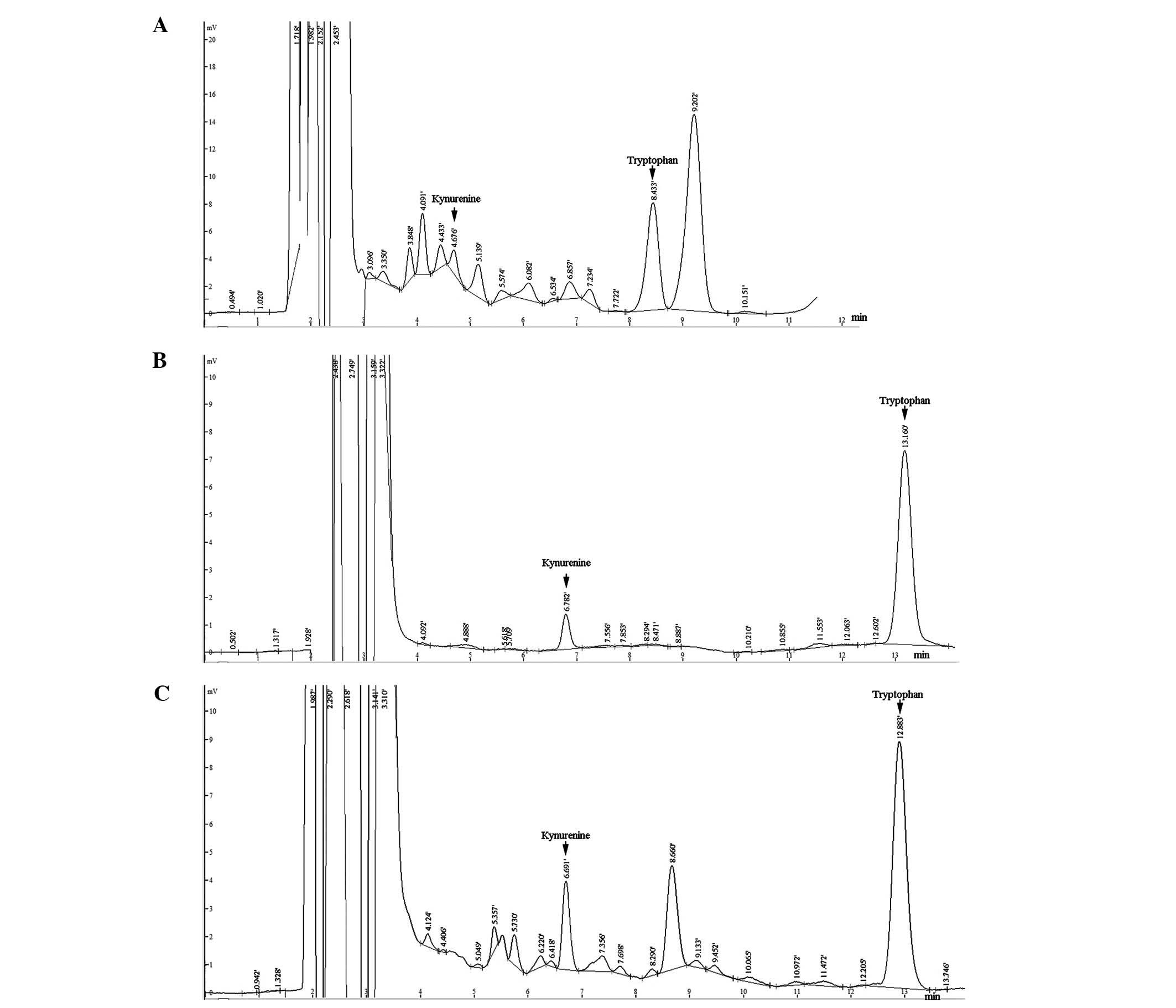
unknown uremic toxin (Fig. 1A).
Additional HPLC methods have been used for uremic patients, but
these methods have tedious and time-consuming procedures (12,13).
The present study aimed to develop a modified method
to measure Trp and Kyn simultaneously in uremic patients for the
evaluation of IDO activity.
Materials and methods
Reference method
The conventional method for simultaneous Trp and Kyn
detection was used as the reference. A SymmetryShield RP-C18 column
(150×3.9 mm; inner diameter, 5 μm) was purchased from Waters
Corporation (Milford, MA, USA). The column had a mobile phase of
2.7% acetonitrile and pH 3.6 (11).
Instruments
An LC-3A high-pressure liquid chromatograph
(Shimadzu, Kyoto, Japan), SCI 100 UV-Vis detector (Daniel Elite
Analytical Industries, Inc., Houston, TX, USA), YH-300 data
processing system (Easeatech, Guangzhou, China) and VICILC 25-μl
manual injector (Shimadzu, Kyoto, Japan) were used in the
procedure.
Reagents
Standard samples of Kyn and L-Trp were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Chromatographic pure
acetonitrile, potassium dihydrogen phosphate, sodium acetate,
analytical-grade acetic acid, 36% perchloric acid and ultra-pure
water were treated with a Milli-Q Pure water device (Millipore
Corporation, Billerica, MA, USA).
Protein precipitant preparation
Perchloric acid solution at a volume fraction of
5.0% was prepared using ultra-pure water.
Mobile phase
Acetic acid (36%) and acetonitrile solutions were
used in the mobile phase. Sodium acetate crystals were weighed and
sodium acetate-acetic acid solution (containing 5% acetonitrile)
was prepared. Prior to use, the solution was ultrasonically
degassed for 40 min, incubated overnight and filtered using a
precolumn (KJO-4282; Phenomenex, Torrance, CA, USA).
Preparation of the stock solution
Trp and Kyn stock solutions, at a concentration of
20 mmol/l, were prepared using 2.5% perchloric acid solution.
Aliquotted stock solutions were then stored at −4°C. Standard
working solutions were prepared using ultra-pure water prior to
each use.
Sample collection and processing
In total, 91 patients that were undergoing
hemodialysis three times a week (single pool Kt/V, >1.2) were
recruited from the Blood Purification Center at Guangdong General
Hospital (Guangzhou, China). An additional 10 healthy volunteers
were also recruited (Table I).
Blood samples (2 ml) were collected from the arteriovenous fistulas
of patients who had undergone maintenance hemodialysis and were
placed into 4-ml EDTA tubes. After 1 h, the blood was centrifuged
at room temperature at 2,200 × g for 10 min to separate the plasma.
Blood sample collection was approved by the Ethics Committee of
Guangdong General Hospital (2013069H) and all patients provided
informed consent.
 | Table ICharacteristics of the control
individuals and patients undergoing hemodialysis. |
Table I
Characteristics of the control
individuals and patients undergoing hemodialysis.
| Characteristics | Controls | Patients |
|---|
| Age, years | 34±10 | 53–77 |
| Gender, male/female,
n | 5/5 | 51/40 |
| Plasma Kyn
concentration, μmol/l | 0.8–0.4 | 1.07±0.10 |
| Plasma Trp
concentration, μmol/l | 31.3±21.2 | 5.62±0.98 |
| Plasma albumin,
g/l | | 31.1±3.5 |
| Hemodialysis time,
days | | 1326.9±317.2 |
| Single pool
Kt/V* | | 1.5±0.3 |
Plasma samples (50 μl) were placed into a
centrifugal tube and the same volume of 5% perchloric acid solution
was added. Next, the samples were placed in a vortex mixer for 30
sec and then maintained at room temperature for 15 min to allow
plasma protein precipitation (11). Finally, the samples were
centrifuged at 12,000 × g for 5 min and 20-μl samples of
supernatant were collected for analysis.
Chromatographic requirements
The procedure involved a SinoChrom ODS-BP C18 column
(4.6×150 mm; inner diameter, 4.5 μm; Dalian Elite Analytical
Instruments Co., Ltd., Dalian, China) and a mobile phase of 15
mmol/l sodium acetate acetic acid solution (containing 5%
acetonitrile, pH 4.8) at a flow rate of 1.0 ml/min. The
SC100-UV-visible detector was operated at 225 nm and the injection
volume was 20 μl. Concentrations were measured at room
temperature.
Preparation of mixed plasma
In total, 20 plasma samples were selected randomly
from the 91 patients undergoing maintenance hemodialysis. The
samples were mixed well and stored in a −20°C freezer for later
precision and recovery measurements (10).
Qualitative and quantitative analyses for
Kyn and Trp
Qualitative analyses for Kyn and Trp were performed
using the peak retention value comparison and superposition
methods, while quantitative analysis was performed using the
external standard method. Data processing was conducted using a
YH-300 Chromatograph workstation (Easeatech). Quantitative results
are expressed as mean ± SD.
Results
Trp and Kyn concentrations
Concentrations of Trp and Kyn were detectable in all
10 healthy volunteers and in 90 of the 91 patients who underwent
maintenance hemodialysis under the modified conditions (Table I). By contrast, the reference
method only detected the concentrations of Trp and Kyn in 14 of the
91 patients undergoing maintenance hemodialysis. Failure of the
reference method primarily arose from peak interference (Fig. 1A).
Standard curve and equation
Standard working solutions were prepared by
combining 2.5% perchloric acid solution with Trp and Kyn (Kyn,
0.004–100 μmol/l; Trp, 0.01–1,000 μmol/l). Each sample was injected
three times and the average result was used for analysis. The
results are shown in Table II,
where y represents the peak area (μV.sec) and x is the sample
concentration (μmol/l). A marked linear correlation was observed
between the peak area and the sample concentrations of Kyn and Trp,
with a wide linear range and a low detection limit, indicating good
sensitivity.
 | Table IIRegression analysis and detection
limits for Kyn and Trp. |
Table II
Regression analysis and detection
limits for Kyn and Trp.
| Substance | Regression
equation | R2 | Linear range,
μmol/l | Detection limit |
|---|
| Kyn | y = 28007x +
3843.9 | 1.0 | 0.08–50 | 0.02 |
| Trp | y = 27331x +
85588 | 1.0 | 0.8–500 | 0.2 |
Precision test
Chromatographic columns and precolumns were washed
daily with formaldehyde and the standard curves were corrected
every two days (10). Intra- and
inter-day precision tests were performed on mixed plasma samples
(Table III).
 | Table IIIPrecision of the intra- and inter-day
measurements of Kyn and Trp (n=20). |
Table III
Precision of the intra- and inter-day
measurements of Kyn and Trp (n=20).
| Substance | Intra-day, mol/l | RSD, % | Inter-day, mol/l | RSD, % |
|---|
| Kyn | 0.7301±0.0910 | 2.60 | 0.7415±0.0250 | 3.38 |
| Trp | 3.0280±0.0403 | 1.33 | 2.9184±0.1279 | 2.38 |
Discussion
Kyn/Trp ratio is a marker that reflects immune
homeostasis and inflammation. Although existing HPLC methods are
able to rapidly and simultaneously detect Trp and Kyn in non-uremic
patients, they fail to do so for uremic patients due to the
presence of unknown uremic substances that have absorption profiles
similar to that of Kyn (Fig. 1).
In the present study, the column diameter, pH value of the mobile
phase and the content of acetonitrile were adjusted to isolate Trp
and Kyn from these interfering components (Fig. 1C). The results indicated that the
modified method was able to simultaneously detect the
concentrations of plasma Trp and Kyn in uremic patients.
The method used in the present study differs from
previously reported HPLC methods. Zhen et al and Koening
et al used a 5-μm C18 column and adjusted the wavelength of
the ultraviolet detector during detection (12,13).
In the current study, a 4.5-μm C18 column was used. Furthermore,
the composition of the mobile phase was adjusted, which allowed for
the detection of Kyn and Trp simultaneously at a fixed wavelength
and with a shortened detection time. In addition, the current
method has a wider detection range which completely covers the
levels of human plasma Trp and Kyn under normal and pathological
states. The modified method is also applicable for in vitro
detection. However, in the present study, Trp and Kyn levels
remained undetected in one sample. The reason for this detection
failure remains to be explored.
In conclusion, the modified HPLC method is able to
detect Trp and Kyn simultaneously in uremic patients, satisfying
the requirements for IDO determination in clinical practice.
Acknowledgements
The authors thank the Medical Research Center
(Guangdong General Hospital, Guangzhou, China) core facilities for
assistance and Tie-feng Chen for technical assistance. The study
was supported by a grant from the National Key Technology R&D
Program (no. 2011BAI10B08) and the National Clinical Key Specialty
Construction Preparatory Projects.
References
|
1
|
Stenvinkel P, Heimbürger O, Paultre F,
Diczfalusy U, Wang T, Berglund L and Jogestrand T: Strong
association between malnutrition, inflammation, and atherosclerosis
in chronic renal failure. Kidney Int. 55:1899–1911. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medzhitov R, Shevach EM, Trinchieri G, et
al: Highlights of 10 years of immunology in Nature Reviews
Immunology. Nat Rev Immunol. 11:693–702. 2011.PubMed/NCBI
|
|
3
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eleftheriadis T, Liakopoulos V, Antoniadi
G, Stefanidis I and Galaktidou G: Indoleamine 2,3-dioxygenase is
increased in hemodialysis patients and affects immune response to
hepatitis B vaccination. Vaccine. 29:2242–2247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schefold JC, Zeden JP, Fotopoulou C, et
al: Increased indoleamine 2,3-dioxygenase (IDO) activity and
elevated serum levels of tryptophan catabolites in patients with
chronic kidney disease: a possible link between chronic
inflammation and uraemic symptoms. Nephrol Dial Transplant.
24:1901–1908. 2009. View Article : Google Scholar
|
|
6
|
Eleftheriadis T, Antoniadi G, Liakopoulos
V, Stefanidis I and Galaktidou G: Plasma indoleamine
2,3-dioxygenase concentration is increased in hemodialysis patients
and may contribute to the pathogenesis of coronary heart disease.
Ren Fail. 34:68–72. 2012. View Article : Google Scholar
|
|
7
|
Pallotta MT, Orabona C, Volpi C, et al:
Indoleamine 2,3-dioxygenase is a signaling protein in long-term
tolerance by dendritic cells. Nat Immunol. 12:870–878. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brandacher G, Cakar F, Winkler C, et al:
Non-invasive monitoring of kidney allograft rejection through IDO
metabolism evaluation. Kidney Int. 71:60–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pawlak D, Tankiewicz A, Mysliwiec P and
Buczko W: Tryptophan metabolism via the kynurenine pathway in
experimental chronic renal failure. Nephron. 90:328–335. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Chen Y, Zhang Y, Wang F and Chen Z:
Determination of tryptophan and kynurenine in human plasma by
liquid chromatography-electrochemical detection with multi-wall
carbon nanotube-modified glassy carbon electrode. Biomed
Chromatogr. 25:938–942. 2011. View
Article : Google Scholar
|
|
11
|
Wang R and Tang A: Simultaneous
determination of kynurenine and tryptophan in serum by high
performance liquid chromatography. Se Pu. 24:140–143. 2006.(In
Chinese).
|
|
12
|
Zhen Q, Xu B, Ma L, Tian G, Tang X and
Ding M: Simultaneous determination of tryptophan, kynurenine and
5-hydroxytryptamine by HPLC: Application in uremic patients
undergoing hemodialysis. Clin Biochem. 44:226–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koenig P, Nagl C, Neurauter G, Schennach
H, Brandacher G and Fuchs D: Enhanced degradation of tryptophan in
patients on hemodialysis. Clin Nephrol. 74:465–470. 2010.
View Article : Google Scholar : PubMed/NCBI
|