Introduction
Preeclampsia (PE) is a multisystem syndrome
affecting pregnant females. PE usually develops after 20 weeks of
gestation and affects 4–10% of pregnant females. PE is
characterized by several symptoms, including hypertension,
proteinuria and additional complications such as liver and kidney
failure and fetal distress. Approximately 25% of babies born to
females with PE are smaller than normal for the particular
gestational age. PE is a predominant cause of maternal morbidity
and mortality worldwide (1,2).
Although the exact determinants of PE remain unclear, placental
ischemia is considered to be important in the development. The
hypoxic placenta may lead to an imbalance in the release of
circulating factors, which may result in widespread vascular
endothelial injury. Certain proteomic factors, including
antiangiogenic factors, may contribute to systemic hypertension,
vascular injury and disorders of the coagulation system (3–5).
Variations in these circulating proteomic factors have been shown
to correlate with pathophysiological changes in the disease.
Early diagnosis of PE is reliant upon the provision
of regular antenatal care prior to delivery. To date, no
biomarker-based laboratory assessment is able to diagnose PE.
Investigations have been conducted to identify non-invasive,
blood-borne or urinary maternal biomarkers that predict the
development of PE and aid in the monitoring of this severe
complication during pregnancy (1,6).
Potential biochemical markers, including soluble fms-like tyrosine
kinase 1 (sflt-1) and placental growth factor (PLGF), have been
identified, however are not considered to be reliable in the
diagnosis of PE (1,5,6).
Therefore, identification of effective markers is required to
predict PE.
In a previous study, serum proteomic analysis of PE
was performed, revealing decreased transthyretin (TTR)
concentrations in the sera of females with PE (7). TTR is a tetrameric serum protein
composed of four identical subunits (55 kDa) and is predominantly
synthesized in the liver, eye and choroid plexus. A protein group
comprising TTR, thyroxin-binding globulin and albumin, bind to and
transport thyroid hormones in the blood; the main function of TTR
is the transport of thyroxin (T4) (8). TTR is synthesized by placental
trophoblasts which are critical to normal fetal development. Thus,
disorders caused by TTR production may result in fetal distress
(9–11). In addition, >100 TTR mutations
have been shown to be associated with amyloid diseases, which
induce tissue-selective deposition of amyloid to various organs
(12,13). In a previous study, TTR was shown
to be upregulated by two-fold in pancreatic cancer, thus, it was
concluded that TTR may be used as a novel tumor marker (14). However, whether TTR may be used as
a biomarker of PE remains unknown.
In the present study, significant changes in TTR
expression levels during severe PE were observed. It was
hypothesized that the differences in TTR concentrations during
severe PE were associated with disease pathophysiology, thus, TTR
may be a candidate biomarker of PE.
Materials and methods
Grouping
Three experiments were conducted to identify the
changes in TTR levels during severe PE. Changes in TTR levels
during healthy pregnancy were observed as follows: A series of
samples were collected from normal pregnant females at different
gestation periods to identify the TTR concentrations during healthy
gestation (before 20 weeks, n=41; after 20 weeks, n=39). TTR levels
in females with severe PE were compared with the levels of those in
the normal control subject group. A total of 43 females after 20
weeks of gestation were selected as participants in the severe PE
group; these females were free of other pregnancy complications. No
subjects had a history of hypertension or renal disease. A total of
37 healthy females were enrolled in the control group and matched
to the females in the severe PE group with regard to gestational
age. TTR levels in the severe PE and control groups were monitored
simultaneously. TTR levels in the early (n=21) and late (n=22)
onset PE patients were compared (all of these cases were included
in the severe PE group). The characteristics of participants are
presented in Tables I–III. The serum samples were all
collected from Chinese females.
 | Table ICharacteristics of the severe PE and
control groups. |
Table I
Characteristics of the severe PE and
control groups.
| Characteristics | Control (n=37) | Severe PE (n=43) |
|---|
| Age, years | 27±3a | 28±4 |
| Gestation length,
weeks (range) | 33±4b (25–38) | 33±3 (25–37) |
| Systolic MAP,
mmHg | 115±10 | 159±18 |
| Diastolic MAP,
mmHg | 71±9 | 99±13 |
| Proteinuria, g/24
h | Negative | 5±1 |
| Placenta weight,
g | 692±135c | 623±125 |
| Infant birth weight,
g | 3,245±525d | 1,917±532 |
 | Table IIICharacteristics of early and late
onset PE. |
Table III
Characteristics of early and late
onset PE.
| Characteristics | Early onset PE
(n=21) | Late onset PE
(n=22) |
|---|
| Systolic MAP,
mmHg | 160±13 | 155±15 |
| Diastolic MAP,
mmHg | 112±10 | 92±15 |
| Proteinuria, g/24
h | 6±1 | 5±0 |
| Placenta weight,
g | 603±123a | 645±130 |
| Infant birth
weight, g | 1,489±542b | 2,200±447 |
Severe PE is defined as an increase in blood
pressure (≥160 mmHg systolic pressure or ≥110 mmHg diastolic
pressure on more than two occasions at an interval of at least 6 h)
that occurs following 20 weeks of gestation in females with normal
blood pressure, accompanied by proteinuria (serum protein ≥5 g/24 h
or ≥2+ calculated via dipstick measurement),
coagulopathy disorders (platelets <100×109/l or
disseminated intravascular coagulation), liver dysfunction and
acute renal disorders.
Sample collection
Samples were collected from the peripheral blood and
prepared by centrifugation at 2,415 × g for 10 min at 4°C within 4
h following acquisition. Samples were subsequently stored at −80°C
until use. The samples were collected from the Department of
Obstetrics and Gynecology of Beijing Chaoyang Hospital affiliated
to Capital Medical University (Beijing, China). This study was
approved by the Ethics Committee of Beijing Chaoyang Hospital
affiliated to Capital Medical University and informed consent was
provided by each participant.
Western blot analysis
TTR expression levels in severe PE and healthy
control subjects were evaluated by western blot analysis (severe
PE, n=43; control subjects, n=37). Total serum protein
concentrations were determined using the bicinchoninic acid assay
method (BCA Protein Assay Reagent; Thermo, Rockford, IL, USA).
Samples of 60 μg serum protein from the two groups were run on 15%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The
proteins were transferred to nitrocellulose membranes (Millipore
Corporation, Billerica, MA, USA) and subjected to 300 mV for 25
min. The membranes were blocked overnight at 4°C in blocking buffer
(containing 5% skimmed milk, 0.1% Tween 20 and 0.01 M tris-buffered
saline) and incubated with primary antibodies against TTR (mouse
monoclonal antibody, 1:1,000 dilution; Abcam, Cambridge, UK) for
120 min at room temperature. The membranes were incubated with goat
anti-mouse IgG secondary antibody (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 50 min. Protein levels were analyzed by
evaluation of the total signal intensities of the western blot
bands.
Identification of TTR levels using an
enzyme-linked immunosorbent assay (ELISA)
Serum TTR levels were determined by ELISA (Assaypro,
St. Charles, MO, USA). Samples were diluted for detection
(1:40,000)and the data were presented using CurveExpert 1.3.
Statistical analysis
Data were analyzed using SPSS 17.0 software (SPSS,
Inc, Chicago, IL, USA) with the independent samples t-test.
P<0.05 was considered to indicate a statistically significant
difference. The diagnostic value of TTR for severe PE was
determined based on receiver operating characteristic (ROC) curves
which were analyzed using MedCalc 9.6.2.0 (MedCalc Software bvba,
Ostend, Belgium).
Results
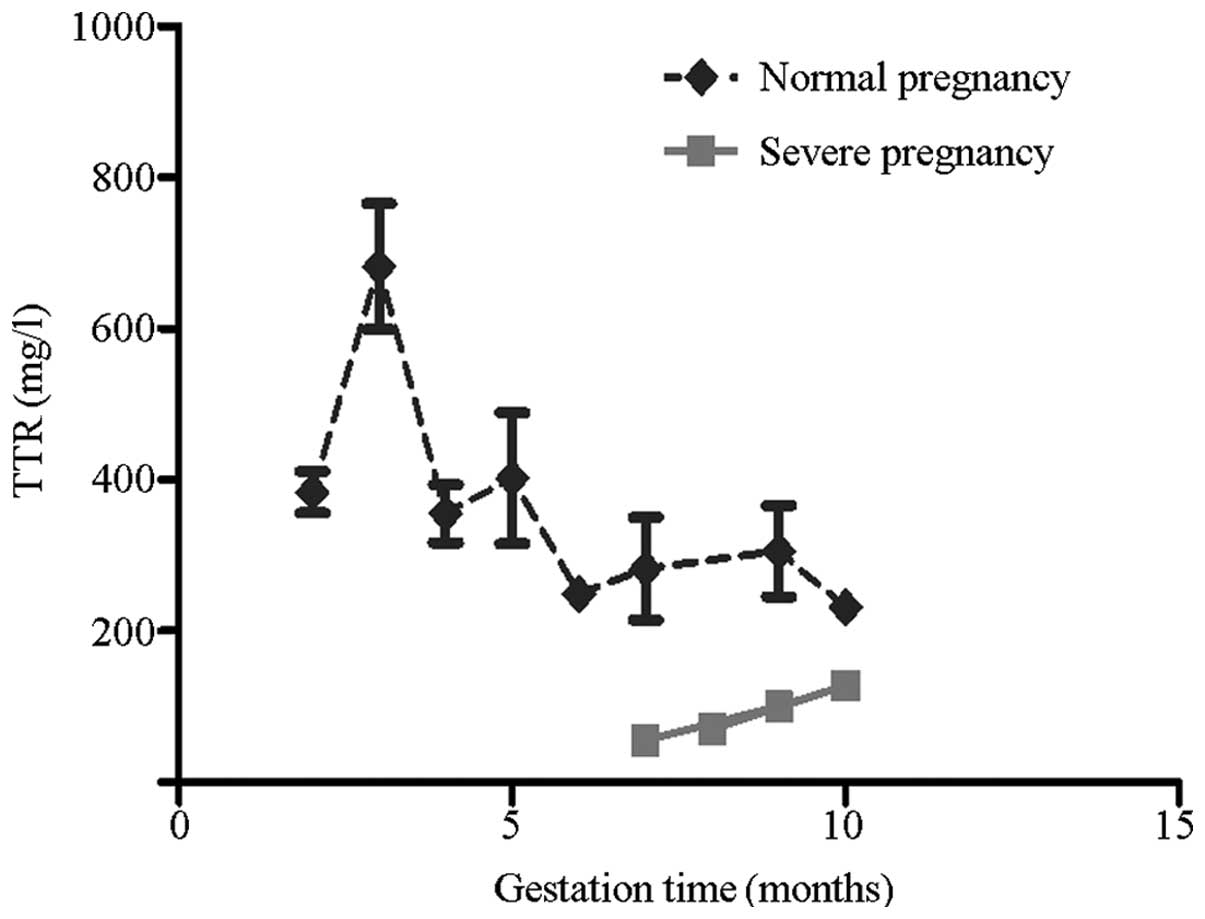
Detection of TTR concentrations during
healthy pregnancy
TTR concentrations in the three trimesters of normal
pregnancy were determined using ELISA kits (Fig. 1). TTR levels significantly
increased in the third month of gestation and rapidly decreased
following 20 weeks of gestation. TTR levels in the initial 20 weeks
of gestation were considerably higher compared with those following
20 weeks of gestation (P<0.001). No further changes in TTR
levels were observed thereafter.
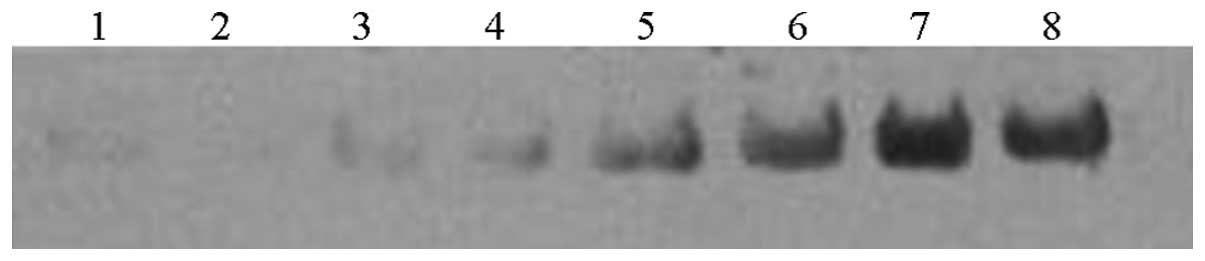
Western blot analysis of TTR changes
during severe PE
TTR expression levels were detected by western blot
analysis to directly monitor the changes in TTR during severe PE. A
total of 43 females with severe PE and 37 control subjects were
enrolled in the present study. Fig.
2 shows the expression levels of TTR in the sera of the two
groups. The single band at ~16 kDa represented the TTR monomer
undergoing dissociation. TTR expression levels were markedly
decreased in the sera of patients with severe PE and were ~2.6
times lower compared with the control subjects (4,867±3,464 vs.
12,517±8,516 OD units in the severe PE and control subjects,
respectively; P<0.001).
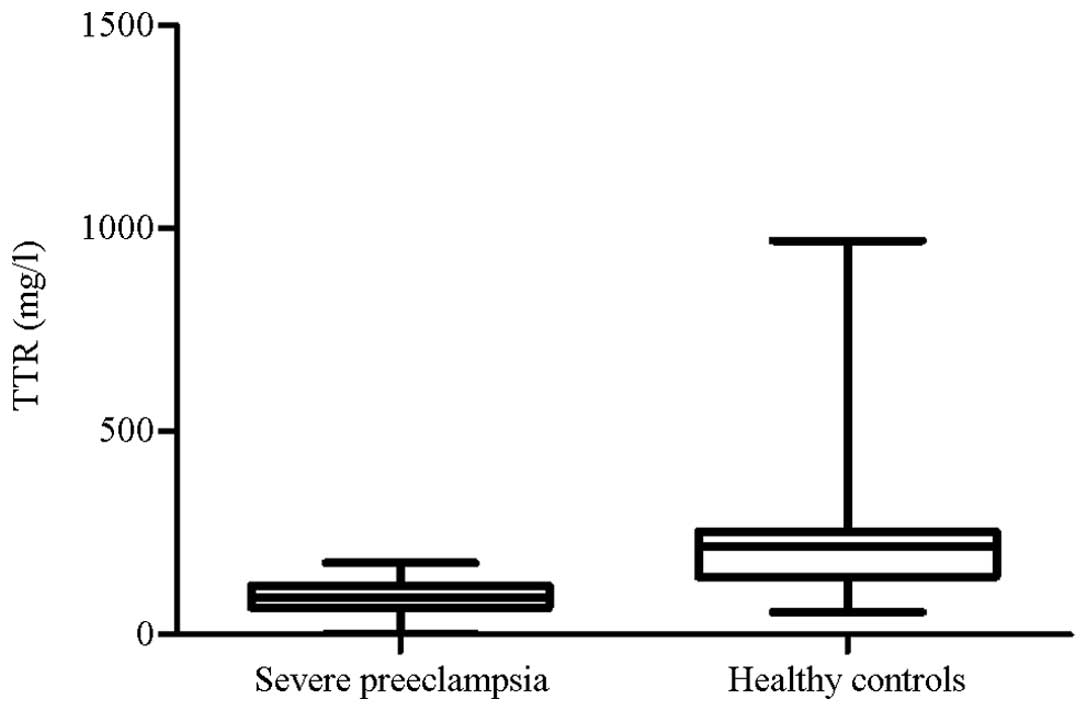
Identification of TTR levels during
severe PE using ELISA
ELISA analysis was conducted to quantify the TTR
levels of the patients in the severe PE (n=43) and control (n=37)
groups. TTR levels significantly decreased in the severe PE group
(P<0.001) and were ~2.4 times lower compared with the control
group. This result is consistent with those of the western blot
analysis. The median TTR concentration of the severe PE group was
significantly lower compared with the control group (Fig. 3). In Fig. 1, the median TTR concentrations in
the severe PE and healthy subjects at the same gestation period
were compared. The curve for the severe PE group was significantly
lower than that for the healthy pregnancy group.
TTR levels in early and late onset
PE
Among the 43 participants in the severe PE group, 21
individuals were assigned to the early onset group and 22
individuals were assigned to the late onset group. TTR levels were
lower in the early onset patients than in the late onset group
(P<0.001).
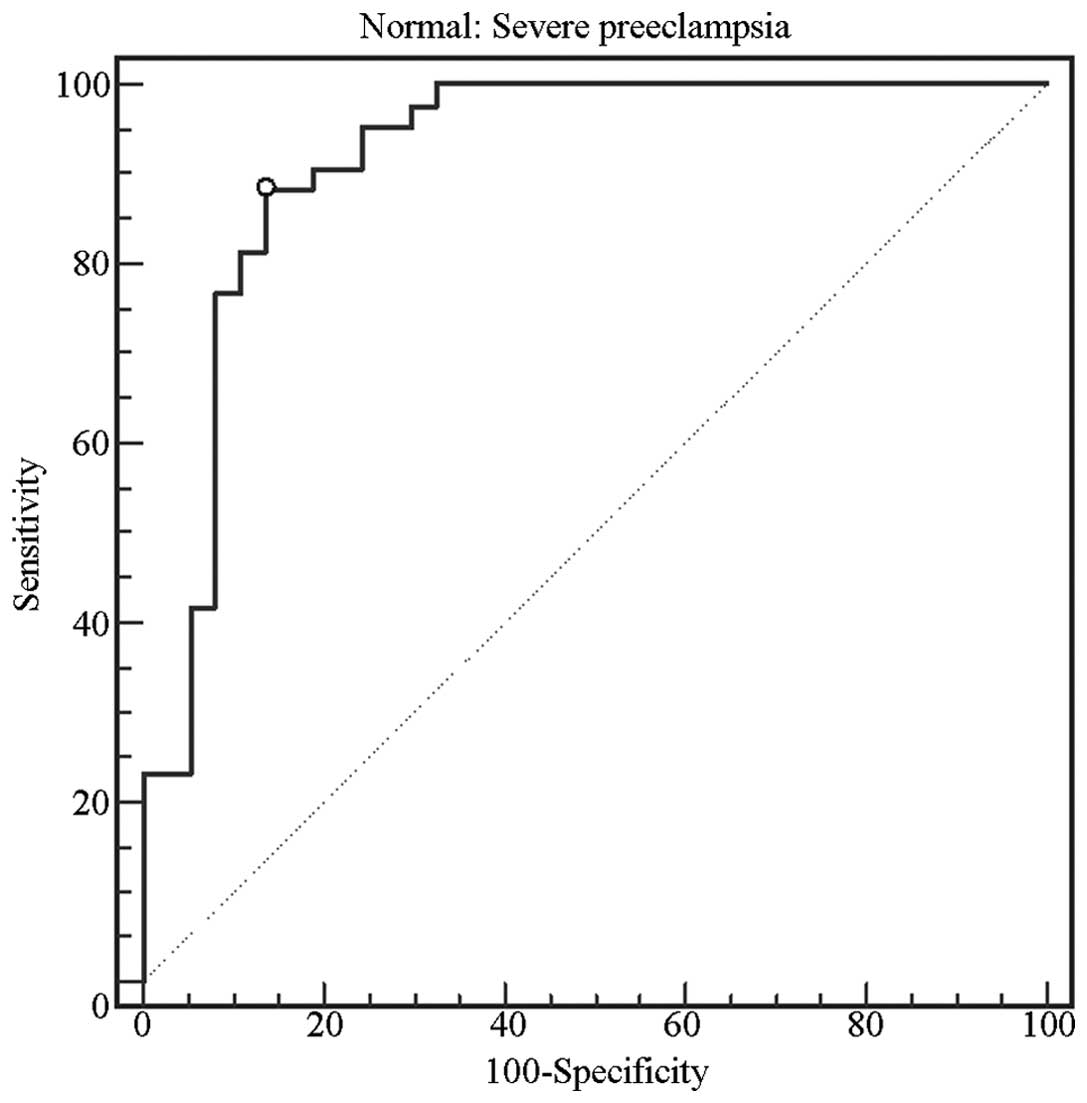
Diagnostic value of TTR for severe
PE
The diagnostic value of TTR in severe PE pregnancies
was examined with ROC curves (Fig.
4). The results indicated that TTR may be a reliable biomarker
for the diagnosis of severe PE, exhibiting sensitivity and
specificity levels of 88.4 and 86.5%, respectively (area under the
curve, 0.917, range, 0.834–0.967), at a diagnostic value of 128.81
mg/l.
Discussion
The identification of novel and effective biomarkers
of PE is critical for early prediction, prognosis, monitoring and
treatment responses. Angiogenic factors, including sflt-1 and PLGF,
have been considered as potential biomarkers of PE. However,
previous studies have presented inconsistencies with regard to the
use of various potential markers to diagnose PE. Therefore, further
studies are required to identify and develop effective and
efficient biomarkers of PE (1,15).
ELISA analysis results of healthy pregnant subjects
identified that TTR concentrations rapidly increased in the third
month of pregnancy (9–12 weeks). TTR levels were higher prior to 20
weeks of gestation. Administration of maternal thyroid hormone to
facilitate fetal development contributes to the rapid increase in
TTR levels in the early stages of gestation (10,11,16).
A previous study identified that fetuses are unable to synthesize
TTR prior to 16 weeks of gestation. In addition, Fig. 3 shows that the highest TTR levels
were observed during the third month (9–12 weeks). Lower TTR levels
were observed following the fifth month (17–20 weeks) of gestation.
Therefore, this result is consistent with results of a previous
study, which indicated that maternal TTR proteins may be important
in transporting thyroid hormone to the fetus and may be required
for fetal development (17). Lower
cord serum levels of T4 in preterm infants have been
shown to correlate with gestation week and weight. In contrast to
term infants, preterm infants frequently experience a decrease in
serum T4 levels, which may account for the increased
rate of morbidity and mortality (18). These results indicate that the
thyroid gland of the fetus does not provide adequate quantities of
T4 prior to full term delivery and the maternal thyroid
hormone compensates for such insufficient T4 levels
during fetal development. Maternal TTR, as a transporter of thyroid
hormone, functions in transporting T4 to the fetus
(11). Although fetuses may
produce TTR proteins, maternal TTR protein may be important in
fetal development.
The present study revealed that TTR levels were
significantly decreased in the sera of females with severe PE. This
result was consistent with that of a previous proteomic analysis,
in which decreased levels of TTR were observed in early onset cases
of severe PE (19). The median
maternal TTR levels were significantly lower in the severe PE group
than in the control group. In addition, the curve for the severe PE
group was markedly lower compared with the control group in the
equivalent gestation month, indicating that the changes in TTR
levels during severe PE may correlate with disease development. ROC
curves were used to evaluate the reliability of TTR as a diagnostic
tool for severe PE (Fig. 4). TTR
levels were capable of discriminating between severe PE and healthy
females (sensitivity and specificity of 88.4 and 86.5%,
respectively) at a cutoff value of 128.81 mg/l.
TTR concentrations in early and late onset cases of
severe PE were compared. Diagnosis at earlier gestational stages
has been reported to indicate a higher risk of maternal and fetal
mortality (20,21). In the present study, TTR levels
were markedly decreased in early onset severe PE cases, indicating
that the changes in TTR levels may correlate with the severity of
PE. Furthermore, these changes may be used to monitor severe
complications.
In the present study, two hypotheses were presented
to explain the changes in TTR levels during severe PE. Initially,
it was hypothesized that the decreased TTR levels may contribute to
the pathology of PE. Maternal vascular dysfunction, that induces
multi-organ disorders, was considered to be the basic pathological
manifestation of PE (3,4,22).
The TTR tetramer dissociates to produce a non-native TTR monomer
with low conformational stability, thereby forming TTR amyloids,
which bind to the vascular wall and lead to changes in membrane
fluidity (23–25). Therefore, TTR may damage the
maternal vascular system via amyloid deposition and this condition
may be attributed to the decreased TTR levels during severe PE. TTR
amyloid fibrils may be selectively deposited in the maternal
vascular system, resulting in organ ischemia of the placenta,
liver, kidney and brain, as well as other clinical manifestations
(26–29). Therefore, TTR concentrations may
change prior to the onset of PE and may be used as a potential
biomarker to predict and monitor PE. The second hypothesis
suggested that the changes in the TTR levels of the severe PE group
may have resulted from reduced production of the TTR protein.
Previous studies have identified that females with PE exhibit a
greater risk of subclinical hypothyroidism during pregnancy, which
is attributed to vascular endothelial growth factor inhibitors that
damage the endothelium of thyroid capillaries; subclinical
hypothyroidism is also involved in reducing the production of
thyroid hormone (30). Decreased
TTR expression may also be responsible for vascular injury of the
placenta. TTR secreted from the placenta is involved in the
transport of the maternal thyroid hormone into the fetal
circulation via the TTR-T4 complex, which is important
in fetal development (10,11,18).
As the predominant pathological mechanism of changes in PE,
placenta necrosis may result in decreased TTR secretion and lead to
disorders during fetal development under severe PE conditions. If
the 2nd hypothesis is correct, the decreased level of TTR should be
a result of PE pathological variations and may be it is not lower
than normal before PE is diagnosed and may not be able to be a
predictor of PE. However, hormonal secretion by the placenta may be
impaired by continued placenta necrosis, resulting in a further
decrease in TTR expression in maternal serum. Therefore, lower
maternal TTR levels may indicate that PE has worsened.
In conclusion, the present study has revealed that
TTR levels are significantly decreased in severe PE and may be
associated with the various changes observed during PE. Therefore,
TTR may be used as a candidate biomarker of PE. However, further
studies are required to confirm whether TTR functions in the
pathology of PE and whether TTR levels change during mild PE. Thus,
changes in TTR concentrations prior to the onset of PE require
further investigation.
Acknowledgements
The authors thank Professor Shengdian Wang for his
technical assistance. The study was supported by grants from the
Sino-US Cooperation Funds (no. 2007DFA31080) and NIDCR/NIH (no. U19
DE018385).
References
|
1
|
Grill S, Rusterholz C, Zanetti-Dällenbach
R, et al: Potential markers of preeclampsia - a review. Reprod Biol
Endocrinol. 7:702009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sibai B, Dekker G and Kupferminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dekker GA and Sibai BM: Etiology and
pathogenesis of preeclampsia: current concepts. Am J Obstet
Gynecol. 179:1359–1375. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hacker NF, Gambone JC and Hobel CJ: Hacker
and Moore’s Essentials of Obstetrics and Gynecology. 5th edition.
Elsevier and Saunders; Philadelphia, PA, USA: 2010
|
|
5
|
Chaiworapongsa T, Romero R, Espinoza J, et
al: Evidence supporting a role for blockade of the vascular
endothelial growth factor system in the pathophysiology of
preeclampsia. Young Investigator Award. Am J Obstet Gynecol.
190:1541–1550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maynard SE, Min JY, Merchan J, et al:
Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may
contribute to endothelial dysfunction, hypertension, and
proteinuria in preeclampsia. J Clin Invest. 111:649–658. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Zhang N, Yu H, Chen Y, Liang Y,
Deng H and Zhang Z: Proteomic analysis of human serum for Finding
pathogenic factors and potential biomarkers in preeclampsia.
Placenta. 32:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fleming CE, Nunes AF and Sousa MM:
Transthyretin: more than meets the eye. Prog Neurobiol. 89:266–276.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su PH, Wang SL, Chen JY, Hu JM, Chang HP
and Chen SJ: Transthyretin levels are not related to Apgar score in
low birth weight and very low birth weight infants. Early Hum Dev.
84:533–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McKinnon B, Li H, Richard K and Mortimer
R: Synthesis of thyroid hormone binding proteins transthyretin and
albumin by human trophoblast. J Clin Endocrinol Metab.
90:6714–6720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Landers KA, McKinnon BD, Li H, Subramaniam
VN, Mortimer RH and Richard K: Carrier-mediated thyroid hormone
transport into placenta by placental transthyretin. J Clin
Endocrinol Metab. 94:2610–2616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wiseman RL, Powers ET and Kelly JW:
Partitioning conformational intermediates between competing
refolding and aggregation pathways: insights into transthyretin
amyloid disease. Biochemistry. 44:16612–16623. 2005. View Article : Google Scholar
|
|
13
|
Saraiva MJ: Transthyretin mutations in
hyperthyroxinemia and amyloid diseases. Hum Mutat. 17:493–503.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv S, Gao J, Zhu F, et al: Transthyretin,
identified by proteomics, is overabundant in pancreatic juice from
pancreatic carcinoma and originates from pancreatic islets. Diagn
Cytopathol. 39:875–881. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conde-Agudelo A, Villar J and Lindheimer
M: World Health Organization systematic review of screening tests
for preeclampsia. Obstet Gynecol. 104:1367–1391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahmed OM, El-Gareib AW, El-Bakry AM, Abd
El-Tawab SM and Ahmed RG: Thyroid hormones states and brain
development interactions. Int J Dev Neurosci. 26:147–209. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haddow JE, Palomaki GE, Allan WC, et al:
Maternal thyroid deficiency during pregnancy and subsequent
neuropsychological development of the child. N Engl J Med.
341:549–555. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
LaFranchi S: Thyroid function in the
preterm infant. Thyroid. 9:71–78. 1999. View Article : Google Scholar
|
|
19
|
Pecks U, Seidenspinner F, Röwer C, Reimer
T, Rath W and Glocker MO: Multifactorial analysis of affinity-mass
spectrometry data from serum protein samples: a strategy to
distinguish patients with preeclampsia from matching control
individuals. J Am Soc Mass Spectrom. 21:1699–1711. 2010. View Article : Google Scholar
|
|
20
|
Espinoza J, Romero R, Nien JK, et al:
Identification of patients at risk for early onset and/or severe
preeclampsia with the use of uterine artery Doppler velocimetry and
placental growth factor. Am J Obstet Gynecol.
196:3262007.PubMed/NCBI
|
|
21
|
Llurba E, Carreras E, Gratacós E, et al:
Maternal history and uterine artery Doppler in the assessment of
risk for development of early- and late-onset preeclampsia and
intrauterine growth restriction. Obstet Gynecol Int.
2009:2756132009.PubMed/NCBI
|
|
22
|
Than NG, Romero R, Hillermann R, Cozzi V,
Nie G and Huppertz B: Prediction of preeclampsia - a workshop
report. Placenta. 29(Suppl A): S83–S85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reixach N, Deechongkit S, Jiang X, Kelly
JW and Buxbaum JN: Tissue damage in the amyloidoses: Transthyretin
monomers and nonnative oligomers are the major cytotoxic species in
tissue culture. Proc Natl Acad Sci USA. 101:2817–2822. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schweigert FJ, Wirth K and Raila J:
Characterization of the microheterogeneity of transthyretin in
plasma and urine using SELDI-TOF-MS immunoassay. Proteome Sci.
2:52004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hughes SE and McKenna WJ: New insights
into the pathology of inherited cardiomyopathy. Heart. 91:257–264.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Connors LH, Prokaeva T, Lim A, et al:
Cardiac amyloidosis in African Americans: comparison of clinical
and laboratory features of transthyretin V122I amyloidosis and
immunoglobulin light chain amyloidosis. Am Heart J. 158:607–614.
2009. View Article : Google Scholar
|
|
27
|
Lavatelli F, Perlman DH, Spencer B, et al:
Amyloidogenic and associated proteins in systemic amyloidosis
proteome of adipose tissue. Mol Cell Proteomics. 7:1570–1583. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou X, Richardson SJ, Aguilar MI and Small
DH: Binding of amyloidogenic transthyretin to the plasma membrane
alters membrane fluidity and induces neurotoxicity. Biochemistry.
44:11618–11627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y and Zhang Z: Does transthyretin
function as one of contributors for preeclampsia? Med Hypotheses.
76:8–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levine RJ, Vatten LJ, Horowitz GL, et al:
Pre-eclampsia, soluble fms-like tyrosine kinase 1, and the risk of
reduced thyroid function: nested case-control and population based
study. BMJ. 339:b43362009. View Article : Google Scholar : PubMed/NCBI
|