Introduction
Percutaneous vertebroplasty (PVP) is a minimally
invasive procedure that involves radiographically guided injection
of bone cement directly into the vertebral body (1). Polymethylmethacrylate (PMMA) and
calcium phosphate cement (CPC) are common types of bone cement used
for PVP. In 1987, Galibert et al (2) described PVP as a treatment for
painful, aggressive, vertebral hemangioma. Although PVP is a
minimally invasive and safe procedure, it is associated with a high
complication rate (3). Cement
leakages account for the majority of symptomatic complications
(1,3,4).
While intradiscal cement leakage is generally asymptomatic, it may
have late mechanical consequences on the adjacent mobile spinal
segments (5,6). Intradiscal cement leakage is commonly
observed following PVP. Mirovsky et al (1) reported intradiscal cement leakage
following PVP in 41% of patients. The age and gender of patients,
the type of fracture, the volume of injected PMMA, and the liquid
consistency of PMMA were not found to be statistically significant
risk factors for intradiscal cement leakage. In a study by Pitton
et al (7), intradiscal
cement leakage was detected in 27.5% of patients who had undergone
PVP, and routes of leakage included fractured endplates, vacuum
clefts or iatrogenic perforation of the endplate by the needle tip.
Although leakage is largely asymptomatic, it may have long-term
effects on the disc and adjacent vertebrae. Recently published data
indicate that large-disc cement leaks may predispose individuals to
the collapse of adjacent vertebrae (8). The association between intradiscal
cement leakage and the occurrence of new adjacent osteoporotic
vertebral compression fractures (OVCF) was substantiated by the
identification of the following volumetric correlation: A higher
intradiscal leakage volume is associated with an increased
likelihood of new adjacent OVCF. This volumetric effect may explain
the diverse findings across studies, i.e., depending on the mean
leakage volume, intradiscal cement leakage may or may not be a risk
factor for new fractures. Therefore, intradiscal cement leakage,
particularly large volumes (≥1 ml), should be avoided (8). Although rates of complications for
PVP are low, cement leakage occurs in up to 90% of patients.
Current evidence indicates that sequelae of cement leakage may be
more common and clinically relevant than previously considered
(9).
The present study aimed to investigate whether bone
cement can lead to intervertebral disc degeneration (IDD), and
evaluate the degree of IDD relative to the type and volume of
cement injected into the disc space.
Materials and methods
Experimental animals
Adult dogs (n=16; each ~16 kg) were supplied by the
Experimental Animal Center of Soochow University (Nantong, China).
The animal use protocol was approved by the Institutional Animal
Care and Use Committee of Soochow University (approval number SVXK;
SU2002-0037). This study was performed in accordance with the Guide
for the Care and Use of Laboratory Animals published by the
National Institutes of Health (1996). The dogs were randomly
divided into two groups according to sacrifice time (12 or 24 weeks
post-cement injection). In each dog, five lumbar intervertebral
discs (L1–L2 to L5–L6) were studied. One disc was untreated and
acted as the control group, whereas the other four discs were
injected with PMMA (Corin Ltd., Cirencester, UK) or CPC (Shanghai
Rebone Biomaterials Co., Ltd, Shanghai, China). A total of 0.1 or
0.3 ml cement was injected. The dogs were randomized into five
groups: A, control; B1, 0.1 ml PMMA; B2, 0.3 ml PMMA; C1, 0.1 ml
CPC; and C2, 0.3 ml CPC (Table
I).
 | Table IDefinition of histological grading
scale. |
Table I
Definition of histological grading
scale.
| Grade | Annulus fibrosus | Border between the
annulus fibrosus and nucleus pulposus | Cellularity of the
nucleus pulposus | Matrix of the nucleus
pulposus |
|---|
| 1 | Normal pattern of
fibrocartilage lamellae (U-shaped in the posterior aspect and
slightly convex in the anterior aspect), without ruptured fibers or
a serpentine appearance anywhere within the annulus | Normal | Normal cellularity
with large vacuoles in the gelatinous structure of the matrix | Normal gelatinous
appearance |
| 2 | Ruptured or
serpentine-patterned fibers in <30% of the annulus | Minimally
interrupted | Slight decrease in
the number of cells and fewer vacuoles | Slight condensation
of the extracellular matrix |
| 3 | Ruptured or
serpentine-patterned fibers in >30% of the annulus | Moderate/severe
interruption | Moderate/severe
decrease (≥50%) in the number of cells and no vacuoles | Moderate/severe
condensation of the extracellular matrix |
Experimental procedure
The dogs were anesthetized with 3% pentobarbital
sodium (1 mg/kg i.v.) and hair was shaved from the left flank and
mid-back. Next, the animals were positioned in left lateral
recumbence on the operating table, and the skin of the spine was
sterilized and then fixed. Under the guidance of fluoroscopy from a
digital subtraction angiography machine (Agniostar Plus; Siemens
AG, Munich, Germany), an 18G spinal needle (Terumo Corporation,
Tokyo, Japan) was inserted into the lumbar intervertebral disc of
the dogs. The magnification function was used to ensure that a
one-shot puncture was achieved. The PMMA was mixed with barium
sulfate, resulting in a 20% ratio of PMMA powder and liquid. The
cement mixture was prepared once the spinal needle was positioned,
with the tip of the needle reaching the center of the disc. The
cement is ready for injection when it drips slowly like melted ice
cream from the tip of the 1 ml syringe. The needle was removed
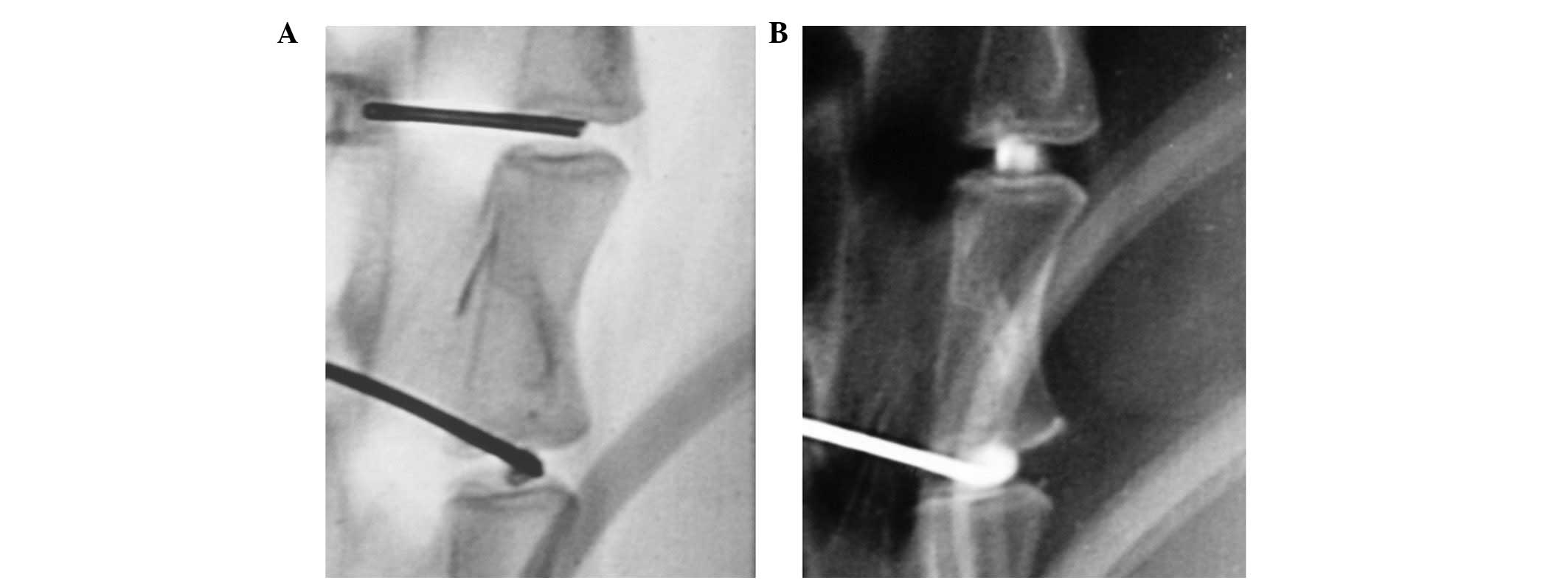
after the chosen cement was injected. Lateral plane radiographs of
the L1–L6 intervertebral discs were obtained prior to and following
injection (Fig. 1). The
collimator-to-film distance was 80 cm, with an exposure of 120 mAs
and penetration power of 54 kVp.
Magnetic resonance imaging (MRI)
examination and analysis
A 1.5-T clinical magnet was used for MRI scans. The
anesthetized dogs were placed in a supine position within the
magnet field, with the lumbar region centered over a 10-inch
diameter circular surface coil (General Electric Healthcare,
Cleveland, OH, USA). A coronal T2-weighted localizer image
[repetition time (TR), 1,445 ms; echo time (TE), 37 ms] was used to
establish the position of the lumbar discs from L1–L2 to L5–L6. A
4-mm thick mid-sagittal section (field of view, 12×9-cm; matrix
size, 256×192 cm) was imaged by using a T2-weighted imaging
sequence (TR, 4,188 ms; TE, 112 ms) to highlight the signal from
the nucleus pulposus. T2-weighted axial images (TR, 3,500 ms; TE,
100 ms) and T1-weighted mid-sagittal images (TR, 324 ms; TE, 12 ms)
were also obtained.
The T2-weighted mid-sagittal images of the discs
were qualitatively analyzed for evidence of degeneration by
calculating the MRI index using Sobajima’s method (10). The MRI outcome, also know as the
MRI index (the product of the nucleus pulposus area and average
signal intensity), was used as a measure of the degenerative
changes in the nucleus pulposus. Quantitative analysis of these
images was performed with a picture archiving and communications
system in which the nucleus pulposus of each disc was outlined on
the screen and the computer mouse was used to define the region of
interest. The intra-observer reliability in classifying disc
degeneration via MRI was assessed by having the same examiner
re-evaluate all images >1 month after the initial assessment. To
assess inter-observer reliability, two senior authors, who have
>15 years of experience as board-certified surgeons,
independently performed the rating. The degree of agreement beyond
chance was determined according to Cohen’s κ statistics (11).
Histology examination
The dogs were sacrificed by intravascular
embolization of air. The L1–L6 spinal segments were removed, and
following 48 h of fixation in 10% formalin, each segment was sawed
open. The distance between the superior and inferior endplates was
1 cm. The intact intervertebral disc, and the superior and inferior
endplates were placed into decalcifying fluid for 4 weeks. Once
decalcification was complete, each segment was cut sagittally into
four parts, in series through the center of the vertebral body.
Each part had a thickness of 3 mm and was dehydrated for 12 h.
Following paraffin embedding, each part was cut into six serial
sections (5 μm each), yielding a total of 24 sections.
To observe the changes in the nucleus pulposus,
annulus fibrosus and cartilage endplate, the specimens were stained
with hematoxylin and eosin. IDD pathological scores were reviewed
according to the standards used by Masuda et al (12).
A histological grading scale based on four
categories of degenerative changes was used to assess the annulus
fibrosus, the border between the annulus fibrosus and the nucleus
pulposus, the cellularity of the nucleus pulposus, and the matrix
of the nucleus pulposus, using mid-sagittal sections (Table I). The grades ranged between 4 and
12. A normal disc was scored as grade 4, scoring 1 point for each
of the four categories listed above for a total of 4 points. Grade
12 was representative of severe degeneration, with 3 being the
maximum number of points that could be obtained in each
category.
Statistical analysis
Data are presented as the mean ± standard error.
Intra- and inter-group variability was accounted for by mixed model
analysis. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA).
Results
MRI findings
The architectures of the annulus fibrosus and
nucleus pulposus were intact in the control group at 12 and 24
weeks. The intervertebral space in the cement groups exhibited
varying levels of stenosis at 12 and 24 weeks. The superior and
inferior edges of the vertebral body at the corresponding
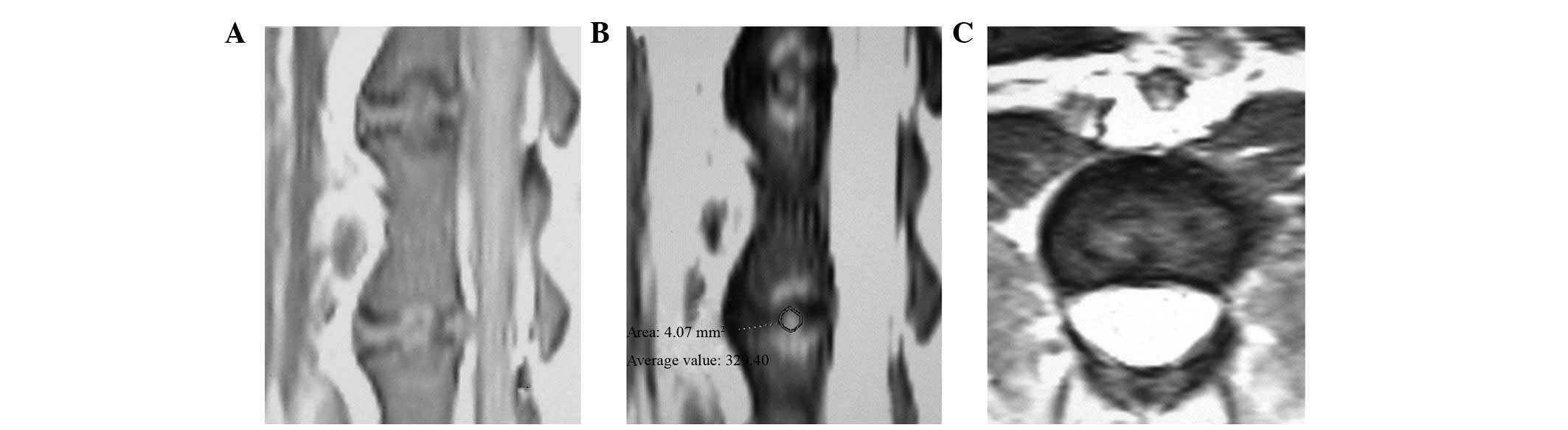
intervertebral space were coarse. In the T2-weighted images,
signals from the nucleus pulposus were not uniform and decreased at
various rates. The center of the nucleus pulposus had a much lower
signal relative to the control group, with an irregular and patchy
appearance. The area of relatively high signal was diminished
compared to control group and the shape of the nucleus pulposus was
irregular (Fig. 2). A patchy
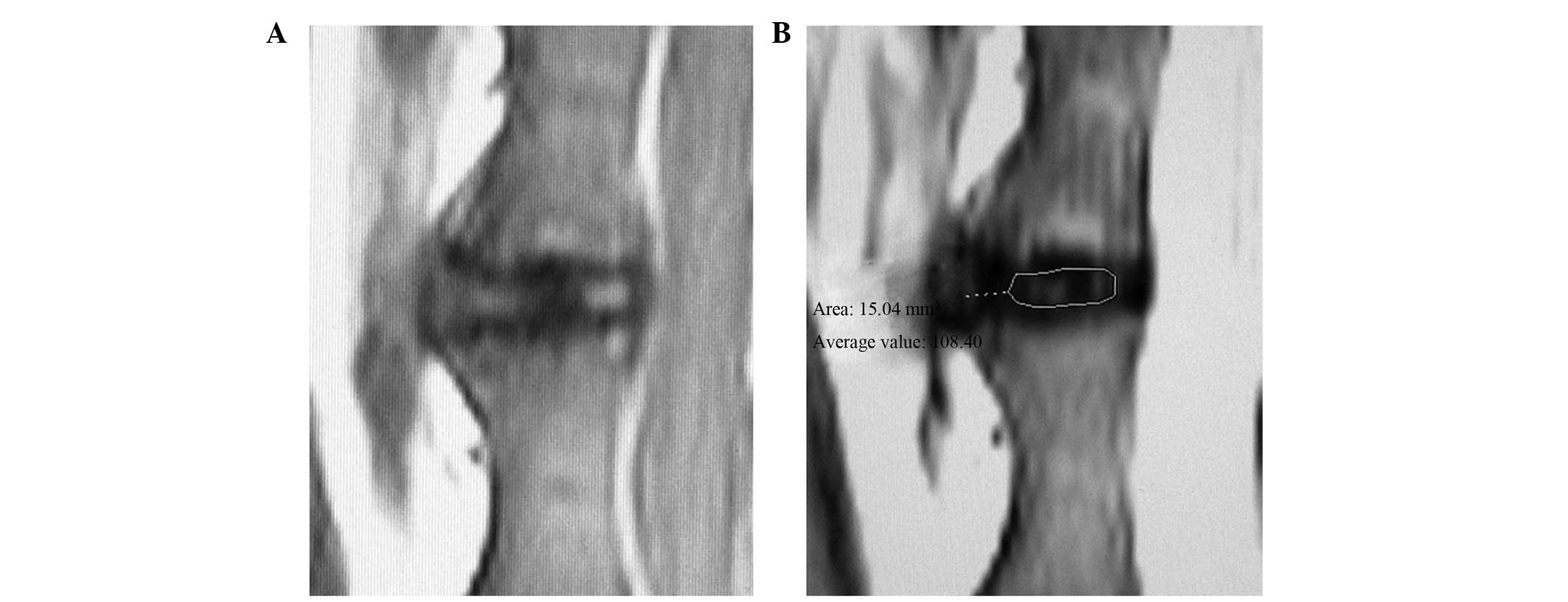
streak of high signals was observed in the annulus fibrosus, which
had a clear boundary with regard to the nucleus pulposus. Irregular
and patchy low signals were observed in the intervertebral space in
the T1-weighted images, and the intervertebral disc had an abnormal
shape (Fig. 3). Figs. 2 and 3 demonstrate that specific vertebral
bodies near the injected discs exhibited medullar edema, fat
degeneration and Schmorl’s nodule formation.
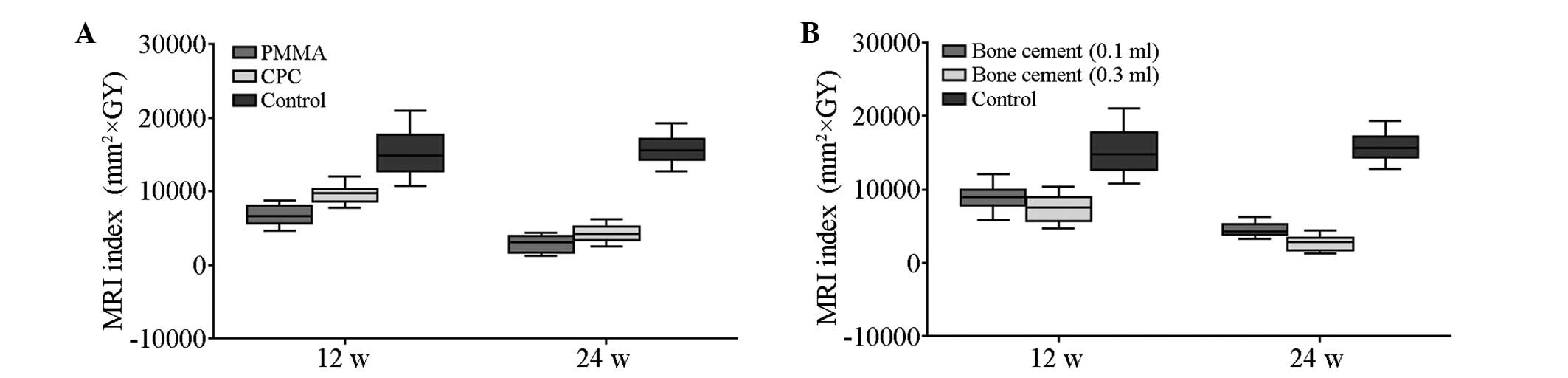
With regard to the MRI index, no significant
difference was observed at 12 and 24 weeks for cement and control
groups (t=0.505; P>0.05; Table
II). A significant difference in the MRI index of all cement
subgroups was observed at 12 and 24 weeks compared with the control
group. A significant difference was also observed between the PMMA
groups and the CPC groups at 12 and 24 weeks (Fig. 4).
 | Table IIMRI indexes (mm2 x GY). |
Table II
MRI indexes (mm2 x GY).
| Group | 12 weeks | 24 weeks |
|---|
| Control group | 15104.60±3312.17 | 15762.88±2079.45 |
| PMMA (0.1 ml) | 7588.55±1069.59 | 3875.71±399.61 |
| PMMA (0.3 ml) | 5942.18±839.27 | 1985.58±593.49 |
| CPC (0.1 ml) | 10303.23±963.87 | 5056.15±800.48 |
| CPC (0.3 ml) | 9010.63±886.36 | 3521.01±678.29 |
Pathology findings
At 12 and 24 weeks, the control group had a normal
boundary between the annulus fibrosus and the nucleus pulposus, and
calcification in the cartilage endplate was not observed. The
vascular bed was not reduced, and the cells of the nucleus pulposus
were normal. The interstitial substance of the nucleus pulposus was
not condensed. Although ruptures were present in certain samples,
disruption was absent in the annulus fibrosus.
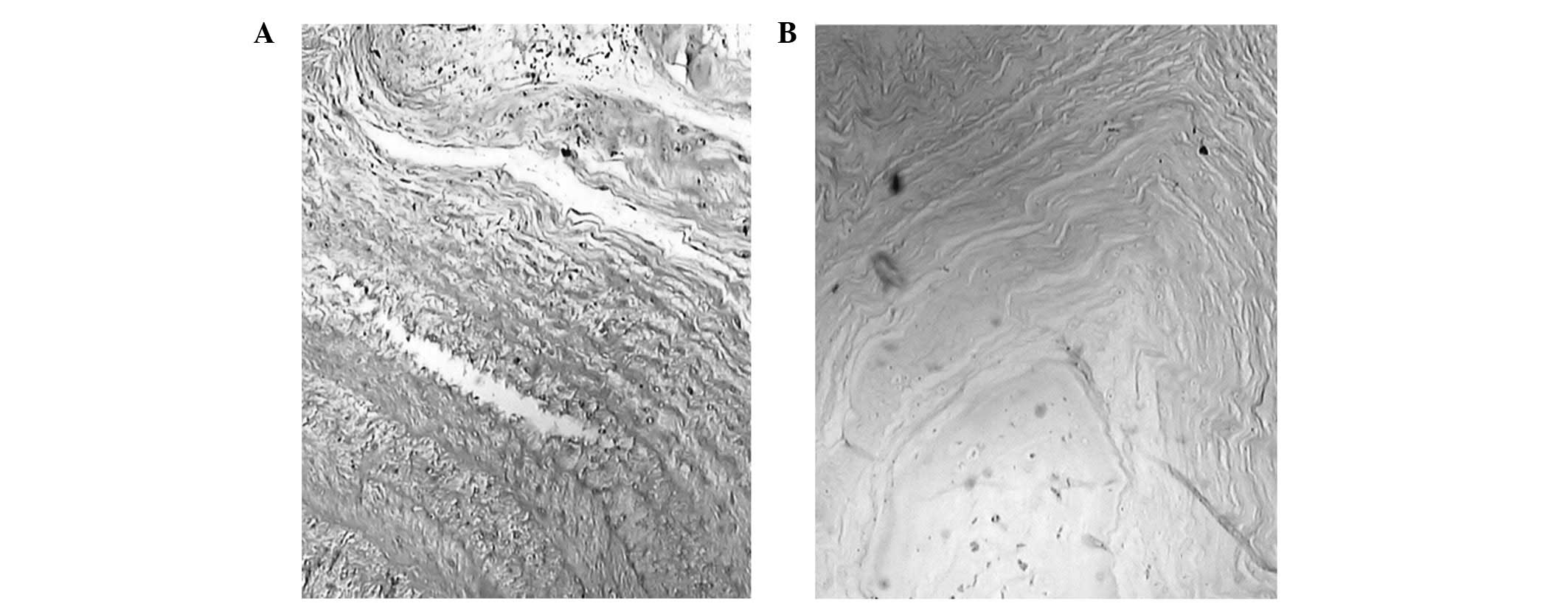
At 12 weeks post-cement injection, a slightly
interrupted border between the annulus fibrosus and nucleus
pulposus, as well as chipping in the nucleus pulposus due to the
condensed matrix, were identified in all histological analyses.
(Fig. 5). The nucleus pulposus of
specific cells was within the adjacent inner annulus fibrosus. The
number of cells and cell vacuoles of the nucleus pulposus
decreased. Extracellular matrix condensation occurred in certain
samples. The cartilage endplate had ruptured or formed
serpentine-patterned fibers; however, no calcification occurred in
the cartilage endplate. The thickness of the vascular bed was not
reduced. Fibrosis was evident in the annulus fibrosus and nucleus
pulposus of specific samples from the PMMA groups.
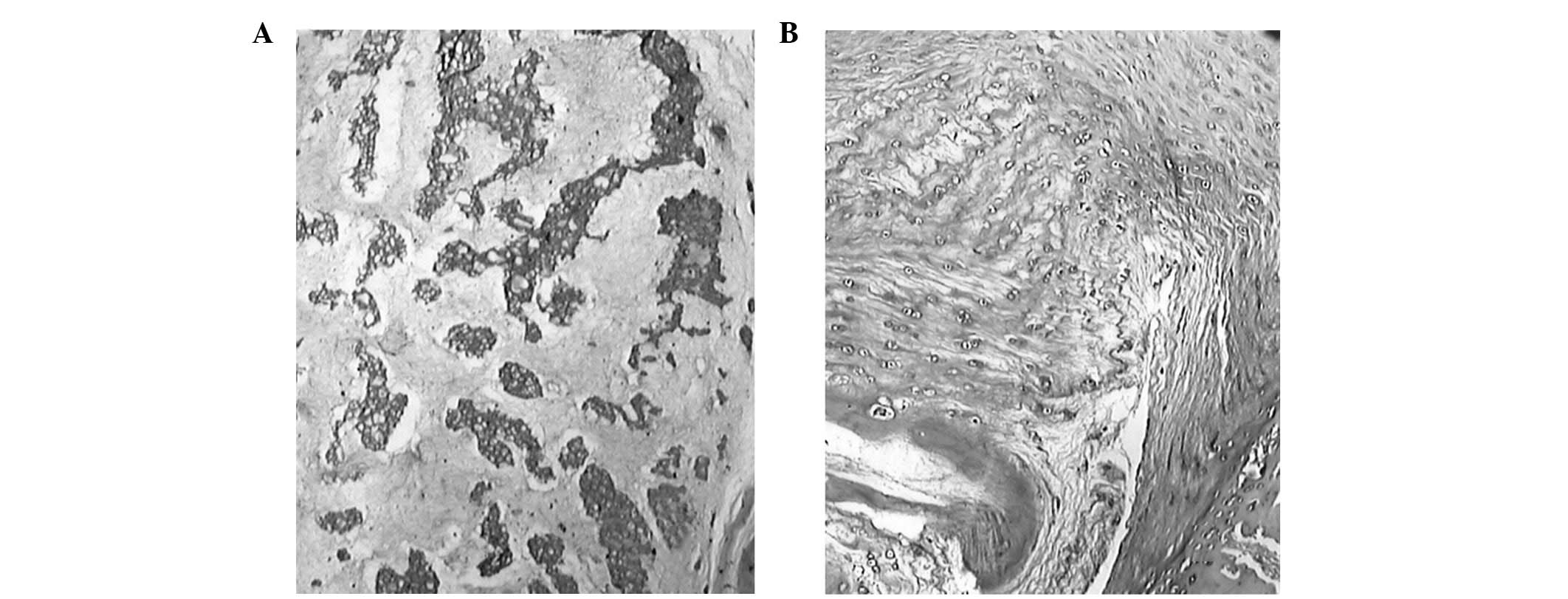
At 24 weeks post-cement injection, no boundary was
observed between the annulus fibrosus and the nucleus pulposus.
Cells from the nucleus pulposus were observed in the adjacent inner
annulus fibrosus, but its vacuoles had shrunk and the number of
cells significantly decreased. Disparity in the condensation of the
extracellular matrix was observed (Fig. 6). The cartilage endplate had
ruptures or serpentine-patterned fibers covering up to 30% of the
annulus fibrosus. Various extents of fibrosis were observed in the
annulus fibrosus and nucleus pulposus in all samples. Calcification
in the cartilage endplate and reduction in the vascular bed were
not found in the CPC groups, whereas a thin area of calcification
in the cartilage endplate was found in the PMMA groups.
Furthermore, necrosis of nucleus pulposus cells, condensation and
degradation of the matrix, and rupture and tumescence were more
severe in the PMMA groups compared with the CPC groups.
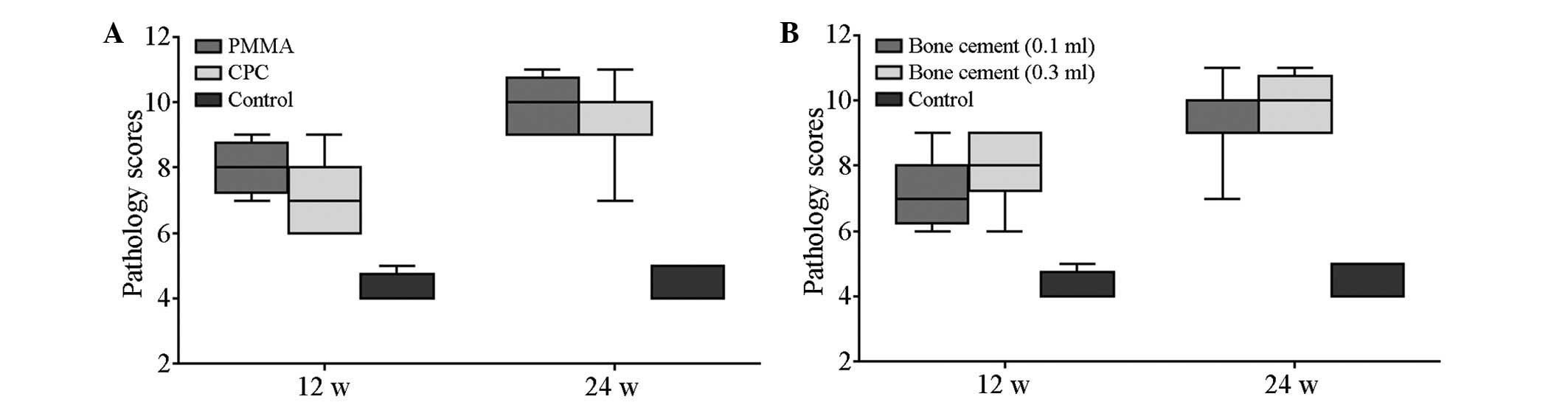
Table III lists
the pathology scores of all groups at the two time points. No
significant difference was observed in the scores of the control
groups at 12 and 24 weeks (t=0.509; P>0.05). The scores of the
cement subgroups at 12 and 24 weeks were significantly different
compared with those of the control group (P<0.05). The scores of
the PMMA group and the CPC group were also significantly different
at 12 and 24 weeks. Inter-group variability was accounted for by
mixed model analysis (F, 91.52, 13.71, 13.71; P<0.05; Fig. 7).
 | Table IIIPathology scores in all groups (mean ±
SEM). |
Table III
Pathology scores in all groups (mean ±
SEM).
| Group | 12 weeks | 24 weeks |
|---|
| Control group | 4.25±0.46 | 4.38±0.52 |
| PMMA (0.1 ml) | 7.75±0.71 | 9.63±0.74 |
| PMMA (0.3 ml) | 8.25±0.71 | 10.25±0.71 |
| CPC (0.1 ml) | 6.75±0.71 | 8.75±1.04 |
| CPC (0.3 ml) | 7.75±1.04 | 9.63±0.74 |
Discussion
The most frequently used cement types in PVP are the
acrylic polymers, PMMA and CPC. CPC, which is a mixture of several
types of calcium phosphate, has osteoconduction and reabsorption
properties (13). PMMA is composed
of methylmethacrylate polymer and the monomer of PMMA eventually
has a toxic effect on cells. PMMA reaches peak temperatures of up
to 113°C in vitro and produces an exothermic reaction during
polymerization (14,15).
During PVP, PMMA may cause thermal necrosis in the
adjacent tissue. In particular, direct contact between PMMA and the
nerve roots or the dural sac causes thermal necrosis in neural
structures. The occurrence and severity of thermal tissue damage
depends on the following factors: i) The magnitude and the duration
of exposure; ii) the type of tissue exposed; and iii) the extent of
local heat convection via blood flow. In the present in vivo
study, temperatures in the vertebral body and epidural space were
not high enough to cause tissue necrosis when only a small quantity
(0.5–0.7 ml) of PMMA was injected (16).
Following PMMA injection in the present study,
histological examination revealed necrosis of nucleus pulposus
cells, condensation and degradation of the matrix, rupture and
tumescence of the annulus fibrosus, and calcification of the
cartilage endplate. These changes were less prominent in the CPC
groups relative to the PMMA groups. Calcification was not detected
in the cartilage endplate in the CPC groups. The difference in
histological changes between the PMMA and CPC groups may be
associated with cell toxicity and the exothermic effect of
PMMA.
Affluent veins are present in the vertebral body.
Venous flow can efficiently reduce temperature levels and the local
concentration of PMMA monomers, preventing structural injury.
However, the intervertebral disc has an avascular architecture
(15). Following cement leakage,
heat convection and toxicity inside the disc cannot dissipate
rapidly due to cell infiltration. In the samples injected with PMMA
in this study, a calcification zone formed at the cartilage
endplate. The cartilage endplate is the main route through which
the disc gains nutrition. Thus, calcification accelerates the
process of disc degeneration.
The present experiments demonstrate that the degree
of disc degeneration positively correlates with the quantity of
bone cement injected into each disc. The intervertebral disc is
known as a closed buffer system. When cement is injected, the
stress in the disc increases as the pressure increases.
Intervertebral discs primarily gain nutrition via passive
diffusion. The increasing stress may cause cell infiltration
hypofunction, which influences nutrient absorption. Furthermore,
the metabolic level of the disc cell decreases under sustained high
stress. As the level of aerobic oxidation decreases, the lactic
acid content increases, and the pH value decreases (17). These changes promote cell death in
the disc. Rannou et al (18) demonstrated that cell apoptosis in
the annulus fibrosus occurred when a mechanical load of 1.3 MPa was
applied to the spine of mice. The application of mechanical load
increased stress to the disc, which in turn resulted in severe disc
degeneration. Another in vitro study indicated that a
cement-filled bone is 36-fold stronger than a cancellous spinal
bone (19).
Injected cement occupies certain spaces in the disc
and causes mechanical changes that compress the nucleus pulposus.
As a result, the nucleus pulposus is displaced, deformed and
broken. The process of disc degeneration is caused by derangement
of cell metabolism and dysfunction of biochemical constituents.
Another reason for the degeneration of the nucleus
pulposus in the PMMA groups is cell apoptosis. Our histological
findings demonstrate that condensation of the extracellular matrix
may be caused by several factors: i) Hydratability subsides with an
increase in stress, which may be an important factor affecting the
extracellular matrix; ii) condensation and degradation of the
extracellular matrix are induced by the thermal reaction and
toxicity of the PMMA monomer, which may also induce apoptosis; and
iii) the quantity and quality of synthesis of the extracellular
matrix is reduced with the loss of healthy nucleus pulposus cells.
These factors contribute to the degeneration of a cement-injected
disc (20).
The present study also indicates that the extent of
disc degeneration is affected by the time period following bone
cement injection. As the observation time was extended, signals
from the nucleus pulposus in T2-weighted images decreased, and the
area of relatively high signal declined, accompanied by the
irregular appearance of the nucleus pulposus. Histological data
reveal the change from a slightly interrupted border at 12 weeks to
the loss of any boundary between the annulus fibrosus and nucleus
pulposus at 24 weeks. Other key findings include the gradual
reduction of the nucleus pulposus cell, the increased significance
in the occurrence of matrix condensation, and the extended degree
of the ruptured or serpentine-patterned fibers in the annulus
fibrosus. These observations, along with the MRI results, strongly
demonstrate that degeneration of injected discs increases over
time.
Cement leakage, which has a potential influence on
the disc during PVP, often occurs under two conditions: Fractured
endplates and vacuum clefts (1,21).
The most common reason for cement leakage is the fracturing of
endplates, which may already exist along with compressive vertebral
fractures. Cement leakage may also occur as a result of excessive
penetration by the needle into the endplate. Vacuum clefts are
caused by a non-union of vertebral fractures. If penetration into
the disc space occurs, the risk of cement leakage increases.
The present study used a direct puncture technique
and employed injection to disc as the model of cement leakage in
PVP. An ideal model for cement leakage should involve injecting
cement into the vertebral body through the endplate; however, this
model is difficult to manipulate as improper management of the
needle tip could injure the endplate. With the trans-vertebral
method, controlling the amount of cement leakage may be impossible.
Direct puncture with a needle may result in IDD. The degree of IDD
has a close correlation with the diameter of the puncture needle,
where the smaller the needle, the lower the extent of IDD (12). By using a 27G needle to puncture
the disc of a rabbit, An et al (22) demonstrated that no histological
abnormality occurs in the annulus fibrosus, nucleus pulposus and
endplate. Similarly, in the present study, IDD was not observed
after using an 18G needle to puncture the discs of dogs in the
control group. The reason for the absence of IDD may be associated
to the size of the animal relative to the needle.
Although the model used in the present study may not
exactly replicate cement leakage during PVP, the data provide
evidence of the consequences of leakage. Therefore, caution should
be exercised to avoid cement extravasation into the disc space
during injection. Prior to cement injection, the operator should
verify that the needle tip is outside the disc by using various
positions of the C arm when the needle tip is located near the
fractured endplate. Cement injection with paste or drainage is
recommended in the treatment of vertebrae with vacuum clefts.
References
|
1
|
Mirovsky Y, Anekstein Y, Shalmon E,
Blankstein A and Peer A: Intradiscal cement leak following
percutaneous vertebroplasty. Spine (Phila Pa 1976). 31:1120–1124.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galibert P, Deramond H, Rosat P and Le
Gars D: Preliminary note on the treatment of vertebral angioma by
percutaneous acrylic vertebroplasty. Neurochirurgie. 33:166–168.
1987.(In French).
|
|
3
|
Cortet B, Cotten A, Boutry N, Flipo RM,
Duquesnoy B, Chastanet P and Delcambre B: Percutaneous
vertebroplasty in the treatment of osteoporotic vertebral
compression fractures: an open prospective study. J Rheumatol.
26:2222–2228. 1999.PubMed/NCBI
|
|
4
|
Jensen ME, Evans AJ, Mathis JM, Kallmes
DF, Cloft HJ and Dion JE: Percutaneous polymethylmethacrylate
vertebroplasty in the treatment of osteoporotic vertebral
compression fractures: technicalaspects. AJNR Am J Neuroradiol.
18:1897–1904. 1997.
|
|
5
|
Ahn Y, Lee JH, Lee HY, Lee SH and Keem SH:
Predictive factors for subsequent vertebral fracture after
percutaneous vertebroplasty. J Neurosurg Spine. 9:129–136. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen JK, Lee HM, Shih JT and Hung ST:
Combined extraforaminal and intradiscal cement leakage following
percutaneous vertebroplasty. Spine (Phila Pa 1976). 32:E358–E362.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pitton MB, Herber S, Bletz C, et al:
CT-guided vertebroplasty in osteoporotic vertebral fractures:
incidence of secondary fractures and impact of intradiscal cement
leakages during follow-up. Eur Radiol. 18:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nieuwenhuijse MJ, Putter H, van Erkel AR
and Dijkstra PD: New vertebral fractures after percutaneous
vertebroplasty for painful osteoporotic vertebral compression
fractures: a clustered analysis and the relevance of intradiskal
cement leakage. Radiology. 266:862–870. 2013. View Article : Google Scholar
|
|
9
|
Nieuwenhuijse MJ, Van Erkel AR and
Dijkstra PD: Cement leakage in percutaneous vertebroplasty for
osteoporotic vertebral compression nfractures: identification of
risk factors. Spine J. 11:839–848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobajima S, Kompel JF, Kim JS, et al: A
slowly progressive and reproducible animal model of intervertebral
disc degeneration characterized by MRI, X-ray, and histology. Spine
(Phila Pa 1976). 30:15–24. 2005.PubMed/NCBI
|
|
11
|
Brennan P and Silman A: Statistical
methods for assessing observer variability in clinical measures.
BMJ. 304:1491–1494. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masuda K, Aota Y, Muehleman C, et al: A
novel rabbit model of mild, reproducible disc degeneration by an
anulus needle puncture: correlation between the degree of disc
injury and radiological and histological appearances of disc
degeneration. Spine (Phila Pa 1976). 30:5–14. 2005.
|
|
13
|
Provenzano MJ, Murphy KP and Riley LH III:
Bone cements: review of their physiochemical and biochemical
properties in percutaneous vertebroplasty. AJNR Am J Neuroradiol.
25:1286–1290. 2004.PubMed/NCBI
|
|
14
|
Dahl OE, Garvik LJ and Lyberg T: Toxic
effects of methymethacrylate monomer on leukocytes and endothelial
cells in vitro. Acta Orthop Scand. 65:147–153. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belkoff SM and Molloy S: Temperature
measurement during polymerization of polymethylmethacrylate cement
used for vertebroplasty. Spine (Phila Pa 1976). 28:1555–1559. 2003.
View Article : Google Scholar
|
|
16
|
Aebli N, Goss BG, Thorpe P, Williams R and
Krebs J: In vivo temperature profile of intervertebral discs and
vertebral endplates during vertebroplasty: an experimental study in
sheep. Spine (Phila Pa 1976). 31:1674–1678. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hutton WC, Elmer WA, Bryce LM, Kozlowska
EE, Boden SD and Kozlowski M: Do the intervertebral disc cells
respond to different levels of hydrostatic pressure? Clin Biomech
(Bristol, Avon). 16:728–734. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rannou F, Lee TS, Zhou RH, et al:
Intervertebral disc degeneration: the role of the mitochondrial
pathway in annulus fibrosus cell apoptosis induced by overload. Am
J Pathol. 164:915–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baroud G, Heini P, Nemes J, Bohner M,
Ferguson S and Steffen T: Biomechanical explanation of adjacent
fractures following vertebroplasty. Radiology. 229:606–608. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walker MH and Anderson DG: Molecular basis
of intervertebral disc degeneration. Spine J. 4:158S–166S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peh WC, Gilula LA and Peck DD:
Percutaneous vertebroplasty for severe osteoporotic vertebral body
compression fractures. Radiology. 223:121–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
An Hs, Takegami K, Kamada H, et al:
Intradiscal administration of osteogenic protein-1 increases
intervertebral disc height and proteoglycan content in the nucleus
pulposus in normal adolescent rabbits. Spine (Phila Pa 1976).
30:25–31. 2005.
|