Introduction
Radiation therapy is an important treatment approach
for locally advanced non-small cell lung cancer (1). However, tumor recurrence and
metastasis are the root causes of radiotherapy failure (2). Numerous studies are aiming to improve
the control rate of radiotherapy on tumor (1–3).
Folkman (4) identified that blood
supplied to tumor blood vessels was significant for solid tumor
growth. A tumor with a diameter in excess of 2 mm requires constant
provision of nutrients and oxygen from neovascularization for
continued growth. Tumor growth may therefore be inhibited via the
prevention of tumor angiogenesis (5). Thus, the present study has proposed a
novel strategy for tumor antiangiogenesis therapy.
In China, Endostar has been developed (also known as
novel recombinant human endostatin), where nine amino acids have
been added to the end of the endostatin peptide chain. Endostar has
been shown to inhibit migration and induce apoptosis in endothelial
cells of new blood vessels. In addition, Endostar exerts an
antiangiogenic effect through the adjustment of a variety of
signaling pathways that lead to tumor growth, which indirectly
results in tumor dormancy or regression (6,7).
Hypoxia inducible factor-1 (HIF-1α) is an important
regulatory factor that enables tumor cells to endure a hypoxic
microenvironment. HIF-1α promotes tumor angiogenesis and growth by
promoting tumor angiogenesis and metabolism-associated gene
transcription (8,9). In addition, HIF-1α indirectly
reflects the extent of tumor oxygenation.
Carbonic anhydrase IX (CA-IX) is a cell-specific
tumor-associated protein which is overexpressed in numerous types
of tumors (10). Studies have
shown that CA-IX is a factor closely correlated with hypoxia, and
it is considered to be a reliable marker of hypoxia (10,11).
Transforming growth factor-β1 (TGF-β1) is a
multifunctional regulating peptide that is considered to be closely
associated with tumor invasion and metastasis via the degradation
of extracellular matrix, the promotion of tumor angiogenesis and
the inhibition of the immune system and other channels.
Basic fibroblast growth factor (bFGF) is a
predominant angiogenic growth factor in tumor angiogenesis
(12). It is a normal
micro-substance in human tissues that aids the regulation of
cellular DNA synthesis, the promotion of cell division and the
stimulation of vascular growth.
In the present study, Endostar was adopted as a
vascular specific inhibitor with the aim of investigating whether a
synergistic effect exists in its conjunctive use with radiotherapy;
possible mechanisms of synergy were also determined.
Materials and methods
Cell culture, tumor model and
irradiation
The A549 human lung adenocarcinoma cancer cell line
(American Type Culture Collection, Manassas, VA, USA) was cultured
in RPMI 1640 medium (Hyclone, Thermo Scientific, Logan, UT, USA)
supplemented with 100 IU/ml penicillin, 100 mg/ml streptomycin and
10% heat inactivated fetal bovine serum (Hangzhou Sijiqing
Biological Engineering Materials Co. Ltd., Hangzhou, China) in a 5%
CO2 humidified atmosphere at 37°C. Logarithmic-phase
cells were collected for use throughout the study.
Cervical dislocation was conducted to sacrifice
primary Lewis lung carcinoma-bearing C57 mice (SLAC, Changsha,
Hunan, China) were used in this study. The mouse tumors were
removed and placed in a homogenizer at a ratio of 1 g tumor sample
to 4 ml saline. A cell suspension, with the quantity of living
cells >95%, was used for the inoculation of subcutaneous tumors
in mice. The left hind armpit of each mouse was inoculated with a
0.2 ml cell suspension.
Tumor-bearing mice were fixed on plate following
intraperitoneal injection of anesthetic using 1% chloral hydrate
and 6 MeV electron beam irradiation. A radiation dose of 2 Gy was
administered daily for five days with a total dose of 10 Gy. The
study was conducted in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
Renmin Hospital of Wuhan University (Wuhan, China).
Experimental grouping and treatment
A549 cells at a logarithmic phase were randomly
divided into four groups. The blank control group did not undergo
cell processing. For the Endostar treatment group (ES), 1 μl
Endostar (Simcere Co., Ltd., Nanjing, Jiangsu, China) was added to
each well of the 6-well cell culture plates. For the radiotherapy
group (RT), a single 2 Gy radiation dose was administered. For the
Endostar combined with radiotherapy group (ES + RT), 1 μl Endostar
was initially added to each well of the 6-well cell culture plates,
which was immediately followed by a 2 Gy radiation dose.
In total, 32 Lewis lung tumor mice were randomly
divided into four groups. For the blank control group, each mouse
was injected with 0.2 ml normal saline for 14 days. For the ES
group, each mouse received a subcutaneous injection of 0.2 ml
Endostar for 14 days. For the RT group, the mice were subjected to
radiotherapy six days after grouping using Co-60 gamma-ray
irradiation. The radiation dose was 3 Gy per day for three days
with a total dose of 9 Gy.
Inhibition of cell growth and
proliferation
A549 cells in a logarithmic growth phase were seeded
in 96-well culture plates. The number of cells in each well was
~5,000. Following 12 h of incubation, the treatment wells were
filled with phosphate-buffered saline. Prior to termination of the
culture, 10 μl cell counting kit-8 solution (Dojindo, Kumamoto,
Japan) was added to each well. The absorbance of each well was
determined using an automatic microplate reader with a 450-nm
wavelength.
Detection of cell apoptosis
A549 cells were stained with 10 mg/ml Hoechst 33258
(Sigma Co., Ltd., St. Louis, MO, USA) at 8, 12 and 24 h following
treatment. Apoptotic cells were defined as cells morphologically
showing cytoplasmic and nuclear shrinkage and chromatin
condensation or fragmentation. At least 400 cells were counted per
sample and the percentage of total apoptotic cells was
calculated.
Enzyme-linked immunosorbent assay
(ELISA)
Following cell processing, the supernatant of the
cells was collected at 0, 6, 12, 18 and 24 h, and specimens were
frozen at −20°C. Once thawed, ELISA (Sigma Co., Ltd.) was used to
measure the HIF-1α, bFGF and TGF-β1 protein concentrations in the
supernatant.
Quantitative polymerase chain reaction
(qPCR)
The following PCR primers were synthesized by Wuhan
JinSirui (JinSirui. Ltd., Wuhan, Hubei, China): HomoTGF-β1 forward,
5′-CACGTGGAGCTGTACCAGAA-3′ and reverse, 5′-GAACCCGTTGATGTCCACTT-3′;
HIF-1α forward, 5′-TGATGACCAGCAACTTGAGG-3′ and reverse,
5′-TGGGGCATGGTAAAAGAAAG-3′; bFGF forward,
5′-GAGAAGAGCGACCCTCACAT-3′ and reverse, 5′-ACTGCCCAGTTCGTTTCAGT-3′;
M-CA-IX forward, 5′-CTCGTGATTCTCGGCTACAACT-3′ and reverse,
5′-ACTGGCTCAGGGCTGCTATC-3′; and e-cadherin forward,
5′-AACGCATTGCCACATACACTC-3′ and reverse,
5′-AGCGATGGCGGCATTGTAG-3′.
Equal quantities of RNA were used for each sample
for reverse transcription PCR into cDNA. qPCR was then conducted
using SYBR®-Green I fluorescent dye (Toyobo Co., Ltd.,
Osaka, Tokyo). PCR amplification and detection were performed using
a LightCycler instrument (ABI7500, Life technologies, NK, USA).
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for data analysis. Results are presented as the mean ± SD.
The Pearson method was used for correlation analysis of HIF-1α and
bFGF expression when following a normal distribution. Numerical
values were calculated and subjected to a significance test based
on paired or unpaired Student’s t-tests. P<0.05 was considered
to indicate a statistically significant difference.
Results
Growth of cells
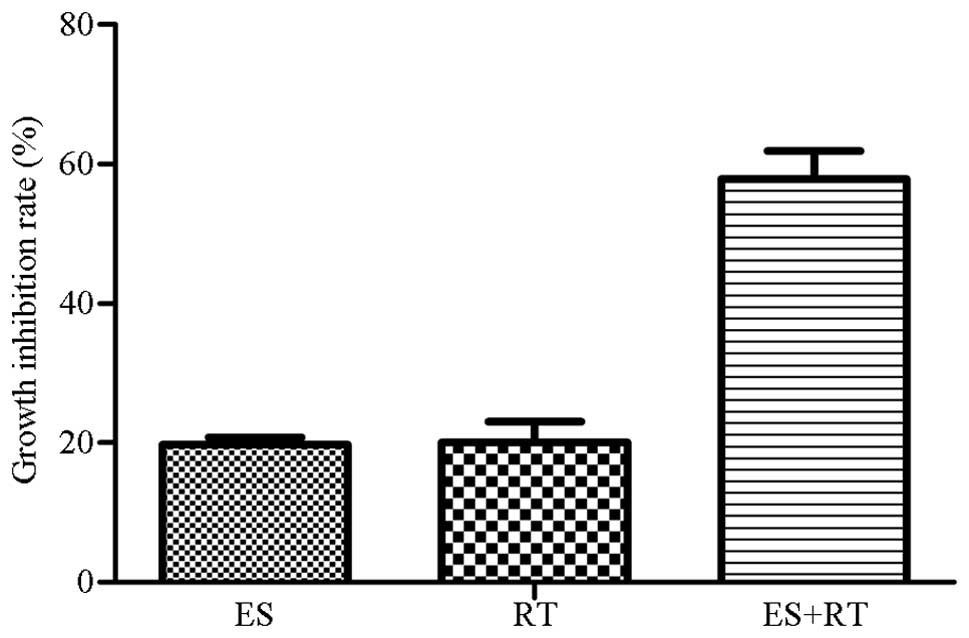
A549 cells were treated for 24 h using various
methods, as described in the materials and methods. The ES, RT and
ES + RT groups exhibited inhibition of A549 cell growth with the ES
+ RT group exhibiting the greatest growth inhibition (Fig. 1).
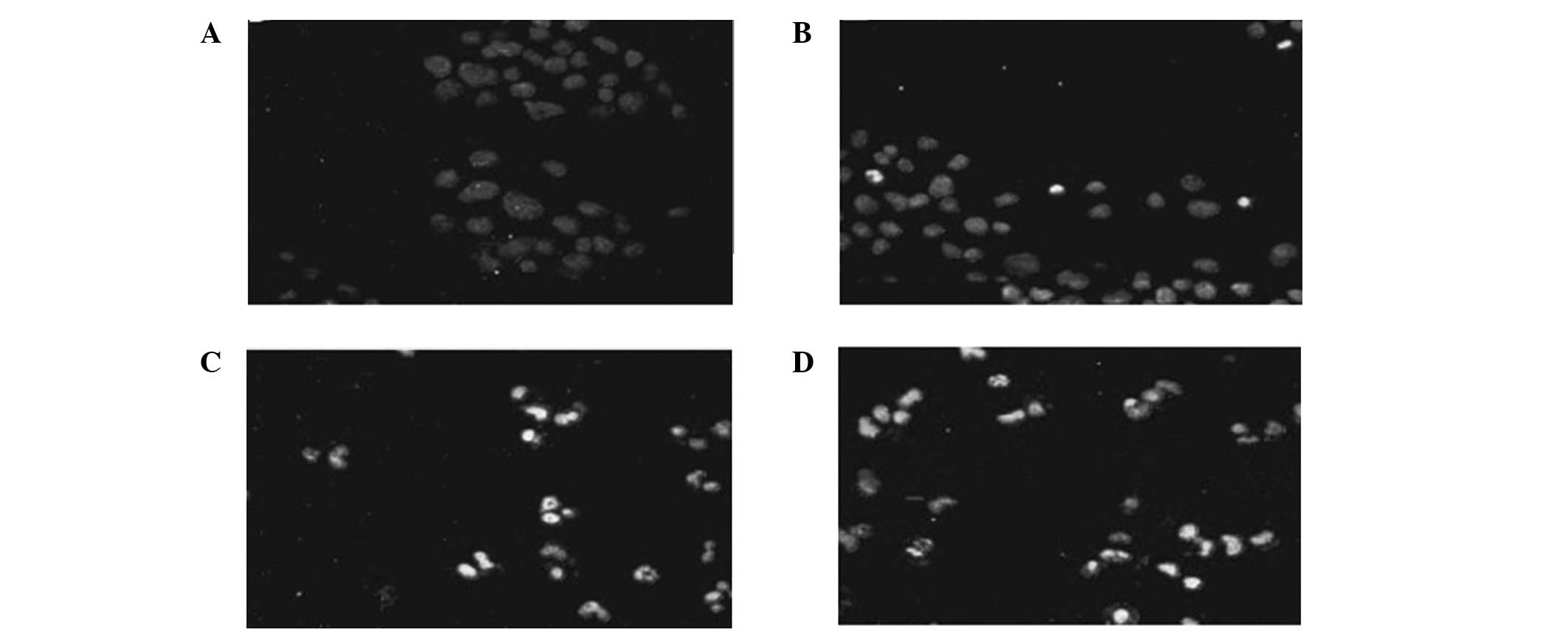
Detection of apoptosis
The ES + RT group exhibited a greater increase in
apoptosis compared with the RT and ES groups (P<0.05). The RT
group had a greater effect in promoting cell apoptosis compared
with the ES group (P<0.05). Compared with the control group, the
apoptosis rate of all the other groups increased (Fig. 2).
HIF-1α, bFGF and TGF-β1 mRNA
expression
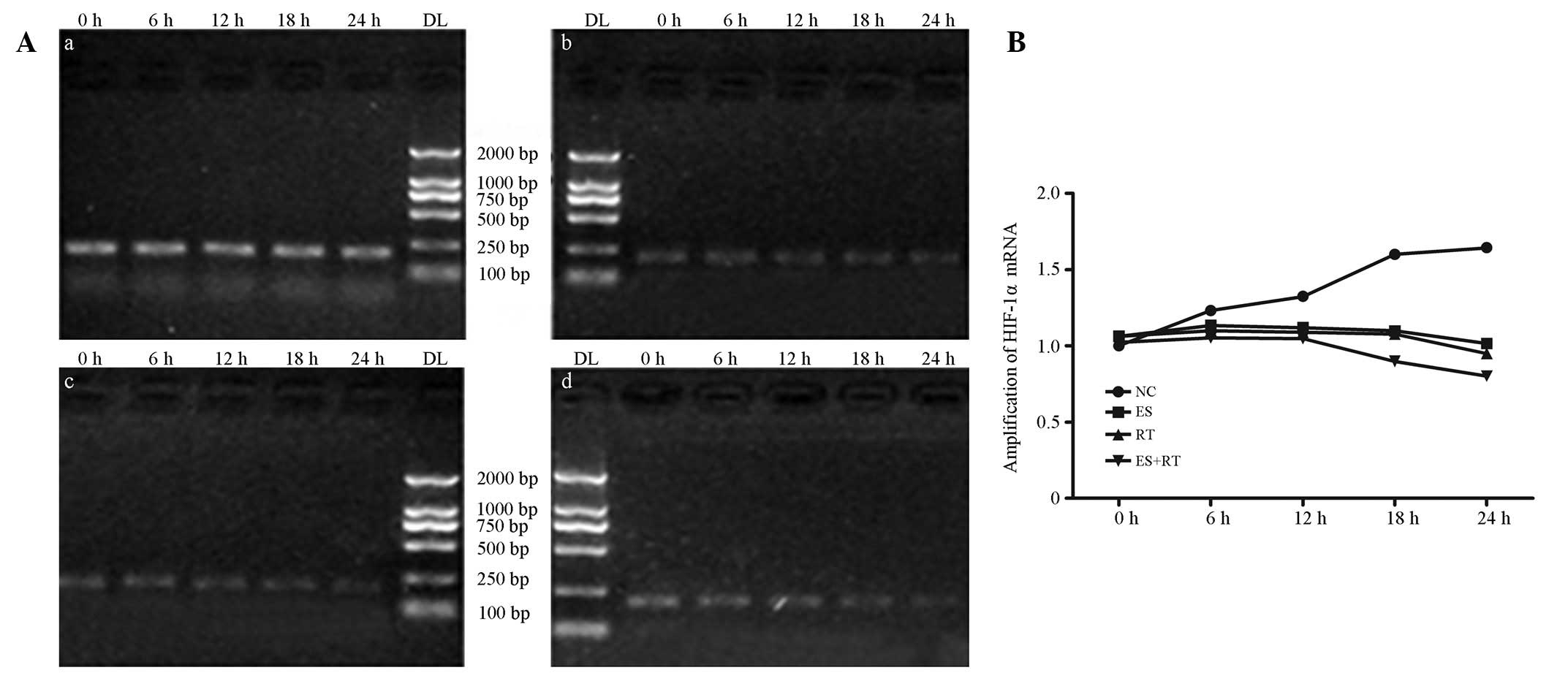
A549 cell DNA was amplified using PCR. The mRNA
expression levels of TGF-β1, bFGF and HIF-1α changed over time
(0–24 h). When compared with the control group, each treatment
group exhibited decreased expression. The mRNA expression levels of
HIF-1α, bFGF and TGF-β1, compared with the blank control and ES
groups, are shown in Figs. 3A,
4A and 5A, respectively. mRNA expression analysis
results are shown in Figs. 3B,
4B and 5B. The levels of HIF-1α, bFGF and TGF-β1
in ES + RT group were all significantly decreased when compared
with control, ES and RT groups at 24 h (P<0.05). HIF-1α and bFGF
expression levels were positively correlated (P<0.01; Figs. 3–5).
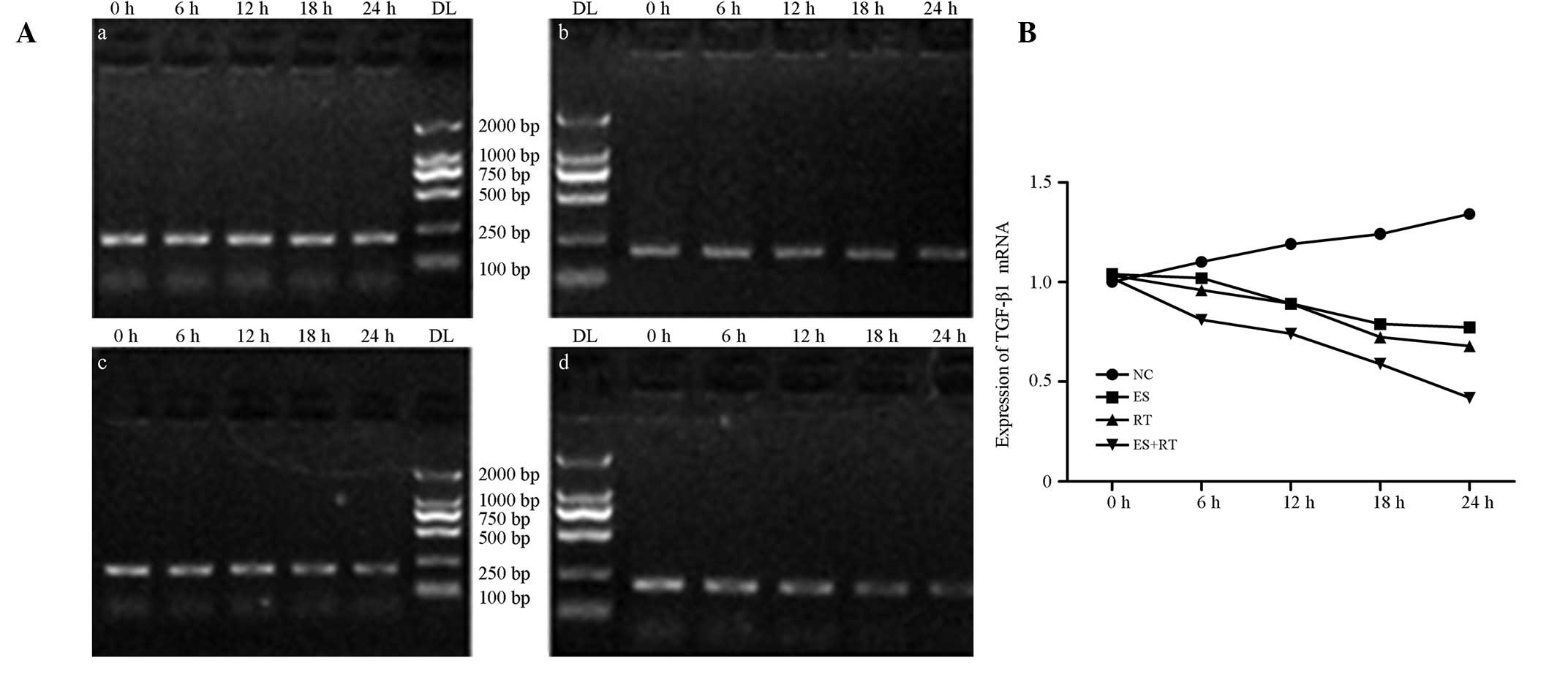
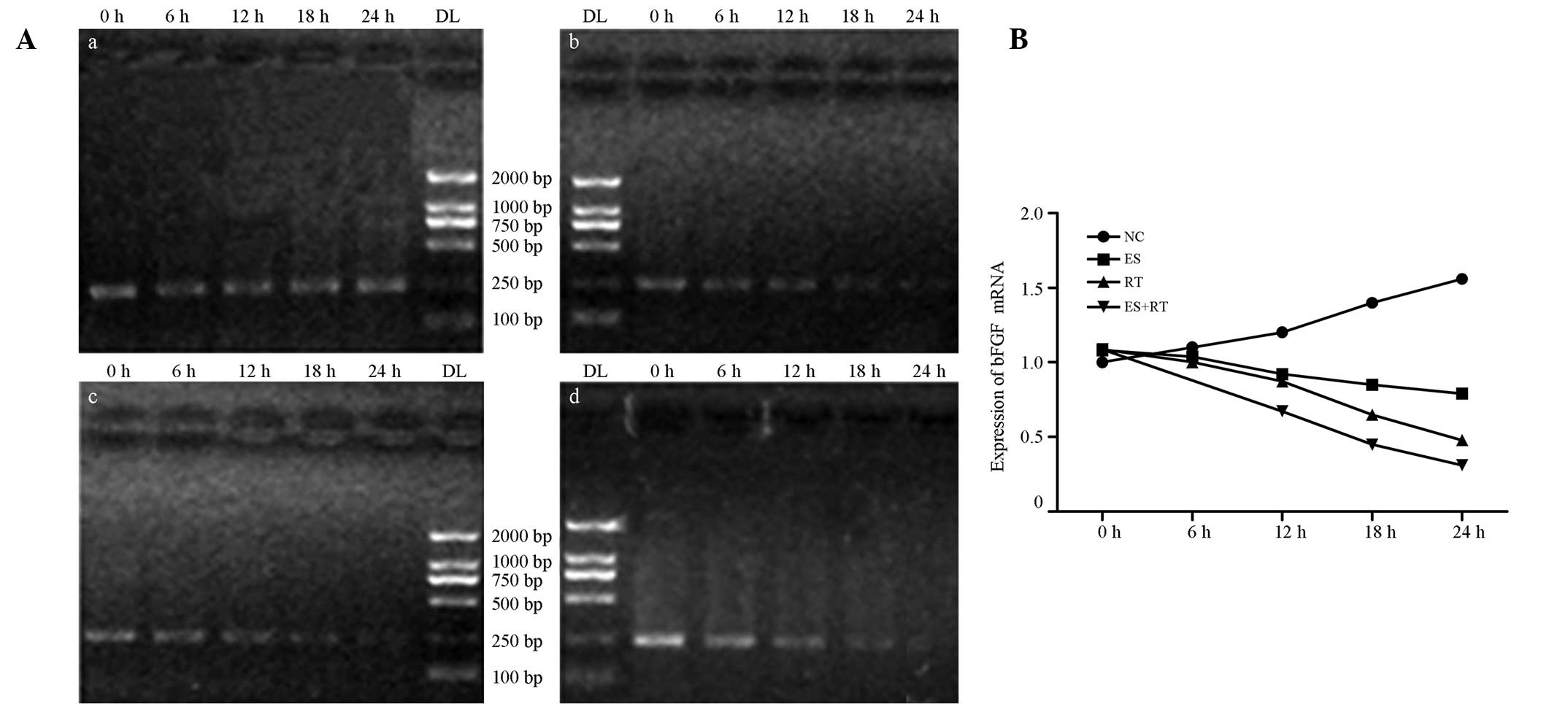 | Figure 4(A) Expression levels of bFGF mRNA in
each treatment group. qPCR electrophoresis results indicate that
the mRNA expression levels of bFGF in the ES, RT and ES + RT groups
decreased over time when compared with the control group. At 24 h,
amplification reached a minimum of 0.6387±0.2182, 0.6451±0.1520 and
0.0644±0.0235 in the ES, RT and ES + RT groups, respectively. (B)
bFGF mRNA amplification in each group. The decrease in bFGF mRNA
expression levels in the ES, RT and ES + RT groups was
statistically significant when compared with the control group
(P<0.05). The ES + RT group exhibited the lowest bFGF mRNA
expression levels; differences were statistically significant when
compared with the RT and ES groups (P<0.05). bFGF, basic
fibroblast growth factor; NC, control; ES, Endostar treatment; RT,
radiotherapy; ES + RT, Endostar combined with radiotherapy
treatment; qPCR, quantitative polymerase chain reaction. |
Protein expression of HIF-1α, bFGF and
TGF-β1
TGF-β1, HIF-1α and bFGF protein expression levels in
the A549 cells of each group exhibited no significant differences
prior to processing. However, TGF-β1, HIF-1α and bFGF protein
expression levels in A549 cells decreased following treatment with
Endostar and/or radiotherapy. Compared with the blank control
group, TGF-β1, HIF-1α and bFGF protein expression levels in the RT
and ES + RT groups decreased significantly after 24 h
(P<0.05).
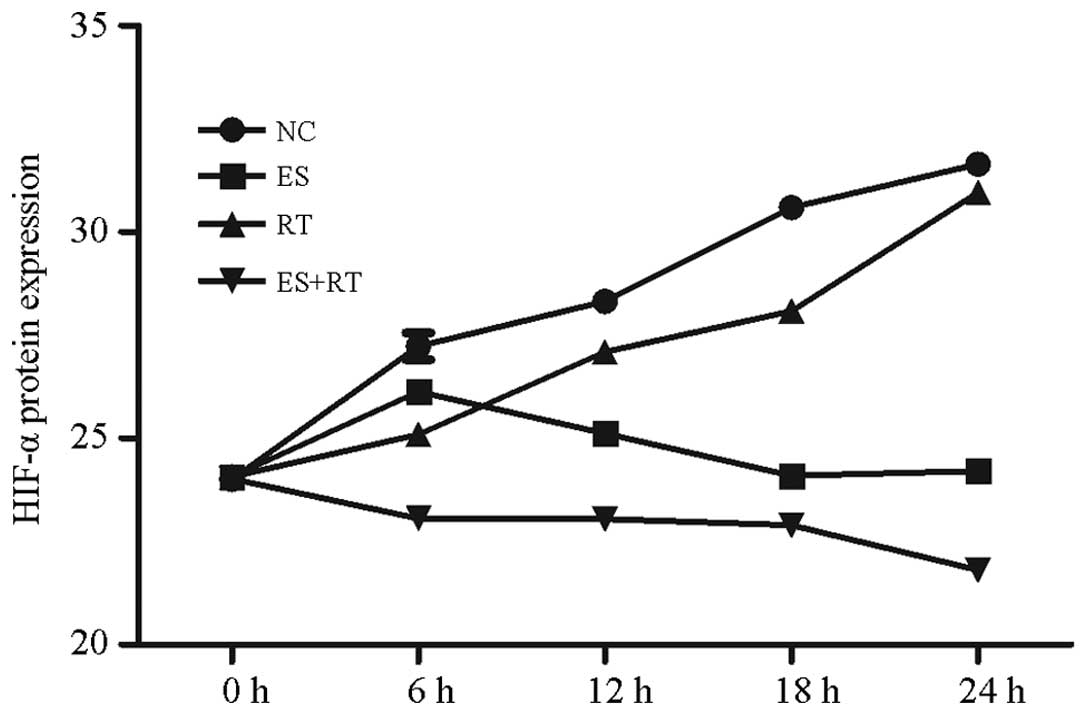
HIF-1α protein expression levels in the supernatants
of the ES, RT and ES + RT groups, as measured by ELISA, were shown
to decrease increasingly over time. Lowest expression was observed
at 24 h (P<0.05; Fig. 6).
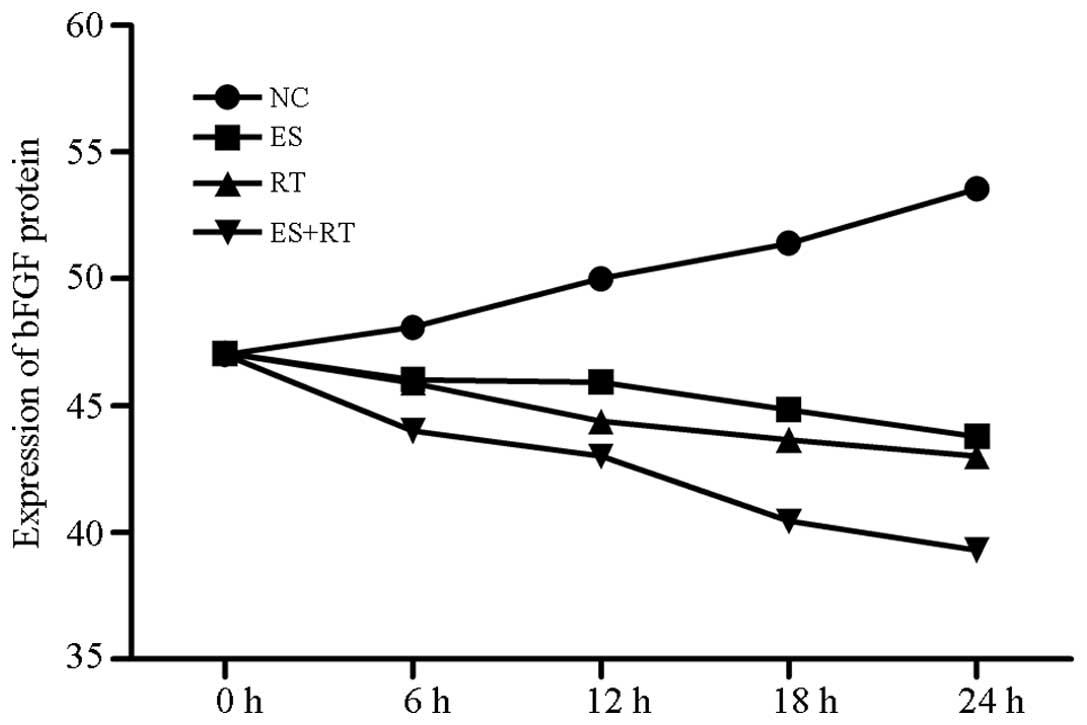
bFGF protein expression levels in the supernatants
of the ES, RT and ES +RT groups, as measured by ELISA, were shown
to decrease increasingly over time. Lowest expression was observed
at 24 h (P<0.05; Fig. 7).
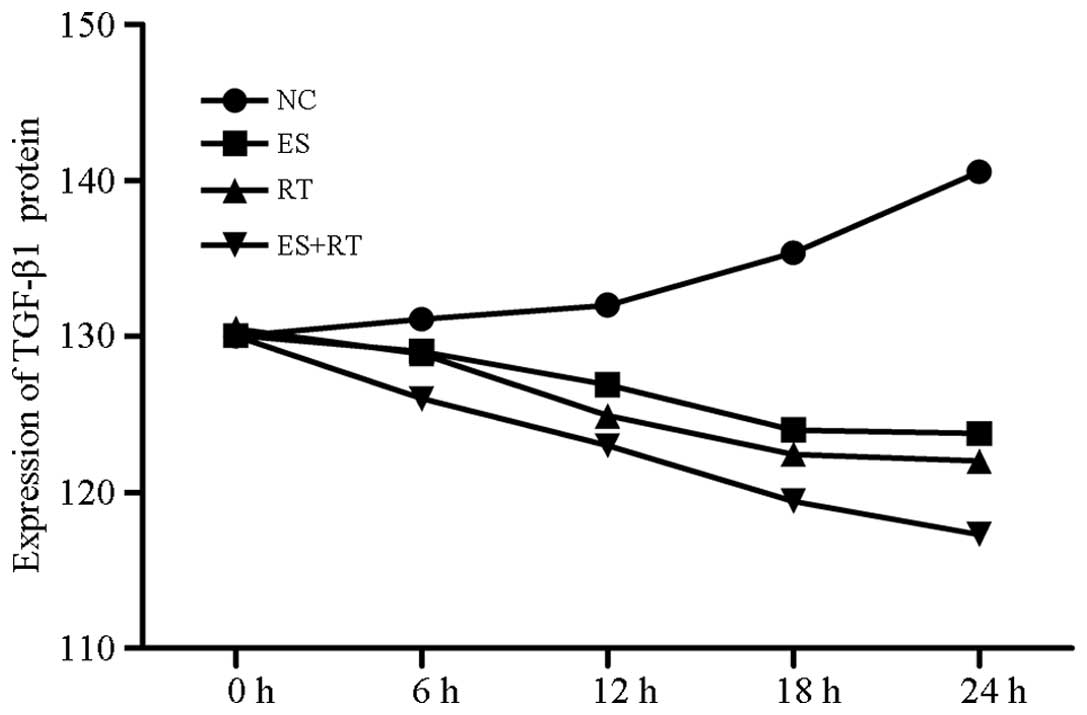
TGF-β1 expression levels in A549 cells also
decreased. TGF-β1 expression levels in the ES and ES + RT groups
significantly decreased within 24 h. According to statistical
analysis, no significant difference was identified in TGF-β1
expression levels among the groups prior to processing (P>0.05;
Fig. 8).
Correlation analysis of HIF-1α and bFGF
expression
SPSS 13.0 statistical software was used to analyze
the correlation between HIF-1α and bFGF expression. A correlation
coefficient of r=−0.80 was derived using the Pearson method
(P<0.01). This result demonstrated that HIF-1α and bFGF
expression were positively correlated, indicating that increased
HIF-1α expression is associated with increased bFGF expression.
Discussion
Antiangiogenesis treatments block the supply of
blood and oxygen to tumors (4,5),
inducing a hypoxic state. In the present study, antiangiogenic
treatment showed a synergistic effect when combined with radiation
therapy. In addition, the present study indicated that
antiangiogenic agents target the proliferation of endothelial
cells, thus, reducing the oxygen consumption of the tumor and
endothelial cells. As a result, oxygenation in tumor
microenvironment is improved, as well as the sensitivity to
radiotherapy (13). Endostar
improves the disordered vascular network of tumors and enables the
structure to function under normal vascular status, referred to as
the ‘tumor vascular normalization time window’. This ‘normalized
vasculature’ improves local tumor blood circulation, reduces tumor
interstitial pressure and improves local oxygen partial pressure
(13,14). Therefore, Endostar enhances
cytotoxic effects on tumor cells within the ‘vascular normalization
time window’.
Blood vessels in tumor tissues differ from those in
normal vasculature. These poorly distributed and functioned vessels
lead to insufficient blood and oxygen supply in tumor tissue
(13,14). In the cell studies, tumor cells did
not lack oxygen. Radiation resulted in tumor cell apoptosis,
however, the extent of apoptosis was increased following Endostar
treatment. Cell proliferation in the ES group was inhibited,
indicating that Endostar exhibited an antitumor effect.
The results of the present study indicate that
radiotherapy combined with Endostar significantly inhibited TGF-β1
and HIF-1α expression. TGF-β1 and HIF-1α serve valuable functions
in tumor angiogenesis and vascular remodeling, invasion and
metastasis. Hypoxia and nutritional deficiency are the predominant
features of solid tumors (15,16).
Neovascularization in the tumor tissue is primarily mediated by
vascular endothelial growth factor (VEGF) and its signaling
pathway. Under hypoxic conditions, VEGF is induced by HIF-1α
expression (17). Previous studies
have indicated that TGF-β1 and HIF-1α promote angiogenesis through
the TGF-β1/PHD2/HIF-1α/VEGF pathway in the process of tumor
angiogenesis. A decrease in TGF-β1 reduces the incidence of cell
epithelial-mesenchymal transition (18,19);
an important biological process involving tumor cell migration and
invasion. Inhibition of TGF-β1 overexpression in tumor cells
impedes cell invasion and metastasis, thereby improving the
efficacy of treatment (20).
In conclusion, Endostar combined with radiotherapy
inhibits tumor cell proliferation and the expression of TGF-β1 and
HIF-1α, which may affect the expression of VEGF and other genes.
VEGF-mediated tumor angiogenesis and TGF-β1-mediated tumor
metastasis may be restricted by this therapeutic strategy. The
present study provides a theoretical basis for the improvement of
treatment in clinical oncology. Improved efficacy may be associated
with combined therapy, which significantly downregulates TGF-β1 and
HIF-1α expression. Furthermore, combined therapy inhibits tumor
cell matrix degradation and reduces cell invasion.
Acknowledgements
The study was supported by a grant from the Key
Program of National Natural Science Foundation of China (no.
30970860).
References
|
1
|
McCloskey P, Balduyck B, Van Schil PE, et
al: Radical treatment of non-small cell lung cancer during the last
5 years. Eur J Cancer. 49:1555–1564. 2013.PubMed/NCBI
|
|
2
|
Chi A, Liao Z, Nguyen NP, et al: Systemic
review of the patterns of failure following stereotactic body
radiation therapy in early-stage non-small-cell lung cancer:
clinical implications. Radiother Oncol. 94:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salama JK and Vokes EE: New radiotherapy
and chemoradiotherapy approaches for non-small-cell lung cancer. J
Clin Oncol. 31:1029–1038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Ge W, Hu K, et al: Endostar
down-regulates HIF-1 and VEGF expression and enhances the
radioresponse to human lung adenocarcinoma cancer cells. Mol Biol
Rep. 39:89–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge W, Cao DD, Wang HM, et al: Endostar
combined with chemotherapy versus chemotherapy alone for advanced
NSCLCs: a meta-analysis. Asian Pac J Cancer Prev. 12:2901–2907.
2011.
|
|
8
|
Agani F and Jiang BH: Oxygen-independent
regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in
cancer. Curr Cancer Drug Targets. 13:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang CM and Yu J: Hypoxia-inducible
factor-1 as a therapeutic target in cancer. J Gastroenterol
Hepatol. 28:401–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monti SM, Supuran CT and De Simone G:
Anticancer carbonic anhydrase inhibitors: a patent review (2008 –
2013). Expert Opin Ther Pat. 23:737–749. 2013.PubMed/NCBI
|
|
11
|
Sedlakova O, Svastova E, Takacova M, et
al: Carbonic anhydrase IX, a hypoxia-induced catalytic component of
the pH regulating machinery in tumors. Front Physiol. 4:4002014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montesano R, Vassalli JD, Baird A, et al:
Basic fibroblast growth factor induces angiogenesis in vitro. Proc
Natl Acad Sci USA. 83:7297–7301. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stylianopoulos T and Jain RK: Combining
two strategies to improve perfusion and drug delivery in solid
tumors. Proc Natl Acad Sci USA. 110:18632–18637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goel S, Duda DG, Xu L, et al:
Normalization of the vasculature for treatment of cancer and other
diseases. Physiol Rev. 91:1071–1121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Favaro E, Nardo G, Persano L, et al:
Hypoxia inducible factor-1alpha inactivation unveils a link between
tumor cell metabolism and hypoxia-induced cell death. Am J Pathol.
173:1186–1201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koul HK, Pal M and Koul S: Role of p38 MAP
Kinase Signal Transduction in Solid Tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Driessen A, Landuyt W, Pastorekova S, et
al: Expression of carbonic anhydrase IX (CA IX), a hypoxia-related
protein, rather than vascular-endothelial growth factor (VEGF), a
pro-angiogenic factor, correlates with an extremely poor prognosis
in esophageal and gastric adenocarcinomas. Ann Surg. 243:334–340.
2006. View Article : Google Scholar
|
|
18
|
Akhurst RJ: TGF-beta antagonists: why
suppress a tumor suppressor? J Clin Invest. 109:1533–1536. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang Q, Li L, Zhang J, et al: CDK5 is
essential for TGF-β1-induced epithelial-mesenchymal transition and
breast cancer progression. Sci Rep. 3:29322013.
|
|
20
|
Chen KC, Chen CY, Lin CJ, et al: Luteolin
attenuates TGF-β1-induced epithelial-mesenchymal transition of lung
cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway.
Life Sci. 93:924–933. 2013.PubMed/NCBI
|