1. Introduction
Han et al performed a cross-sectional study
on a group of human immunodeficiency virus (HIV)-1-infected
patients who underwent long-term highly active antiretroviral
therapy. The results indicated that various fragments of the HIV-1
genome were detectable in tears, in the absence of a detectable
plasma viral load (1). Earlier in
the 1980s, studies isolated HIV viruses from tears, cornea, aqueous
humor, conjunctiva, retinal vascular endothelium and even contact
lenses (2–4). Pathanapitoon et al analyzed
the aqueous and vitreous humor samples from HIV-1-infected patients
and observed that several patients had intraocular HIV-1 RNA levels
that were higher than the corresponding HIV-1 RNA plasma levels,
which indicated a largely elevated ocular-to-plasma HIV ratio
(5). Thus, the mechanisms by which
HIV invades the eye and exists in the tissues in the absence of a
detectable plasma virus level were questioned. To date, there has
been no explanation of these circumstances. A growing number of
studies have shown that the central nervous system (CNS) is a
sanctuary for HIV, which crosses the blood-brain barrier (BBB)
early in the course of systemic infection and resides in brain
macrophages and microglia (6,7). One
hypothesis is that HIV persists in these sanctuaries during
antiretroviral treatment and may cause the generation and
dissemination of drug-resistant viruses (8). Another hypothesis is that the
breakdown of the blood-retinal barrier (BRB), which is associated
with the changes in the tight junctions, contributes to the
trafficking of HIV into the eye (9,10).
Therefore, the present review focused on the key breakdown
mechanisms of tight junctions.
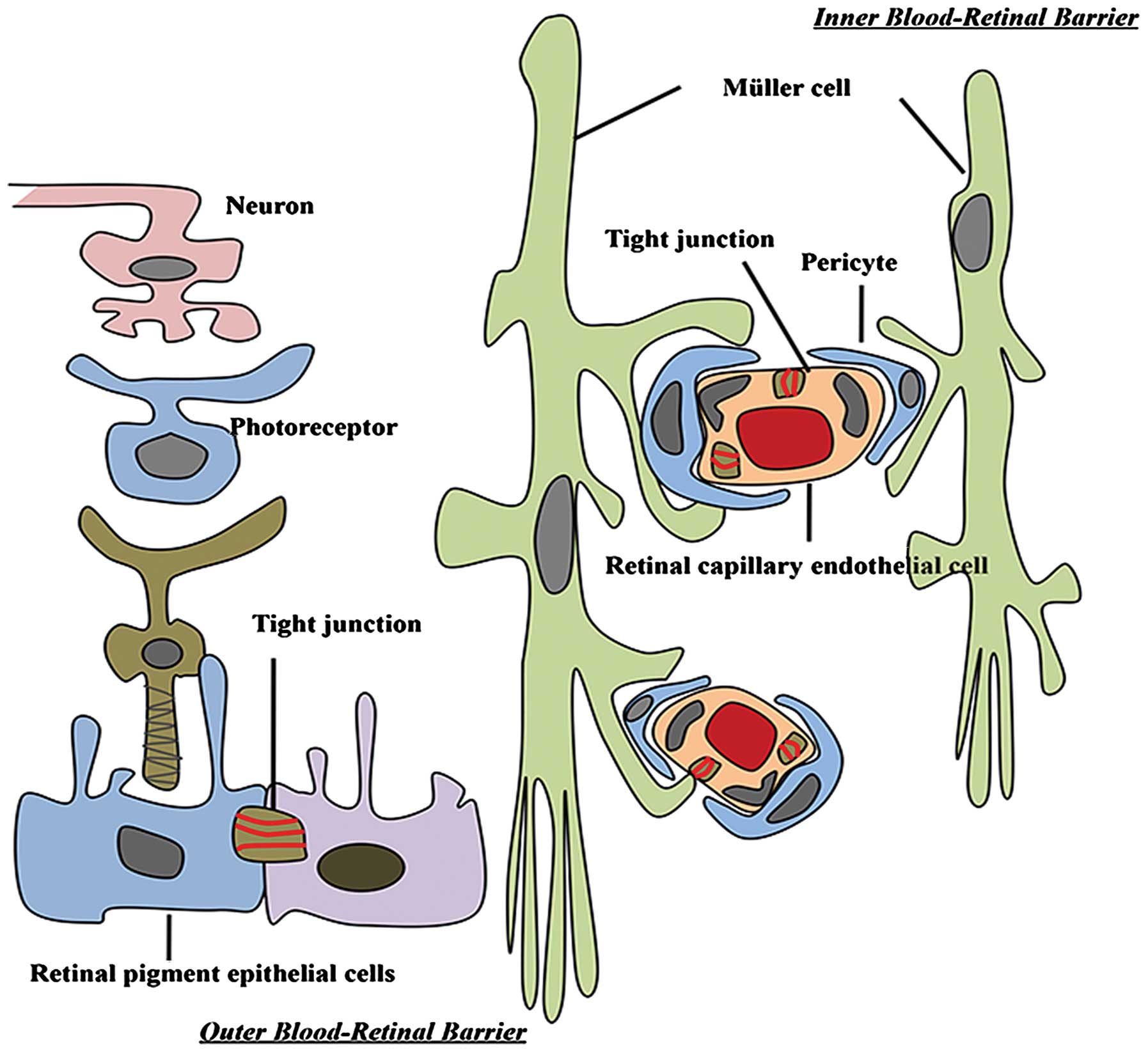
2. Components of the blood-retinal
barrier
The BBB provides significant protection against
microbial invasion of the brain (11). The BRB and BBB are derived from the
same embryonic primordium. Brain endothelial cells form extremely
tight cell-cell junctions that are distinct from the tight
junctions of endothelia and epithelia elsewhere in the body. Brain
endothelial cells lack fenestrations and have a high number of
mitochondria, which are characteristics associated with their
specialized functions. For example, a high mitochondrial content is
likely to be important for providing the energy required to
maintain the structure and function of the BBB (12). For BBB capillaries, the
transendothelial electrical resistance, an indicator of
permeability, ranges between 1,000 and 2,000 Ω/cm2.
However, for systemic capillaries this value is only 5–10
Ω/cm2. The BRB, which maintains eye homeostasis, has a
similar nature to the BBB (13).
The BRB is composed of retinal capillary endothelial cells (inner
BRB) and retinal pigment epithelium (RPE) cells (outer BRB)
(14). These two cell types
develop tight junctions that confer a high degree of control of
solute and fluid permeability between the circulating blood and the
neural retina (Fig. 1).
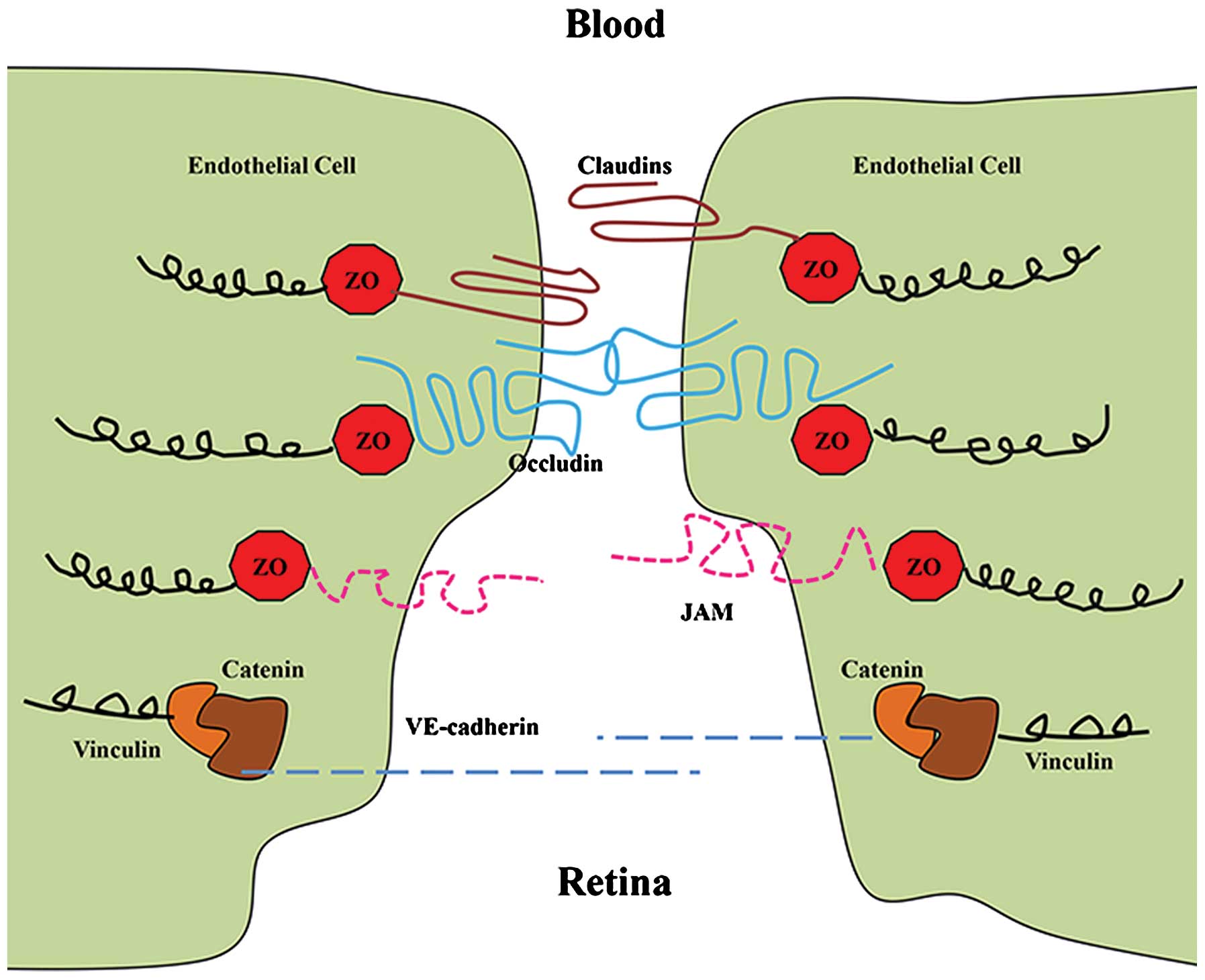
3. Tight junctions in the eye
The transmembrane proteins of tight junctions
include occludin, junction adhesion molecules and claudins. These
proteins extend into the paracellular space, acting in concert to
affect barrier properties (15).
Occludin and claudins have external loops that mediate
intercellular adhesion by interaction with occludin and claudins of
neighboring cells (16). In
addition, claudins and occludin interact with zonula occludens
(ZOs) −1, −2 and −3, which in turn associate with the actin
cytoskeleton (Fig. 2). The 220-kDa
phosphoprotein ZO-1, in particular, is able to bind to a wide
variety of protein partners and allow for the control of tight
junction assembly (17). During
viral infections and other pathological conditions, altering the
localization or cleavage of the tight junction proteins is the main
pathological change, which results in the increasing permeability
of the barrier (18).
4. Claudins
Claudin-5 is expressed predominantly in endothelial
cells (19). A study using
claudin-5-deficient mice demonstrated that it is necessary to
preserve the vascular barrier to small (<0.8 kDa) molecules in
the brain (20). As claudin-5 is
expressed in the retinal vasculature (21), it is likely to contribute to the
function of the BRB. The expression of claudin-3, −10 and −19 has
been detected in the human fetal RPE (22).
5. Occludin
Increased expression of occludin has been observed
to correlate with increased barrier function and decreased
paracellular permeability (23,24).
In addition, changes in the content and localization of occludin in
diabetic retinas have been demonstrated to be associated with
alterations in barrier function (25). A study of bovine retinal
endothelial cells treated with vascular endothelial growth factor
(VEGF) revealed a decreased occludin content and immunostaining at
cell borders concomitant with increased BRB permeability (26). Similarly, diabetes reduces occludin
content in the brain vasculature; this reduction correlates with
the incidence of vascular diseases (27).
6. Zonula occludens-1
ZO proteins are intracellular proteins that
associate with the cytoplasmic surface of tight junctions and
organize the tight junction complex. The presence of ZO-1 is
readily observed in retinal vascular endothelial and RPE cells. In
these cell types, agents that induce permeability, including VEGF
or hepatocyte growth factor, induce the redistribution of ZO-1 from
the cell border to the cell interior (28,29).
7. Mechanism for the disruption of tight
junctions
Increased permeability of the BRB may occur through
two pathways, the paracellular or the transcellular pathway. The
paracellular route is governed by tight junctions and is usually
the main route of increased endothelial barrier permeability
(30).
8. Tat-induced caveolae-associated
signaling
Tat is the only protein actively secreted by HIV-1
infected cells. It circulates in the blood at high levels during
HIV infection and crosses the BBB with large quantities entering
the CNS (31). The VEGF receptor
has been hypothesized to serve as a high-affinity receptor for Tat
in endothelial cells (32). Tat
specifically interacts with VEGF and surface molecules that belong
to the large family of G-protein-coupled receptors localized to
caveolae, to activate several protein kinases, including certain
kinases involved in Ras signaling (33). Ras proteins are small GTPases that
cycle between inactive GDP-bound and active GTP-bound
conformations. Several elements of the Ras signaling cascades are
localized in caveolae, the dominant type of lipid rafts in
endothelial cells (34). Zhong
et al focused on the breakdown mechanism of the BBB and
found that Tat diminished the expression of several tight junction
proteins, including occludin, ZO-1 and ZO-2, in the caveolar
fraction of human brain microvascular endothelial cells (HBMECs)
(35). These effects were
effectively protected against by the pharmacological inhibition of
Ras signaling and by the silencing of caveolin-1. Lin et al
demonstrated that HIV infection in primary human monocyte-derived
macrophages results in a marked upregulation of caveolin-1
expression mediated by the HIV Tat protein (36). Nag et al assessed the
sequential expression of caveolae and occludin over a period of 12
h to 6 days post-lesion in a rat cortical cold injury model. The
study demonstrated a significant increase in endothelial caveolin-1
expression in arterioles and large veins, particularly those with
BBB breakdown to proteins (37).
In addition, the HIV-1 Tat protein causes the paracellular
permeability of RPE cells to increase in vitro, concomitant
with changes in the expression of tight junctions. Therefore, the
effects of Tat on the outer BRB may be mediated by Ras/ERK1/2
pathways (9).
9. Disruption of tight junctions and the
basal lamina by secreted matrix metalloproteinases
The basal lamina of the BBB contains extracellular
matrix molecules, including laminin, type IV collagen and
fibronectin. The majority of these molecules are substrates for a
family of neutral proteases called matrix metalloproteinases
(MMPs), in particular MMP-2 and −9 (38). MMPs contribute to interactions
between cells and the matrix, allowing movement and shape changes
in CNS development and neuronal plasticity. MMPs are key mediators
of tight junction protein alterations, which lead to BBB
dysfunction (39,40). These zinc-dependent enzymes have
proteolytic activity that acts on the extracellular matrix,
including the basal laminae. MMPs are associated with tight
junction disruption not only by basement membrane degradation, but
also by cleavage of tight junction proteins (41,42).
Elevated levels of MMP-9 have been reported in the cerebrospinal
fluid of HIV-1-infected children (43) and adult patients (44). The overexpression of MMP-2 and
MMP-9 was also reported in the brain of a severe combined
immunodeficiency mouse model of HIV-1 encephalitis (45). In a study in rats, within 30 min of
HIV-1 glycoprotein 120 (gp120) injection into the caudate-putamen
(CP), MMP-2 co-localized with laminin and by 6 h there was a
significant reduction in the number of laminin-positive structures
in the injected CP. Similarly, the levels of vascular tight
junction proteins, claudin-5 and occludin, were significantly
decreased in the experimental group compared with those in the
controls (46). In one study,
primary HBMECs were exposed to HIV-1 Tat proteins. Tat induced
MMP-9 expression, and RNA interference targeting MMP-9 reduced the
paracellular permeability of Tat-treated HBMECs and the
concentration of soluble occludin in the cell supernatant (47). In a diabetic rat model, the
transepithelial electrical resistance (TER) was measured in the
retinal endothelium and RPE following treatment with MMPs. The two
cell types showed decreased TER and degradation of the tight
junction proteins, indicating that elevated expression levels of
MMPs in the retina may facilitate the change in BRB permeability
(48).
10. Disruption of tight junctions by
alterations in the actin cytoskeleton
Tight junctions may also be disrupted from within
cells. Changes in the actin cytoskeleton are likely to occur upon
alteration of the tight junction proteins, resulting in
paracellular permeability changes (49). Reactive oxygen species play a role
in disrupting tight junctions from within cells via the induction
of the RhoA small GTPase, phosphoinositide 3-kinase and protein
kinase B signaling pathways, concomitant with the rearrangement of
the actin cytoskeleton and altered localization of occludin and
claudin-5 (50). In addition, the
alteration of the actin cytoskeleton induced by hypoxic stress
correlates with changes in BBB permeability and ZO-1 localization
(51). Bruban et al showed
that disorganization of cytoskeletal actin filament models was
accompanied by decreased expression of tight junction proteins by
the RPE (52).
11. Inflammatory cytokines induce the
destruction of tight junctions
A number of cytokines have been reported to be
upregulated in the plasma of HIV-infected individuals or in plasma
treated ex vivo. In vitro, the interaction of HIV or the HIV
gp120 envelope with CD4 molecules induces the secretion of tumor
necrosis factor (TNF)-α, interleukin (IL)-1 and other cytokines
(53). Several studies have noted
a marked increase in membrane permeability following exposure to
vasoactive cytokines, including TNF-α, IL-1β, interferon-γ,
histamine 65 and growth factors (54). When activated, inflammatory cells
initiate the cellular release of free radicals, cytokines and
growth factors (55). HIV-1 Tat is
a strong proinflammatory agent that recruits and induces the
transendothelial migration of monocytes (56). When Tat was injected into the
hippocampi of mice, reductions in the levels of ZO-1 and its
continuity were observed, in addition to inflammatory cell
accumulation in the choroid plexus (57). It has also been shown that the
expression of the occludin promoter is affected by TNF-α or
interferon treatment (58). These
studies indicate that the expression of HIV genes or proteins may
alter the capacity of the cells to secrete important cytokines.
Therefore, cytokines are likely to play a vital role in the
pathology of HIV-associated complications.
12. Concluding remarks
The interplay between HIV-1 and hosts at the BRB is
complex. The present review has shown that specific viral genes
affect signaling pathways, the expression of enzymes, including
MMPs, and cytokines that affect inflammation, which leads to the
disruption of tight junctions. These effects are directly caused by
HIV-1-associated proteins or HIV-1-induced inflammatory factors.
This accumulating damage results in BRB breakdown. Advances in the
understanding of HIV-host interactions are likely to be forthcoming
as researchers apply effective approaches to their studies of this
challenging topic. The present study reveals insights into the
molecular mechanisms underlying BRB regulation and may provide
opportunities for the treatment of ocular complications.
Acknowledgments
The study was supported by a grant from the National
Nature Science Foundation of China (no. 81070757).
References
|
1
|
Han Y, Wu N, Zhu W, Li Y, Zuo L, Ye J, Qiu
Z, Xie J and Li T: Detection of HIV-1 viruses in tears of patients
even under long-term HAART. Aids. 25:1925–1927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujikawa LS, Salahuddin SZ, Palestine AG,
Masur H, Nussenblatt RB and Gallo RC: Isolation of human
T-lymphotropic virus type III from the tears of a patient with the
acquired immunodeficiency syndrome. Lancet. 2:529–530. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tervo T, Lähdevirta J, Vaheri A, Valle SL
and Suni J: Recovery of HTLV-III from contact lenses. Lancet.
1:379–380. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ablashi DV, Sturzenegger S, Hunter EA,
Palestine AG, Fujikawa LS, Kim MK, Nussenblatt RB, Markham PD and
Salahuddin SZ: Presence of HTLV-III in tears and cells from the
eyes of AIDS patients. J Exp Pathol. 3:693–703. 1987.PubMed/NCBI
|
|
5
|
Pathanapitoon K, Riemens A, Kongyai N,
Sirirungsi W, Leechanachai P, Ausayakhun S, Kalinina Ayuso V,
Kunavisarut P, de Groot-Mijnes JD and Rothova A: Intraocular and
plasma HIV-1 RNA loads and HIV uveitis. Aids. 25:81–86. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
González-Scarano F and Martín-García J:
The neuropathogenesis of AIDS. Nat Rev Immunol. 5:69–81. 2005.
|
|
7
|
Persidsky Y and Poluektova L: Immune
privilege and HIV-1 persistence in the CNS. Immunol Rev.
213:180–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Luca A, Ciancio BC, Larussa D, Murri R,
Cingolani A, Rizzo MG, Giancola ML, Ammassari A and Ortona L:
Correlates of independent HIV-1 replication in the CNS and of its
control by antiretrovirals. Neurology. 59:342–347. 2002.PubMed/NCBI
|
|
9
|
Bai L, Zhang Z, Zhang H, Li X, Yu Q, Lin H
and Yang W: HIV-1 Tat protein alter the tight junction integrity
and function of retinal pigment epithelium: an in vitro study. BMC
Infect Dis. 8:772008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pomerantz RJ, Kuritzkes DR, de la Monte
SM, Rota TR, Baker AS, Albert D, Bor DH, Feldman EL, Schooley RT
and Hirsch MS: Infection of the retina by human immunodeficiency
virus type I. N Engl J Med. 317:1643–1647. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abbott NJ, Patabendige AA, Dolman DE,
Yusof SR and Begley DJ: Structure and function of the blood-brain
barrier. Neurobiol Dis. 37:13–25. 2010. View Article : Google Scholar
|
|
12
|
Wislocki GB and Ladman AJ: The
demonstration of a blood-ocular barrier in the albino rat by means
of the intravitam deposition of silver. J Biophys Biochem Cytol.
1:501–510. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engelhardt B and Sorokin L: The
blood-brain and the blood-cerebrospinal fluid barriers: function
and dysfunction. Semin Immunopathol. 31:497–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cunha-Vaz JG: The blood-ocular barriers:
past, present, and future. Doc Ophthalmol. 93:149–157. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fanning AS, Mitic LL and Anderson JM:
Transmembrane proteins in the tight junction barrier. J Am Soc
Nephrol. 10:1337–1345. 1999.PubMed/NCBI
|
|
16
|
Hawkins BT and Davis TP: The blood-brain
barrier/neurovascular unit in health and disease. Pharmacol Rev.
57:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stamatovic SM, Keep RF and Andjelkovic AV:
Brain endothelial cell-cell junctions: how to ‘open’ the blood
brain barrier. Curr Neuropharmacol. 6:179–192. 2008.
|
|
18
|
Contreras-Ruiz L, Schulze U,
García-Posadas L, Arranz-Valsero I, López-García A, Paulsen F and
Diebold Y: Structural and functional alteration of corneal
epithelial barrier under inflammatory conditions. Curr Eye Res.
37:971–981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morita K, Sasaki H, Furuse M and Tsukita
S: Endothelial claudin: claudin-5/TMVCF constitutes tight junction
strands in endothelial cells. J Cell Biol. 147:185–194. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H,
Hashimoto N, Furuse M and Tsukita S: Size-selective loosening of
the blood-brain barrier in claudin-5-deficient mice. J Cell Biol.
161:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Antonetti DA, Barber AJ, Khin S, Lieth E,
Tarbell JM and Gardner TW; Penn State Retina Research Group.
Vascular permeability in experimental diabetes is associated with
reduced endothelial occludin content: vascular endothelial growth
factor decreases occludin in retinal endothelial cells. Diabetes.
47:1953–1959. 1998. View Article : Google Scholar
|
|
22
|
Peng S, Adelman RA and Rizzolo LJ: Minimal
effects of VEGF and anti-VEGF drugs on the permeability or
selectivity of RPE tight junctions. Invest Ophthalmol Vis Sci.
51:3216–3225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harhaj NS and Antonetti DA: Regulation of
tight junctions and loss of barrier function in pathophysiology.
Int J Biochem Cell Biol. 36:1206–1237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feldman GJ, Mullin JM and Ryan MP:
Occludin: structure, function and regulation. Adv Drug Deliv Rev.
57:883–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erickson KK, Sundstrom JM and Antonetti
DA: Vascular permeability in ocular disease and the role of tight
junctions. Angiogenesis. 10:103–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Behzadian MA, Windsor LJ, Ghaly N, Liou G,
Tsai NT and Caldwell RB: VEGF-induced paracellular permeability in
cultured endothelial cells involves urokinase and its receptor.
FASEB J. 17:752–754. 2003.PubMed/NCBI
|
|
27
|
Chehade JM, Haas MJ and Mooradian AD:
Diabetes-related changes in rat cerebral occludin and zonula
occludens-1 (ZO-1) expression. Neurochem Res. 27:249–252. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fischer S, Wobben M, Marti HH, Renz D and
Schaper W: Hypoxia-induced hyperpermeability in brain microvessel
endothelial cells involves VEGF-mediated changes in the expression
of zonula occludens-1. Microvasc Res. 63:70–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin M, Barron E, He S, Ryan SJ and Hinton
DR: Regulation of RPE intercellular junction integrity and function
by hepatocyte growth factor. Invest Ophthalmol Vis Sci.
43:2782–2790. 2002.PubMed/NCBI
|
|
30
|
Lum H and Malik AB: Regulation of vascular
endothelial barrier function. Am J Physiol. 267:L223–L241.
1994.PubMed/NCBI
|
|
31
|
Davidson DC, Hirschman MP, Sun A, Singh
MV, Kasischke K and Maggirwar SB: Excess soluble CD40L contributes
to blood brain barrier permeability in vivo: implications for
HIV-associated neurocognitive disorders. PloS One. 7:e517932012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Albini A, Benelli R, Presta M, Rusnati M,
Ziche M, Rubartelli A, Paglialunga G, Bussolino F and Noonan D:
HIV-tat protein is a heparin-binding angiogenic growth factor.
Oncogene. 12:289–297. 1996.PubMed/NCBI
|
|
33
|
Wennerberg K, Rossman KL and Der CJ: The
Ras superfamily at a glance. J Cell Sci. 118:843–846. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cohen AW, Hnasko R, Schubert W and Lisanti
MP: Role of caveolae and caveolins in health and disease. Physiol
Rev. 84:1341–1379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhong Y, Zhang B, Eum SY and Toborek M:
HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling
and cAMP response element-binding protein activation. J Neurosci.
32:143–150. 2012. View Article : Google Scholar
|
|
36
|
Lin S, Wang XM, Nadeau PE and Mergia A:
HIV infection upregulates caveolin 1 expression to restrict virus
production. J Virol. 84:9487–9496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nag S, Venugopalan R and Stewart DJ:
Increased caveolin-1 expression precedes decreased expression of
occludin and claudin-5 during blood-brain barrier breakdown. Acta
Neuropathol. 114:459–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clark IM, Swingler TE, Sampieri CL and
Edwards DR: The regulation of matrix metalloproteinases and their
inhibitors. Int J Biochem Cell Biol. 40:1362–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng S, Cen J, Huang Y, Shen H, Yao L,
Wang Y and Chen Z: Matrix metalloproteinase-2 and −9 secreted by
leukemic cells increase the permeability of blood-brain barrier by
disrupting tight junction proteins. PloS One. 6:e205992011.
|
|
40
|
Yang Y, Estrada EY, Thompson JF, Liu W and
Rosenberg GA: Matrix metalloproteinase-mediated disruption of tight
junction proteins in cerebral vessels is reversed by synthetic
matrix metalloproteinase inhibitor in focal ischemia in rat. J
Cereb Blood Flow Metab. 27:697–709. 2007.
|
|
41
|
Gurney KJ, Estrada EY and Rosenberg GA:
Blood-brain barrier disruption by stromelysin-1 facilitates
neutrophil infiltration in neuroinflammation. Neurobiol Dis.
23:87–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reijerkerk A, Kooij G, van der Pol SM,
Khazen S, Dijkstra CD and de Vries HE: Diapedesis of monocytes is
associated with MMP-mediated occludin disappearance in brain
endothelial cells. FASEB J. 20:2550–2552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McCoig C, Castrejon MM, Saavedra-Lozano J,
Castano E, Baez C, Lanier ER, Saez-Llorens X and Ramilo O:
Cerebrospinal fluid and plasma concentrations of proinflammatory
mediators in human immunodeficiency virus-infected children.
Pediatr Infect Dis J. 23:114–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sporer B, Paul R, Koedel U, Grimm R, Wick
M, Goebel FD and Pfister HW: Presence of matrix metalloproteinase-9
activity in the cerebrospinal fluid of human immunodeficiency
virus-infected patients. J Infect Dis. 178:854–857. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Persidsky Y, Limoges J, Rasmussen J, Zheng
J, Gearing A and Gendelman HE: Reduction in glial immunity and
neuropathology by a PAF antagonist and an MMP and TNFalpha
inhibitor in SCID mice with HIV-1 encephalitis. J Neuroimmunol.
114:57–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Louboutin JP and Strayer DS: Blood-brain
barrier abnormalities caused by HIV-1 gp120: mechanistic and
therapeutic implications. Scientific World Journal.
2012:4825752012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu R, Feng X, Xie X, Zhang J, Wu D and Xu
L: HIV-1 Tat protein increases the permeability of brain
endothelial cells by both inhibiting occludin expression and
cleaving occludin via matrix metalloproteinase-9. Brain Res.
1436:13–19. 2012. View Article : Google Scholar
|
|
48
|
Giebel SJ, Menicucci G, McGuire PG and Das
A: Matrix metalloproteinases in early diabetic retinopathy and
their role in alteration of the blood-retinal barrier. Lab Invest.
85:597–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lai CH, Kuo KH and Leo JM: Critical role
of actin in modulating BBB permeability. Brain Res Brain Res Rev.
50:7–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schreibelt G, Kooij G, Reijerkerk A, van
Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA,
Couraud PO, Piontek J, et al: Reactive oxygen species alter brain
endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB
signaling. FASEB J. 21:3666–3676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hicks K, O’Neil RG, Dubinsky WS and Brown
RC: TRPC-mediated actin-myosin contraction is critical for BBB
disruption following hypoxic stress. Am J Physiol Cell Physiol.
298:C1583–C1593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bruban J, Glotin AL, Dinet V, Chalour N,
Sennlaub F, Jonet L, An N, Faussat AM and Mascarelli F:
Amyloid-beta(1–42) alters structure and function of retinal
pigmented epithelial cells. Aging Cell. 8:162–177. 2009.
|
|
53
|
D’Addario M, Wainberg MA and Hiscott J:
Activation of cytokine genes in HIV-1 infected myelomonoblastic
cells by phorbol ester and tumor necrosis factor. J Immunol.
148:1222–1229. 1992.PubMed/NCBI
|
|
54
|
Mohammad G, Siddiquei MM, Othman A,
Al-Shabrawey M and Abu El-Asrar AM: High-mobility group box-1
protein activates inflammatory signaling pathway components and
disrupts retinal vascular-barrier in the diabetic retina. Exp Eye
Res. 107:101–109. 2013. View Article : Google Scholar
|
|
55
|
Keshari RS, Jyoti A, Dubey M, Kothari N,
Kohli M, Bogra J, Barthwal MK and Dikshit M: Cytokines induced
neutrophil extracellular traps formation: implication for the
inflammatory disease condition. PloS One. 7:e481112012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Weiss JM, Nath A, Major EO and Berman JW:
HIV-1 Tat induces monocyte chemoattractant protein-1-mediated
monocyte transmigration across a model of the human blood-brain
barrier and up-regulates CCR5 expression on human monocytes. J
Immunol. 163:2953–2959. 1999.
|
|
57
|
Pu H, Tian J, Andras IE, Hayashi K, Flora
G, Hennig B and Toborek M: HIV-1 Tat protein-induced alterations of
ZO-1 expression are mediated by redox-regulated ERK 1/2 activation.
J Cereb Blood Flow Metab. 25:1325–1335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mankertz J, Tavalali S, Schmitz H,
Mankertz A, Riecken EO, Fromm M and Schulzke JD: Expression from
the human occludin promoter is affected by tumor necrosis factor
alpha and interferon gamma. J Cell Sci. 113:2085–2090.
2000.PubMed/NCBI
|