Introduction
Angioplasty is an effective method to
arteriosclerosis. Restenosis (RS) is a common adverse event of
angioplasty, which is a complicated formation. In the present
study, the preventive effects of basic fibroblast growth factor
(bFGF) on RS were observed. In addition, the effects of bFGF
administration on endothelium-dependent and -independent
vasorelaxation were analyzed during angiopoiesis.
Materials and methods
Animals
A total of 40 male experimental dogs (weight, 12.5
kg) were fed a specific diet each morning (cholesterol, 5 g,
Sigma-Aldrich, St. Louis, MO, USA; fat, 15 g, Sigma-Aldrich; egg
yolk, 50 g; common mixed feed, 500 g). After 3 weeks, the animals
were administered 0.5 mg/kg chloral hydrate (Medical Isotopes,
Inc., Pelham, NH, USA) diluted in 10% alcohol (Anyang General
Chemical Co., Ltd., Anyang, China) via an intravenous drip.
Following endotracheal intubation, a breathing apparatus was
connected to perform ventilation. Animals were anesthetized with
ketamine hydrochloride (25 μg/kg−1/min, Betaphase
Chemicalz, New York, NY, USA) and flaccidity was sustained with
suxamethonium chloride (25 μg/kg−1/min, Chemical Point
UG, Deisenhofen, Germany). Lidocaine (Changzhou Longterm
Biotechnology Co., Ltd., Changzhou, China) was used to lower the
heart rate and avoid arrhythmia. The femoral artery and vein were
dissected, arterial pressure was determined and fluid replacement
was maintained through the femoral vein. Following right iliac
endarterectomy (size, 30 mm; Takumi balloon, Schneider Corporation,
Indianapolis, IN, USA), the animals were fed a hypercholesterolemic
diet for 15 weeks. The study was performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institute of Health. The animal
use protocol was reviewed and approved by the Institutional Animal
Care and Use Committee of the Affiliated Drum Tower Hospital of
Nanjing University Medical School, (Nanjing, China).
Level of atherosclerotic stenosis
In total, 10 dogs were used to examine the level of
atherosclerotic stenosis in the iliac arteries. In order to
maintain the size, the iliac arteries were perfused with 4%
polyoxymethylene (Ticona GmbH, Frankfurt, Germany) diluted in
phosphate-buffered saline (Lorad Chemical Corporation, Petersburg,
VA, USA) for 40 min with a constant pressure of 25.0 kPa through a
duct from the right carotid artery to the abdominal aorta.
Subsequently, the samples were further fixed with 4%
polyoxymethylene, embedded in paraffin and cut into sections. The
slides were stained with hematoxylin and eosin (Dudley Chemical
Corp, Lakewood, NJ, USA). Images were captured to detect the
cross-sectional areas of the iliac arteries on both sides and to
identify atherosclerotic stenosis using a digital image analyzer
(Quantiment-520; Leica, Cambridge, UK).
Balloon dilation
A balloon (size, 30 mm; Takumi) was placed at the
stenosis region via the femoral artery and inflated to 706.6 kPa
for 3 min. This was conducted three times consecutively at 1 min
intervals and then the balloon was removed. Following angioplasty,
10 experimental dogs were randomly selected from 30 dogs. These
dogs were used to analyze the instant changes of the lumen areas
following angiopoiesis. The remaining 20 dogs were randomly divided
into the control and bFGF groups (n=10 per group). The dogs in the
bFGF group were administered 5 μg recombinant bFGF (Sigma-Aldrich,
St. Louis, MO, USA) dissolved in 1 ml albumin (0.5%, w/v) via an
intravenous drip on days 1, 4, 7, 10 and 14 following angioplasty.
The experimental dogs in the control group were treated with 1 ml
albumin (0.5%, w/v). Dogs in the two groups were sacrificed 42 days
after angioplasty to analyze the vascular reactivity and histology
in vitro.
Histology
A total of 10 dogs were divided into two groups
(n=5) and the samples were harvested, fixed, stained and detected
as aforementioned.
In vitro vascular reactivity
Six weeks following angioplasty, iliac arteries on
both sides (angiopoietic and non-angiopoietic) were harvested and
divided into groups (n=5 in each group). The arteries were cut into
vascular circles, 10-mm in length. The vascular circles were dipped
in 40 ml K-H solution (pH 7.4; Sigma-Aldrich) and incubated in
conditions of 95% O2 and 5% CO2 at 37°C.
Vascular circles were connected with the pressure translator, which
was connected to a physiological recorder (VM-180G,
Optical-Electric Co., Hyogo, Japan). Prior to administration, the
vascular circles were stabilized for 60 min at an optimal resting
tension of 4 kPa. All the arterial circles were pretreated with
phenylephrine (Phe; 1×10−9–3×10−5 mol/l;
Pechiney, Stamford, CT, USA), which resulted in stenosis. Following
stabilization of the Phe-induced contractile response, all arterial
circles were treated with endothelium-dependent and -independent
vasodilator. With the administration of acetylcholine (ACh;
1×10−8–3×10−5 mol/l; Angene International
Limited, Hong Kong, China), the arterial circles were rinsed and
stabilized at resting tension when the maximal relaxation occurred.
The smooth muscle relaxant, sodium nitroprusside (SNP;
1×10−9–3×10−5 mol/l; Angene International
Limited), was added to the prestenotic arterial circles treated
with Phe (3×10−5 mol/l).
Statistical analysis
Results are expressed as the mean ± SD and data were
analyzed using the t-test. P<0.05 was considered to indicate a
statistically significant difference. ACh- and SNP-induced
relaxation was calculated as a percentage of the contraction to
Phe. Emax was the maximal vascular reactivity and
EC50 was 50% of the maximal reaction concentration.
Results
Iliac artery area
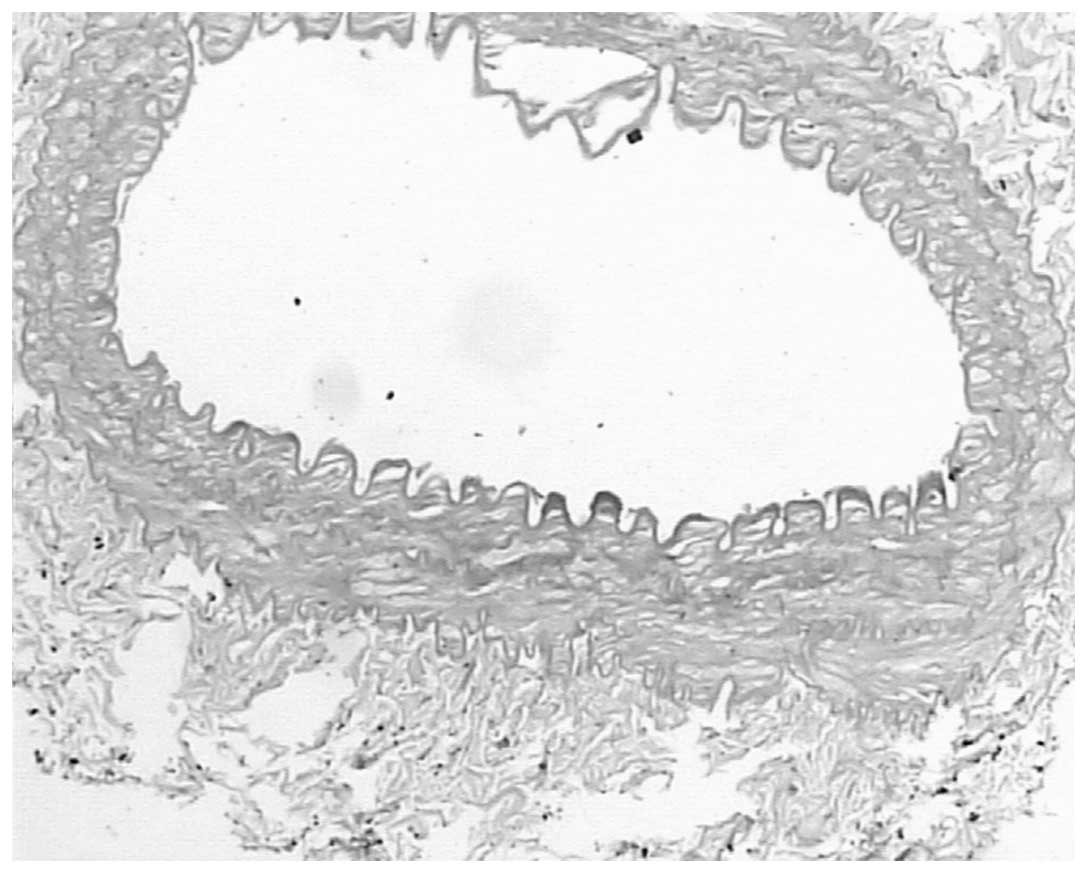
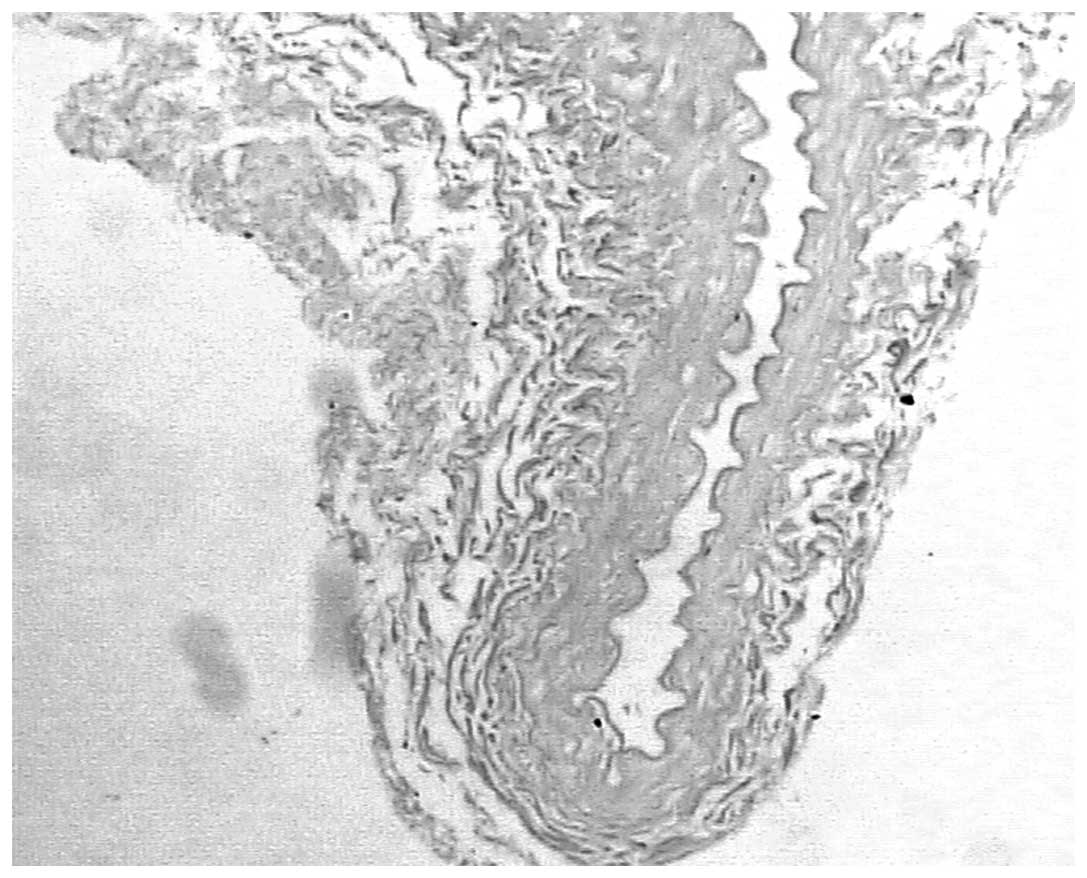
Following angioplasty, cross-sectional areas of the
intimas following endarterectomy on the right side were 60.5%
smaller compared with the intimas that had not undergone
endarterectomy (0.89±0.79 vs. 2.88±0.89 mm2; P<0.05).
Instant cross-sectional areas on the right side after angioplasty
had an average area of 2.10±0.34 mm2, which was
significantly greater compared with the area before angioplasty
(0.89±0.79 mm2; P<0.05). Changes in cross-sectional
areas of the right iliac arteries 6 weeks after angioplasty are
indicated in Table I and Figs. 1 and 2.
 | Table IChanges in the cross-sectional areas
of the right iliac arteries 6 weeks after angioplasty in the two
groups (mm2). |
Table I
Changes in the cross-sectional areas
of the right iliac arteries 6 weeks after angioplasty in the two
groups (mm2).
| Group | Angiopoietic
side | Non-angiopoietic
side |
|---|
| bFGF | 2.01±0.78a | 2.89±0.78b |
| Control | 1.0±0.11 | 2.68±0.88 |
In vitro vascular reactivity
The contractile response resulting from Phe
administration exhibited no significant differences between the
angiopoietic and non-angiopoietic sides of the iliac arterial
circles in the control and bFGF groups. In addition, there was no
significant difference in the endothelium-independent SNP-induced
vasorelaxation response of the non-angiopoietic iliac arterial
circles between the control and bFGF groups (Emax,
93±5.5 vs. 91±4.5%; P>0.05; EC50, 8.9±4.3 vs.
8.8±4.1×10−7 mol/l; P>0.05). Additionally, there was
no significant difference in the endothelium-dependent Ach-induced
vasorelaxation (Emax, 53±7.5 vs. 55±6.7%; P>0.05;
EC50, 7.8±2.1×10−7 mol/l vs.
7.5±2.1×10−7 mol/l; P>0.05). Six weeks after
angioplasty in the right iliac arteries, the maximal
endothelium-dependent Ach-induced relaxation of the bFGF-treated
animals (Emax, 43±8.5%) was significantly greater
compared with the control group (Emax, 11±9%;
P<0.05). Furthermore, Emax and EC50 values
of the right side after endarterectomy recovered to the level of
the opposite side without endarterectomy in the bFGF group
(Emax, 50±8 vs. 45±7%; P>0.05; EC50,
8.1±2.0 vs. 7.8±2.1×10−7 mol/l; P>0.05). Following
angioplasty, the maximal endothelium-independent SNP-induced
relaxation response of the angiopoietic sides was markedly weaker
compared with the non-angiopoietic sides of the iliac arterial
circles in the bFGF group (90±2 vs. 93±5.5%; P<0.05) and the
control group (60±2 vs. 91±4.5%; P<0.05). However, the
Emax of the bFGF group was significantly greater
compared with the control group (P<0.05).
Discussion
Following angioplasty, vascular injury, which is
analogous to generalized wound healing, may be differentiated into
three implicated vascular injury repair stages (1). Firstly, early thrombus formation
where the elastic components of the vessel wall are dragged and
torn during angioplasty, resulting in intimal and deep dissection
extending into the medial and adventitial layers. Dissection planes
with endothelial stripping induce exposure of components under the
intima, platelet adhesion, thrombus formation, acute inflammatory
response and the infiltration of neutrophils, monocytes,
macrophages and T lymphocytes (2–4).
Secondly, there is the cell granulation stage where relevant cells
migrate into the vascular injury region. Cells staining for HHF-35
are present, filling in the original dissection planes and in
certain arteries, areas of organized thrombus with interdigitated
smooth muscle cells (SMCs) are observed (5,6).
Collagenous and elastic fibers are few and foam cells are generally
observed (2). Following activation
with growth factors, including bFGF and platelet derived growth
factor, SMCs metastasize to the injury region, proliferate and
secrete extracellular matrix (7–9).
Finally, there is the vascular remodeling stage where intimal SMCs
are unable to proliferate further. SMCs and adventitial fibroblasts
secrete extracellular matrix (10,11).
An increased density of intimal-medial fibrosis is observed.
Collagen proteins constitute 50% of components in the vessel wall
and play an important role in vascular RS. In addition, the
glycosaminoglycan, hyaluronan, is also a major component of the
vessel wall (12).
bFGF is a single-stranded polypeptide (16 ku) that
exhibits mitogenic activity and stimulates angiogenesis (13). In the present study, administration
of bFGF increased endothelium-dependent vasorelaxation and also
restored endothelium-independent vasorelaxation, further indicating
that bFGF may be effective in treating experimental vascular
injuries. Whether bFGF results in neointima pachynsis and/or
proliferation of medial smooth muscle depends on the dose of bFGF.
The aforementioned conditions may be observed following
administration of a large dose of bFGF, whereas low dose bFGF is
beneficial to endothelial cells, indicating that bFGF is
double-edged and highly-efficient as a selective standard.
Therefore, low-dose bFGF was applied in this study. Administration
of bFGF not only restored the functions of endothelial cells, but
also improved the sensitivity of vessel SMCs to endothelium-derived
relaxing factor, also known as nitrogen monoxidum (14–17).
In the present study, there was no significant difference in the
sensitivity of angiopoietic iliac arteries to SNP (EC50)
between the bFGF and control groups. bFGF upregulates the
expression of vascular endothelial growth factor and promotes the
function of vasorelaxation to produce a synergistic effect of
hypoxia (18,19). Thus, the mechanisms of bFGF in
improving endothelium-dependent and -independent vasorelaxation
require further study. Six weeks after angioplasty, the
cross-sectional areas of the control group dramatically decreased,
whereas those of the bFGF group showed no change, indicating that
bFGF is critical in preventing the formation of RS following
angiopoiesis. Therefore, it is possible that the mechanisms behind
restoring endothelial cell function and improving vasorelaxation
may be associated with the administration of bFGF (20). In addition, there was no change in
the cross-sectional areas of the intima without denudation,
indicating that normal endothelial cells are essential for
preventing atherosclerotic stenosis.
References
|
1
|
Kibos A, Campeanu A and Tintoiu I:
Pathophysiology of coronary artery in-stent restenosis. Acute Card
Care. 9:111–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zargham R: Preventing restenosis after
angioplasty: a multistage approach. Clin Sci (Lond). 114:257–264.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie C, Guo Y, Zhu T, Zhang J, Ma PX and
Chen YE: Yap1 protein regulates vascular smooth muscle cell
phenotypic switch by interaction with myocardin. J Biol Chem.
287:14598–14605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mulder HJ, Schalij MJ, Kauer B, et al:
Pravastatin and endothelium dependent vasomotion after coronary
angioplasty: the PREFACE trial. Heart. 86:533–539. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pozo M, Izquierdo MC, de Nicolás R, Egido
J, Ortiz A and González-Cabrero J: Gliotoxin inhibits neointimal
hyperplasia after vascular injury in rats. J Vasc Res. 46:278–289.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weakley SM, Wang X, Mu H, et al:
Ginkgolide A-gold nanoparticles inhibit vascular smooth muscle
proliferation and migration in vitro and reduce neointimal
hyperplasia in a mouse model. J Surg Res. 171:31–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Segev A, Aviezer D, Safran M, Gross Z and
Yayon A: Inhibition of vascular smooth muscle cell proliferation by
a novel fibroblast growth factor receptor antagonist. Cardiovasc
Res. 53:232–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Majesky MW, Dong XR, Hoglund V, Daum G and
Mahoney WM Jr: The adventitia: a progenitor cell niche for the
vessel wall. Cells Tissues Organs. 195:73–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song Z, Jin R, Yu S, Nanda A, Granger DN
and Li G: Crucial role of CD40 signaling in vascular wall cells in
neointimal formation and vascular remodeling after vascular
interventions. Arterioscler Thromb Vasc Biol. 32:50–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chadjichristos CE, Matter CM, Roth I, et
al: Reduced connexin43 expression limits neointima formation after
balloon distension injury in hypercholesterolemic mice.
Circulation. 113:2835–2843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Makiyama Y, Toba K, Kato K, et al:
Imatinib mesilate inhibits neointimal hyperplasia via growth
inhibition of vascular smooth muscle cells in a rat model of
balloon injury. Tohoku J Exp Med. 215:299–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sadowitz B, Seymour K, Gahtan V and Maier
KG: The role of hyaluronic acid in atherosclerosis and intimal
hyperplasia. J Surg Res. 173:63–72. 2012. View Article : Google Scholar
|
|
13
|
Backes A, Seay U, Sedding DG, Tillmanns HH
and Braun-Dullaeus RC: Inhibition of matrix deposition: a new
strategy for prevention of restenosis after balloon angioplasty. J
Cardiovasc Pharmacol. 55:213–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooney R, Hynes SO, Sharif F, Howard L and
O’Brien T: Effect of gene delivery of NOS isoforms on intimal
hyperplasia and endothelial regeneration after balloon injury. Gene
Ther. 14:396–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mnjoyan ZH, Doan D, Brandon JL, et al: The
critical role of the intrinsic VSMC proliferation and death
programs in injury-induced neointimal hyperplasia. Am J Physiol
Heart Circ Physiol. 294:H2276–H2284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao EH, Fukuda N, Ueno T, et al: A
pyrrole-imidazole polyamide targeting transforming growth
factor-beta1 inhibits restenosis and preserves endothelialization
in the injured artery. Cardiovasc Res. 81:797–804. 2009. View Article : Google Scholar
|
|
17
|
Indolfi C, Torella D, Coppola C, et al:
Physical training increases eNOS vascular expression and activity
and reduces restenosis after balloon angioplasty or arterial
stenting in rats. Circ Res. 91:1190–1197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malabanan KP, Kanellakis P, Bobik A and
Khachigian LM: Activation transcription factor-4 induced by
fibroblast growth factor-2 regulates vascular endothelial growth
factor-A transcription in vascular smooth muscle cells and mediates
intimal thickening in rat arteries following balloon injury. Circ
Res. 103:378–387. 2008. View Article : Google Scholar
|
|
19
|
Cheng B, Fu X and Sheng Z: Effect of
exogenous basic fibroblast growth factor on proliferation and
migration of endothelial cells of partial thickness scald in rats.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 18:200–204. 2004.(In
Chinese).
|
|
20
|
Chang L, Zhang C, Wu YJ and Zhu RZ:
Effects of recombinant human basic fibroblast growth factor on
restenosis after arterial endothelial injury in rats. Acta
Pharmacol Sin. 22:876–880. 2001.PubMed/NCBI
|