Introduction
Invasive fungal infection is a major cause of
morbidity and mortality in immunocompromised patients. Amphotericin
B (AMB) possesses broad-spectrum antifungal activity and
well-documented efficacy against Candida, Aspergillus and
Cryptococcus infections (1).
Liposomal-AMB (L-AMB), which was developed as a drug delivery
system for AMB to reduce its adverse events (e.g., nephrotoxicity),
replaced AMB (2) and is commonly
used in clinical practice worldwide. L-AMB is recommended as a
first-line drug for hematological patients in the guidelines of the
Infectious Diseases Society of America (3,4).
However, treatment with L-AMB may necessitate potassium
supplementation to prevent hypokalemia. A previous study (5) reported the risk factors contributing
to the occurrence of hypokalemia following L-AMB administration.
The study revealed that patient’s serum albumin levels (≥2.82
mg/dl) at the start of L-AMB administration and history of
hypokalemia prior to L-AMB administration were independent risk
factors significantly contributing to the occurrence of hypokalemia
(5). However, proper potassium
supplementation for hypokalemia had not been sufficiently
investigated. The present study therefore retrospectively examined
proper potassium supplementation for hypokalemia induced by
L-AMB
Subjects and methods
Subjects
The subjects were 100 hematological patients who
received L-AMB for the first time at Ogaki Municipal Hospital
(Ogaki-shi, Japan) between April 2012 and March 2013. Seven
patients were excluded due to prior or ongoing treatments that
affect potassium levels (e.g., furosemide or fluid replacement
during L-AMB administration), or as they had serum potassium levels
<3.0 mEq/l (hypokalemia higher than grade 3) prior to the L-AMB
administration. Of the remaining 93 patients, 48 (51.6%) were
assigned to the group receiving supplemental potassium
(supplementation group), and 45 (48.4%) were assigned to the group
without potassium supplementation (non-supplementation group)
(Fig. 1). L-AMB was administered
once a day for 1–2 h. This study was reviewed and approved by the
Ethics Committee at Ogaki Municipal Hospital.
Background of the subjects
The backgrounds of the subjects treated with L-AMB
were investigated for their gender, age, serum creatinine levels,
treatment L-AMB dose, performance status (ECOG), underlying disease
and prior use of antifungal drugs for a primary infection episode.
The backgrounds between the supplementation or non-supplementation
groups were compared.
Incidence of hypokalemia and potassium
supplementation
Incidences of hypokalemia greater than grade 3
(serum potassium levels <3.0 mEq/l) were identified and compared
between the groups. Treatment dosing for potassium supplementation
in the supplementation group was investigated.
Change in the serum potassium levels
The change and the minimum levels of serum potassium
during L-AMB administration were identified and compared between
the groups.
Investigation of the factors affecting
the potassium supplementation during L-AMB administration
The factors affecting the potassium supplementation
during L-AMB administration were examined between the groups.
Investigation of the factors affecting
proper potassium supplementation in the supplementation group
The serum potassium levels following the potassium
supplementation were investigated, and the factors affecting proper
potassium supplementation were examined. The proper potassium
supplementation was defined as serum potassium levels that were
maintained at >3.0 mEq/l (higher than grade 2) following the
potassium supplementation.
Method of data collection
Laboratory values in the form of biochemistry
results were retrospectively identified from electronic medical
charts. Data (including the age, serum creatinine levels, and L-AMB
or potassium supplementation doses of the patients) were presented
as the mean ± standard deviation. The grade of the hypokalemia was
assessed in accordance with the Japan Clinical Oncology Group/Japan
Society of Clinical Oncology Japanese version of the Common
Terminology Criteria for Adverse Events, v4.0.
Statistical analysis
Analyses were performed using JMP software (version
5.0.1J; SAS Institute Japan Ltd., Tokyo, Japan). Wilcoxon
signed-rank test was used for comparison of the serum potassium
levels prior to and following L-AMB administration. The
Mann-Whitney U test was used for comparison of the backgrounds of
the subjects between the groups. The recorded P-values were
two-sided and values of <0.05 were considered to indicate a
statistically significant difference. The areas under the
receiver-operator characteristic (ROC) curves were calculated to
estimate the accuracy and cut-off values for the continuous
variables obtained by univariate logistic regression analysis.
Subsequently, the data were analyzed using multivariate logistic
regression analysis.
Results
Background of subjects
Table I summarizes
the backgrounds of the subjects. The total L-AMB dosages for the
supplementation and non-supplementation groups were 2485.1±1730.6
and 1485.6±1345.6 mg, respectively, and the treatment L-AMB doses
were 125±30 and 110±25 mg/day, respectively. In addition, the
duration of the L-AMB treatment was 19.8±14.2 and 12.9±8.4 days,
respectively. The L-AMB was administered as a second-line therapy
(following micafungin or caspofungin and others) in 61.0% (57/93)
of the subjects and as a first-line therapy in 39.0% (36/93) of the
subjects.
 | Table IPatient demographics and baseline
characteristics. |
Table I
Patient demographics and baseline
characteristics.
| Potassium
supplementation | |
|---|
|
| |
|---|
| Demographics and
characteristics | With | Without | P-value |
|---|
| Gender |
| Male | 29 | 25 | 0.63 |
| Female | 19 | 20 | |
| Age (years;
median) | 68.3±12.3 | 67.2±14.9 | 0.61 |
| Serum creatinine
levels (mg/dl) |
| Prior to L-AMB
administration | 0.70±0.62 | 0.76±0.63 | 0.65 |
| Following L-AMB
administration | 0.91±0.71 | 0.86±0.81 | 0.75 |
| L-AMB |
| Total dosage
(mg) | 2485.1±1730.6 | 1485.6±1345.6 | 0.01 |
| Treatment dose
(mg/day) | 125±30 | 110±25 | 0.01 |
| Duration of
treatment (days) | 19.8±14.2 | 12.9±8.4 | 0.01 |
| Potassium
supplementation |
| Total dosage
(mEq) | 519.6±506.1 | - | |
| Treatment dose
(mEq/day) | 32.6±13.4 | - | |
| Duration of
treatment (days) | 14.4±14.0 | - | |
| Minimum serum
potassium levels | | | <0.01 |
| K ≥3.0 mEq/l | 15 | 28 | |
| K <3.0 mEq/l | 33 | 17 | |
| Performance status
(ECOG) | | | 0.82 |
| 0 | 12 | 14 | |
| 1 | 12 | 8 | |
| 2 | 9 | 8 | |
| 3 | 9 | 11 | |
| 4 | 6 | 4 | |
| Underlying
disease |
| ML | 12 | 19 | |
| AML | 22 | 10 | |
| ALL | 2 | 2 | |
| MDS | 3 | 3 | |
| MM | 4 | 8 | |
| AA | 3 | 3 | |
| Others | 2 | 0 | |
| Prior antifungal
drugs for the primary infection episode |
| Micafungin | 23 | 18 | |
| Caspofungin | 5 | 1 | |
| Voriconazole | 4 | 1 | |
| Fluconazole | 2 | 1 | |
| Itraconazole | 2 | 0 | |
| Nothing | 12 | 24 | |
Incidence of hypokalemia and potassium
supplementation
The incidence of patients with hypokalemia greater
than grade 3 (serum potassium levels <3.0 mEq/l) was 53.8%
(50/93 subjects). Potassium supplementation was used to treat 51.6%
of the subjects (48/93). The total potassium supplementation dosage
was 519.6±506.1 mEq, and the treatment potassium supplementation
dosage was 32.6±13.4 mEq/day. The duration of the potassium
supplementation treatment was 14.4±14.0 days.
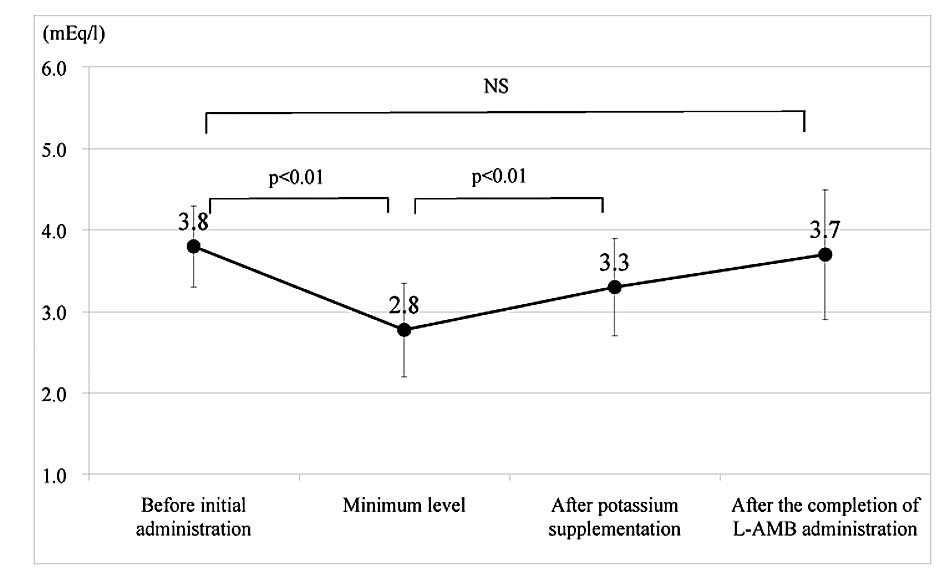
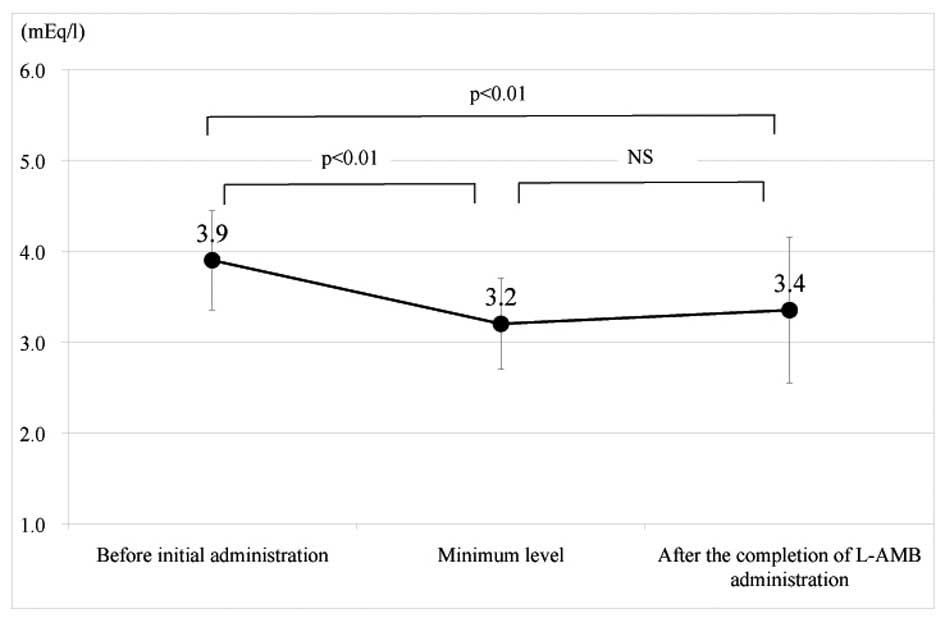
Change in the serum potassium levels
The change in the serum potassium levels during the
L-AMB administration is shown in Figs.
2 and 3, divided into the two
groups. In the supplementation group, the serum potassium levels
significantly decreased from 3.8±0.5 to 2.8±0.6 mEq/l (P<0.01),
when comparing the levels prior to the initial L-AMB administration
with the minimum levels (at the time of the initialization of
potassium supplementation). Subsequently, the levels significantly
increased to 3.3±0.6 mEq/l following the potassium supplementation
(P<0.01). In addition, the levels recovered to 3.7±0.8 mEq/l
following completion of the L-AMB administration (2.9±2.6 days
after completion). In the non-supplementation group, the serum
potassium levels markedly decreased from 3.9±0.5 to 3.2±0.5 mEq/l
(P<0.01), when comparing the levels prior to the initial L-AMB
administration with the minimum levels. In addition, the levels
only recovered to 3.4±0.8 mEq/l following completion of the L-AMB
administration (2.0±1.5 days after completion) and a significant
difference was identified compared with the levels prior to the
L-AMB administration (P<0.01).
Investigation of the factors affecting
potassium supplementation during L-AMB administration
Nine factors affecting the differences between the
groups were analyzed using univariate logistic regression analysis.
The independent variables of the dosage data were analyzed as a
continuous variable, and the results are shown in Table II. The total L-AMB dosage [odds
ratio (OR), 67.97; 95% confidence interval (CI), 4.34–<1000;
P<0.01], treatment L-AMB dose (OR, 25.57; 95% CI, 2.31–395.36;
P=0.01), duration of the L-AMB treatment (OR, 224.62; 95% CI,
5.26–<1000; P<0.01), and minimum serum potassium levels
during the L-AMB administration (OR 0.07; 95% CI 0.01–0.55; P=0.01)
showed significant differences between the two groups. The areas
under the ROC curves of these factors were 0.75, 0.63, 0.71 and
0.66, and the cut-off values were 2001.4 mg, 118.5 mg/day, 16.4
days and 2.98 mEq/l, respectively. Table III shows the results of the
multivariate analysis based on the factors with P<0.25 by
univariate logistic regression analysis. It revealed that the
minimum serum potassium levels during L-AMB administration (≤2.98
mEq/l) were an independent factor significantly contributing to the
effectiveness of potassium supplementation (OR, 3.62; 95% CI,
1.44–9.59; P<0.01).
 | Table IIUnivariate analysis of the factors
affecting potassium supplementation during L-AMB administration
(n=93). |
Table II
Univariate analysis of the factors
affecting potassium supplementation during L-AMB administration
(n=93).
| Factor | OR | 95% CI | P-value | AUC | Cut-off |
|---|
| Gender (female) | 0.81 | 0.35–1.86 | 0.63 | | |
| Age | 1.64 | 0.25–11.18 | 0.60 | | |
| Serum creatinine
levels prior to L-AMB administration (mg/dl) | 0.51 | 0.01–9.84 | 0.65 | | |
| Total L-AMB dosage
(mg) | 67.97 | 4.34–<1000 | <0.01 | 0.75 | 2001.4 |
| Treatment L-AMB dose
(mg/day) | 25.57 | 2.31–395.36 | 0.01 | 0.63 | 118.5 |
| Duration of L-AMB
treatment (days) | 224.62 | 5.26–<1000 | <0.01 | 0.71 | 16.40 |
| Serum potassium
levels prior to L-AMB administration (mEq/l) | 0.51 | 0.06–3.78 | 0.51 | | |
| Minimum serum
potassium levels during L-AMB administration (mEq/l) | 0.07 | 0.01–0.55 | 0.01 | 0.66 | 2.98 |
| PS ≥2 | 1.15 | 0.34–3.83 | 0.81 | | |
 | Table IIIMultivariate analysis of the factors
affecting potassium supplementation during L-AMB administration
(n=93). |
Table III
Multivariate analysis of the factors
affecting potassium supplementation during L-AMB administration
(n=93).
| Factor | OR | 95% CI | P-value |
|---|
| Total L-AMB dosage
(≥2001.4 mg) | 3.23 | 0.67–17.47 | 0.14 |
| Treatment dose
(≥118.5 mg/day) | 2.01 | 0.76–5.88 | 0.14 |
| Duration of treatment
(≥16.4 days) | 1.39 | 0.29–6.17 | 0.66 |
| Minimum serum
potassium levels during L-AMB administration (≤2.98 mEq/l) | 3.62 | 1.44–9.59 | <0.01 |
Investigation of the factors affecting
proper potassium supplementation in the supplementation group
Twelve factors affecting the proper potassium
supplementation were analyzed using univariate logistic regression
analysis. The independent variables of dosage data were analyzed as
a continuous variable, and the results are shown in Table IV. The serum potassium levels
prior to the potassium supplementation showed a significant
difference in the effectiveness of proper potassium supplementation
(OR, 151.51; 95% CI, 12.60–733.52; P<0.01). The area under the
ROC curve of this factor was 0.81 and the cut-off value was 2.83
mEq/l. Table V shows the results
of the multivariate analysis based on the factors with P<0.25 by
univariate logistic regression analysis. The serum potassium levels
prior to the potassium supplementation (≥2.83 mEq/l) again showed a
significant difference (OR, 14.21; 95% CI, 1.95–310.72; P=0.02).
However, the duration of the potassium supplementation and the
treatment L-AMB dose showed no significant difference in the
effectiveness of the proper potassium supplementation.
 | Table IVUnivariate analysis of the factors
affecting proper potassium supplementation (n=48). |
Table IV
Univariate analysis of the factors
affecting proper potassium supplementation (n=48).
| Factor | OR | 95% CI | P-value | AUC | Cut-off |
|---|
| Gender
(female) | 1.43 | 0.38–6.16 | 0.61 | | |
| Age | 0.44 | 0.11–10.25 | 0.62 | | |
| Serum creatinine
levels prior to L-AMB administration (mg/dl) | 6.07 | 0.04–>1000 | 0.61 | | |
| Total L-AMB dosage
(mg) | 0.15 | 0.01–2.72 | 0.19 | | |
| Treatment dose
(mg/day) | 0.14 | 0.01–1.48 | 0.11 | | |
| Duration of
treatment (days) | 0.23 | 0.01–8.71 | 0.39 | | |
| Potassium
supplementation dose (mEq/day) | 2.03 | 0.11–52.03 | 0.65 | | |
| Day of potassium
supplementation start (days) | 0.27 | 0.01–7.01 | 0.41 | | |
| Duration of
potassium supplementation (days) | 0.11 | 0.01–4.49 | 0.24 | | |
| Serum potassium
levels prior to L-AMB administration (mEq/l) | 2.60 | 0.21–37.67 | 0.46 | | |
| Serum potassium
levels prior to potassium supplementation (mEq/l) | 151.51 | 12.6–733.52 | <0.01 | 0.81 | 2.83 |
| PS ≥2 | 4.19 | 1.05–21.41 | 0.06 | | |
 | Table VMultivariate analysis of the factors
affecting proper potassium supplementation (n=48). |
Table V
Multivariate analysis of the factors
affecting proper potassium supplementation (n=48).
| Factor | OR | 95% CI | P-value |
|---|
| Total L-AMB dosage
(mg) | 182.00 | 0.01–262.65 | 0.91 |
| Treatment dose
(mg/day) | 0.74 | 0.12–4.08 | 0.73 |
| Duration of
potassium supplementation (days) | 0.14 | 0.01–80.64 | 0.57 |
| Serum potassium
levels prior to potassium supplementation (≥2.83 mEq/l) | 14.21 | 1.95–310.72 | 0.02 |
| PS ≥2 | 3.04 | 0.62–18.23 | 0.18 |
Discussion
Immunocompromised hematological patients frequently
develop febrile neutropenia, thus empirical treatment with
antifungal drugs is initiated prior to confirmation of a definitive
diagnosis of a fungal infection (1). L-AMB possesses broad-spectrum
antifungal activity and is a first-line indication against
unconfirmed fungal infections in empirical therapy (2). Competitive studies with other
antifungal drugs have demonstrated the efficacy of L-AMB (6,7).
The present study investigated hematological
patients who were receiving L-AMB for the first time. Prior
antifungal drugs used for the primary infection episode in the
patients of the present study (61% of them used L-AMB in the change
from other antifungal drugs) included micafungin and caspofungin.
In addition, L-AMB was used in combination with intensive
antibiotics, including carbapenem (82%) or glycopeptides (50%; data
not shown). Infectious diseases in hematological patients may lead
to a fatal outcome. Thus, infection control with antibiotics and
antifungal drugs is critical.
L-AMB may reduce the levels of blood potassium by
damaging the renal tubules (8,9). The
incidence of hypokalemia in patients treated with L-AMB was
reported as 36% by Ringden in 1994 (10) and as 51.3% by Sunakawa in 2012
(11). In the present study,
hypokalemia greater than grade 3 occurred in 53.8% of the patients,
making it an adverse event that requires attention. By comparing
the serum potassium levels in patients of the two groups, the
minimum levels in the supplementation group were significantly
lower than those of the non-supplementation group, although no
difference in them prior to the L-AMB administration was observed
(mean±SD, 3.8±0.5 vs. 3.9±0.5 mEq/l; P=0.51). However, the serum
potassium levels following the L-AMB administration were higher in
the supplementation group than those in the non-supplementation
group (3.7±0.8 vs. 3.4±0.8 mEq/l; P=0.04). Recovery of the patients
from hypokalemia was more rapid in those in the supplementation
group. Altogether, 51.6% of the subjects were treated with
potassium supplementation. The supplementation group had a longer
duration of L-AMB treatment and a greater total L-AMB dosage
compared with those of the non-supplementation group. Following
investigation of the factors affecting potassium supplementation
during L-AMB administration, it was revealed that the minimum serum
potassium levels during L-AMB administration (≤2.98 mEq/l) were an
independent factor significantly contributing to the effectiveness
of treatment with supplemental potassium. Investigation of the
factors affecting the effectiveness of proper potassium
supplementation in the supplementation group revealed that serum
potassium levels prior to the potassium supplementation showed a
significant difference between the patients that were successfully
treated for hypokalemia and those that did not receive this
treatment. This means that it is necessary to initiate potassium
supplementation prior to reduction of the serum potassium levels to
<2.83 mEq/l. Potassium supplementation from an early stage is
important to maintain serum potassium levels at >3.0 mEq/l
(higher than grade 2), thereby preventing proper potassium
supplementation.
In conclusion, a periodic serum potassium levels
monitor from the beginning of L-AMB administration and potassium
supplementation from an early stage are important to prevent severe
electrolyte abnormalities. Invasive opportunistic fungal infections
are a serious cause of morbidity and mortality for
immunocompromised hematological patients. Therefore, adverse events
management of L-AMB is essential.
References
|
1
|
Pizzo PA, Robichaud KJ, Gill FA and
Witebsky FG: Empiric antibiotic and antifungal therapy for cancer
patients with prolonged fever and granulocytopenia. Am J Med.
72:101–111. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walsh TJ, Finberg RW, Arndt C, Hiemenz J,
Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN,
Dummer S, Schuster M and Holcenberg JS: Liposomal amphotericin B
for empirical therapy in patients with persistent fever and
neutropenia. National Institute of Allergy and Infectious Diseases
Mycoses Study Group. N Engl J Med. 340:764–771. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh
MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA and Wingard
JR; Infectious Diseases Society of America. Clinical practice
guideline for the use of antimicrobial agents in neutropenic
patients with cancer: 2010 update by the Infectious Diseases
Society of America. Clin Infect Dis. 52:427–431. 2011. View Article : Google Scholar
|
|
4
|
Maertens J, Marchetti O, Herbrecht R,
Cornely OA, Flückiger U, Frêre P, Gachot B, Heinz WJ, Lass-Flörl C,
Ribaud P, Thiebaut A and Cordonnier C; Third European Conference on
Infections in Leukemia. European guidelines for antifungal
management in leukemia and hematopoietic stem cell transplant
recipients: summary of the ECIL 3 - 2009 update. Bone Marrow
Transplant. 46:709–718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuoka T, Usami E, Yoshimura T, Takada H
and Yasuda T: Risk factors contributing to occurrence of
hypokalemia after liposomal-amphotericin B administration. Jpn J
Pharm Health Care Sci. 37:487–493. 2011. View Article : Google Scholar
|
|
6
|
Kuse ER, Chetchotisakd P, da Cunha CA,
Ruhnke M, Barrios C, Raghunadharao D, et al; Micafungin Invasive
Candidiasis Working Group. Micafungin versus liposomal amphotericin
B for candidaemia and invasive candidosis : a phase III randomized
double-blind trial. Lancet. 369:1519–1527. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herbrecht R, Denning DW, Patterson TF,
Bennett JE, Greene RE, Oestmann JW, et al; Invasive Fungal
Infections Group of the European Organisation for Research and
Treatment of Cancer and the Global Aspergillus Study Group.
Voriconazole versus amphotericin B for primary therapy of invasive
aspergillosis. N Engl J Med. 347:408–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nichols AJ, Koster PF, Brooks DP and
Ruffolo RR Jr: Effect of fenoldpam on acute and subacute
nephrotoxicity produced by amphotericin B in the dog. J Pharmacol
Exp Ther. 260:269–274. 1992.PubMed/NCBI
|
|
9
|
Tasset C, Preat V, Bernard A and Roland M:
Comparison of nephrotoxicities of different polyoxyethylenglycol
formulations of amphotericin B in rats. Antimicrob Agents
Chemother. 36:1525–1531. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ringdén O, Andström E, Remberger M, et al:
Safety of liposomal amphotericin B (AmBisome) in 187 bone marrow
transplant recipients treated with cyclosporin. Bone Marrow
Transplant. 14(Suppl 5): S10–S14. 1994.PubMed/NCBI
|
|
11
|
Sunakawa K, Tsukimoto I, Tsunematu Y,
Honda M, Iwai N, Maniwa T, Haigo H, Suzuki K and Mori T: Evaluation
of the safety and efficacy of liposomal amphotericin B (L-AMB) in
children. J Infect Chemother. 18:456–465. 2012. View Article : Google Scholar : PubMed/NCBI
|