Introduction
Microglia are the cells responsible for innate
immunity in the central nervous system and accumulating evidence
suggests that activated microglia modulate the development and/or
progression of Alzheimer’s disease, Parkinson’s disease and
amyotrophic lateral sclerosis, among other diseases (1–3).
Exposure to lipopolysaccharide (LPS), β-amyloid or interferon
(IFN)-γ activates microglia, inducing the secretion of a variety of
pro-inflammatory mediators and potentially neurotoxic compounds
(4,5). Receptor binding of cytokines
stimulates a variety of intracellular signaling pathways that have
been implicated in neurodegenerative disorders, including
activation of nuclear transcription factor-κB (NF-κB), the
mitogen-activated protein kinase (MAPK) family and protein kinase B
(Akt). These are the most important molecules that control the
synthesis and release of pro-inflammatory substances from activated
microglia (6–8). Epidemiological studies suggest that
inhibition of microglial activation attenuates the severity of
neurotoxicity (9,10). The inhibition of activated
microglia is therefore an important therapeutic target for
neurodegenerative disorders.
Luteolin (3′,4′,5,7-tetrahydroxyflavone), a flavone
that has been identified in celery, green pepper, perilla leaves
and seeds, and at high concentrations in chamomile, exhibits strong
anti-inflammatory, antioxidant and free-radical scavenging
properties. In addition, luteolin has been shown to inhibit the
LPS-induced production of tumor necrosis factor alpha (TNF-α) and
nitric oxide (NO) in an activated macrophage-like cell line
(11). Luteolin also reduces the
production of LPS-induced pro-inflammatory cytokines in intestinal
epithelial cells, mouse bone marrow-derived dendritic cells
(12), rat fibroblasts (13) and human gingival fibroblasts
(14). Furthermore, in a previous
study we observed that luteolin inhibited the secretion of several
pro-inflammatory enzymes and pro-inflammatory cytokines by
activated microglia (15).
However, the mechanism by which luteolin inhibits microglial
inflammation is not completely understood. Much less is known about
the role of luteolin in neuroprotection and regulation of the
underlying signaling pathways.
In the present study, the effects of luteolin on
Toll-like receptor-4 (TLR-4) expression and the NF-κB, MAPK and Akt
signaling pathways were investigated using LPS-stimulated BV2
cells, a murine microglial cell line. In further experiments using
a microglial-neuronal coculture system, the protective effects of
luteolin against microglial-mediated LPS neurotoxicity, and
therefore its potential role in the prevention of neurodegenerative
diseases were investigated.
Materials and methods
Materials
Luteolin (purity > 98%; molecular weight, 286.24;
chemical formula C15H10O6), LPS,
dimethylsulfoxide (DMSO) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma (St. Louis, MO, USA). Antibodies against
NF-κB p65, p38, phosphorylated p38 (p-p38), JNK, phosphorylated JNK
(p-JNK), ERK, phosphorylated ERK (p-ERK), Akt and phosphorylated
Akt (p-Akt) were obtained from Cell Signaling Technology (Beverly,
MA, USA). Antibodies against TLR-4 were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Mouse anti-β-actin antibody
was purchased from Sigma.
Cell culture
BV2 immortalized murine microglia were provided by
the Cell Culture Center of the Chinese Academy of Medical Sciences
(Beijing, China). The human neuroblastoma cell line SH-SY5Y was
donated by Dr Enxiang Tao. The cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin in a
humidified atmosphere of 5% CO2 at 37°C. In all
experiments, the BV2 microglia were pretreated with the indicated
concentrations of luteolin for 1 h prior to the addition of LPS
(1.0 μg/ml) in serum-free DMEM.
Immunofluorescence staining
For immunofluorescence staining, the cells were
fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1%
Triton X-100 for 10 min and blocked with 5% bovine serum albumin
(BSA) for 30 min. The cells were then incubated with primary
antibody to NF-κB p65 (1:100 dilution) overnight at 4°C. After
washing three times with PBS, the cells were incubated with
secondary antibody conjugated to rhodamine for 1 h (Cell Signaling
Technology). The nuclei were stained with Hoechst 33258.
Fluorescent images were captured using a laser scanning confocal
microscope (LSM 510 META; Carl Zeiss, Stuttgart, Germany).
Total RNA isolation and quantitative PCR
(qPCR) analysis
Total RNA was isolated with TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Total RNA (1.0 μg) was reverse
transcribed using M-MLV reverse transcriptase (Promega, Madison,
WI, USA) to produce cDNA. The primers used for qPCR were as
follows: TLR-4, 5′-GCT TTC ACC TCT GCC TTC AC-3′ and 5′-CCA
ACG GCT CTG AAT AAA GTG-3′; and GAPDH, 5′-TCA CCA CCA TGG
AGA AGG C-3′ and 5′-GCT AAG CAG TTG GTG GTG CA-3′. The following
qPCR conditions were used: 40 cycles of denaturation at 94°C for 20
sec, annealing at 62°C for 30 sec and extension at 72°C for 30 sec.
SYBR Green qPCR Master mix 2 (Takara Bio, Inc., Shiga, Japan) was
used in all samples and the reactions were carried out in a 20-μl
reaction volume using a LightCycler LC480 qPCR instrument (Roche
Diagnostics, Basel, Switzerland). The mRNA expression levels of
target genes relative to glyceraldehyde-3-phosphate dehydrogenase
(GAPDH, a housekeeping gene used as an endogenous control)
were calculated according to the standard curves.
Western blot analysis
BV2 microglia were harvested and lysed in RIPA
buffer [1 mM ethylenediaminetetraacetic acid (EDTA), 150 mM NaCl,
1% igepal (CA-630), 0.1% sodium dodecyl sulfate (SDS), 0.5 % sodium
deoxycholate and 50 mM Tris HCl; pH 8.0 (Sigma)]. Equal amounts of
protein were separated by 8–12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE), transferred to
polyvinylidene fluoride (PVDF) membranes, blocked with 5% nonfat
milk for 2 h and incubated with primary antibodies at 4°C
overnight. Following incubation with appropriate secondary
antibodies conjugated to horseradish peroxidase (goat anti-rabbit
secondary antibody was obtained from Cell Signaling Technology),
immunoblots were exposed on film using electrochemiluminescence
(ECL) western detection reagent (Amersham Pharmacia Biotech,
Amersham, UK). The bands were quantified by the optical density
ratio using β-actin as a control.
Cytotoxicity assay in a coculture of
microglia and neurons
SH-SY5Y cells were grown in the bottom of wells, BV2
cells were then grown in culture inserts (pore size 0.4 μm;
Corning, New York, NY, USA) and 1.0 μg/ml LPS was added to the
culture insert. In this coculture system, the microglia were able
to communicate with the neurons through a semipermeable membrane,
which avoids direct contact between the two cellular systems
(16). After coculture for 24 h,
the SH-SY5Y cells were incubated with MTT solution (0.5 mg/ml in
PBS) for 4 h at 37°C. The culture supernatants were then removed,
the resulting formazan crystals were dissolved in DMSO and the
absorbance was read at 570 nm with a microplate reader (ReTiSoft
Inc., Mannedorf, Switzerland). Cell survival was expressed as the
ratio of absorbance (percentage survival) compared with a DMSO
control.
Detection of apoptosis in a coculture of
microglia and neurons
In the coculture system described above, apoptotic
SH-SY5Y cells were detected by the terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay.
Following each treatment, the TUNEL assay was performed according
to manufacturer’s instructions (Roche Diagnostics Corporation,
Indianapolis, IN, USA) and all nuclei were counterstained with 5
mg/ml Hoechst 33342 for 10 min at 37°C. The labeled SH-SY5Y cells
were examined with a laser scanning confocal microscope. SH-SY5Y
cells were considered to be apoptotic when their nuclei were
costained with Hoechst 33342 and TUNEL. The number of apoptotic
cells was counted among 100 randomly chosen neurons observed on
several optic fields.
Statistical analysis
Quantitative data are presented as the mean ±
standard error of the mean (SEM) of at least three independent
experiments. Comparisons between two groups were analyzed using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant result.
Results
Luteolin suppresses TLR-4 expression in
LPS-stimulated BV2 microglia
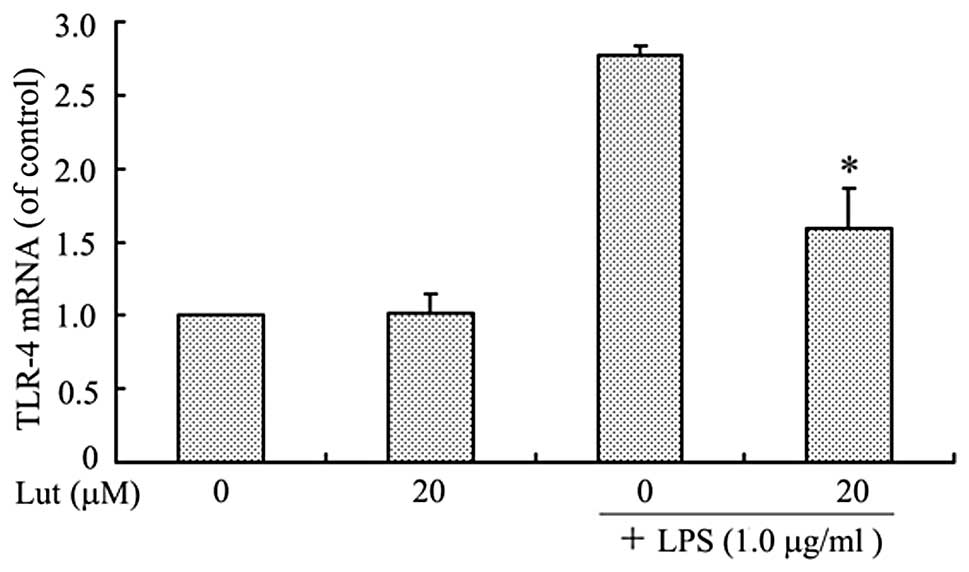
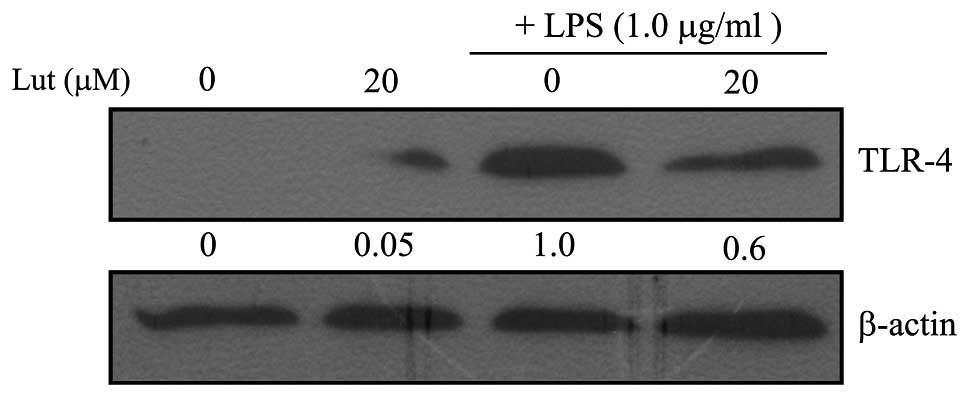
To examine the effect of luteolin on TLR-4
expression, the levels of TLR-4 mRNA and protein in LPS-stimulated
BV2 microglia were measured. The BV2 microglia were pretreated with
luteolin for 1 h and then stimulated with LPS for 1 h prior to qPCR
or for 24 h prior to western blotting. As shown in Figs. 1 and 2, TLR-4 mRNA and protein levels increased
in LPS-stimulated BV2 microglia, but both were markedly suppressed
by treatment with luteolin. This result indicates that luteolin
suppressed TLR-4 expression and therefore may lead to inhibition of
activation of the NF-κB, MAPK and Akt pathways.
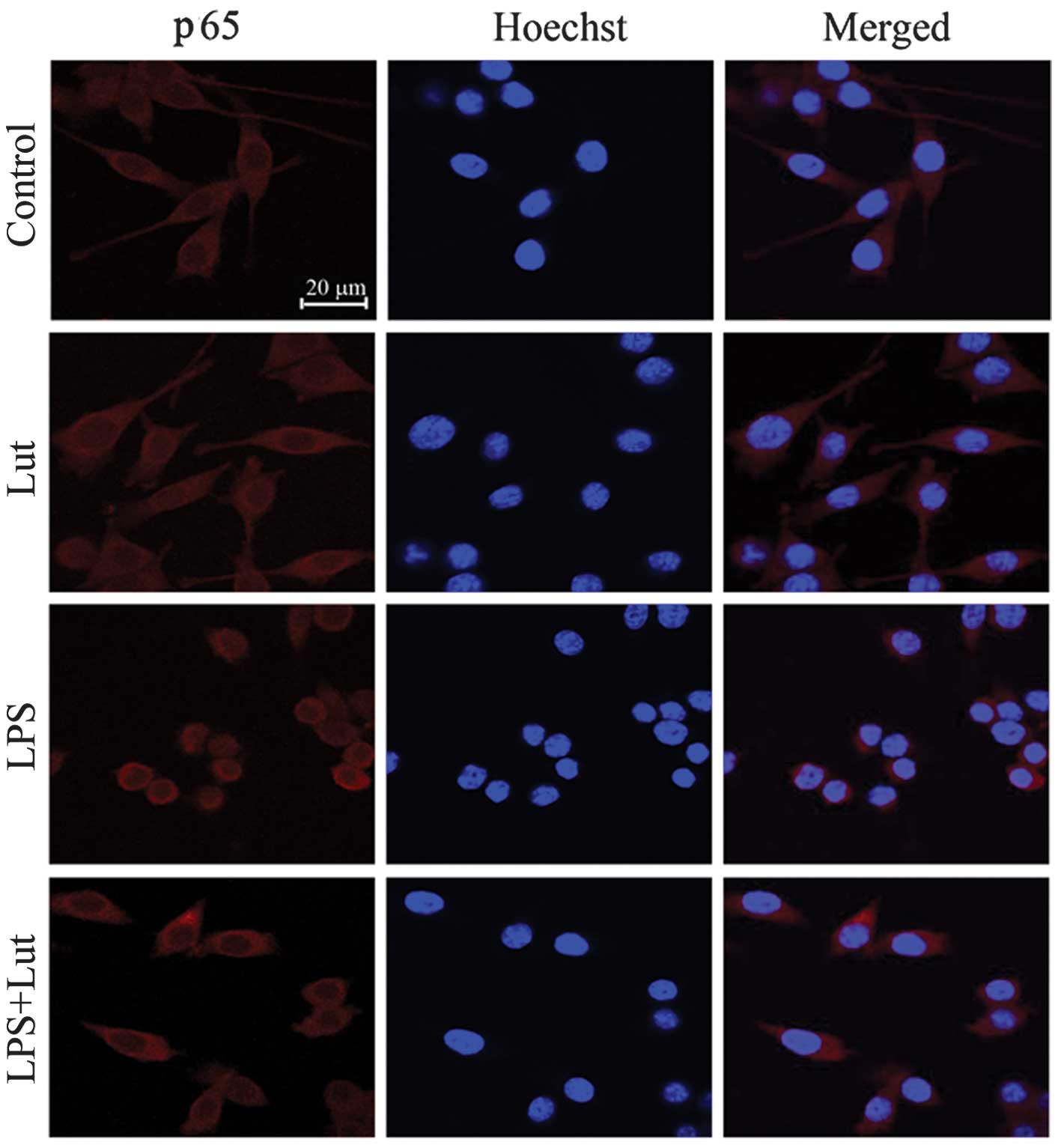
Effects of luteolin on the NF-κB
signaling pathway
Activation of NF-κB leads to its translocation to
the nucleus where it mediates the transcriptional regulation of
pro-inflammatory genes. The activation and nuclear translation of
NF-κB is a key step in LPS-stimulated microglial activation. The
regulation of NF-κB by luteolin was investigated using
immunofluorescence staining. As shown in Fig. 3, the NF-κB p65 subunit was
primarily retained in the cytoplasm in unstimulated cells; however,
following stimulation with LPS, cytoplasmic NF-κB p65 levels were
reduced, with a corresponding increase in nuclear NF-κB p65.
Treatment with 20 μM luteolin significantly blocked the activation
of NF-κB p65 nuclear translocation in LPS-stimulated BV-2 cells.
This result suggests that luteolin suppresses pro-inflammatory
enzymes and pro-inflammatory cytokines by inhibiting NF-κB
activation.
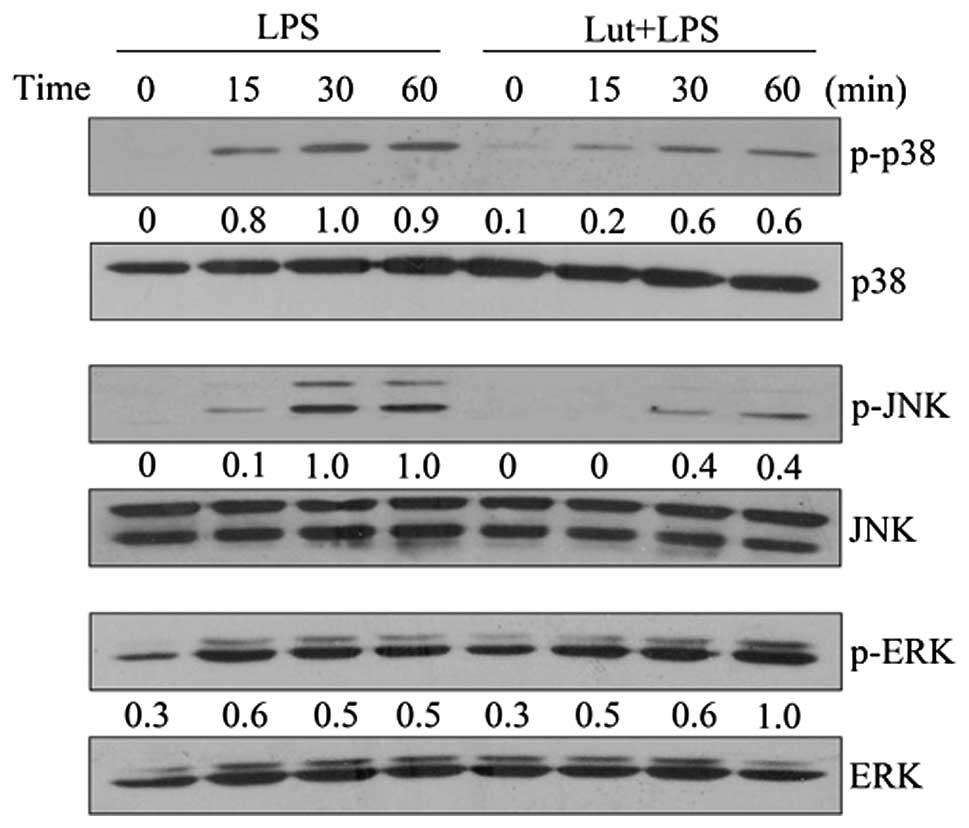
Effects of luteolin on the MAPK signaling
pathway
To investigate whether the inhibition of NF-κB
activation by luteolin is mediated via the MAPK pathway, the
phosphorylation of three MAPK molecules, p38 MAPK, JNK and ERK1/2,
was examined in LPS-stimulated BV-2 cells. As shown in Fig. 4, LPS rapidly activated MAPKs within
15 min of LPS stimulation, while luteolin at 20 μM markedly
inhibited the LPS-induced phosphorylation of p38 and JNK, but had
no effect on ERK phosphorylation. The levels of non-phosphorylated
p38, JNK and ERK were unaffected by LPS or luteolin treatment.
Effects of luteolin on the Akt signaling
pathway
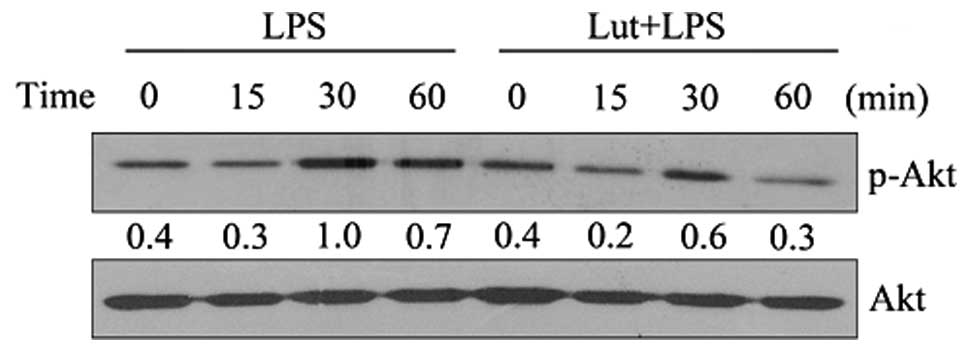
The effect of luteolin on Akt was then examined. As
shown in Fig. 5, luteolin
significantly inhibited the LPS-induced activation of Akt. The
results suggest that inhibition of Akt by luteolin may contribute
to the suppression of LPS-induced NF-κB activation and the
expression of inflammatory mediators in BV2 cells.
Luteolin decreases microglial-induced
SH-SY5Y cell death in a coculture system
In order to investigate whether luteolin protects
against the neuronal death induced by microglial activation, a
coculture system with SH-SY5Y neuronal cells and BV2 microglia was
used. LPS at concentrations of 0.1, 1.0 or 10.0 μg/ml were not
observed to induce cell death in the SH-SY5Y cells (data not
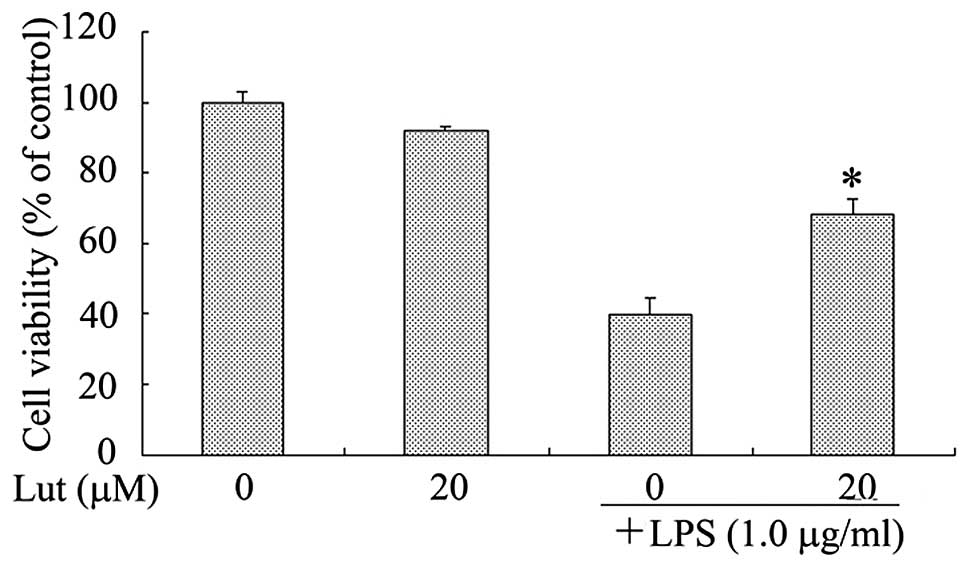
shown). Next, the SH-SY5Y cell viability following coculture with
LPS-activated BV2 microglia was examined using the MTT assay. As
shown in Fig. 6, SH-SY5Y cells in
control inserts in the absence of LPS-stimulated BV2 microglia did
not undergo cell death. By contrast, LPS treatment alone led to a
high level of SH-SY5Y cell death in the coculture, suggesting that
the LPS-activated microglia secreted pro-inflammatory cytokines
that were able to migrate through the insert, inducing the death of
the neuronal cells. Treatment with luteolin markedly reduced the
death of the SH-SY5Y cells; cell viability was increased by ~28.6%
when the LPS-stimulated BV2 microglia were pretreated with luteolin
(Fig. 6).
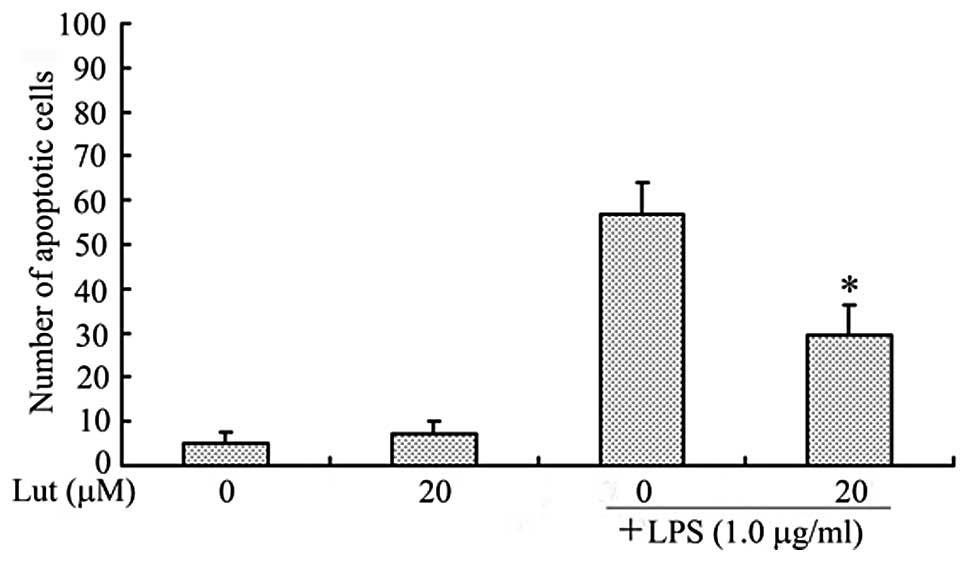
Apoptosis was determined by the TUNEL
assay
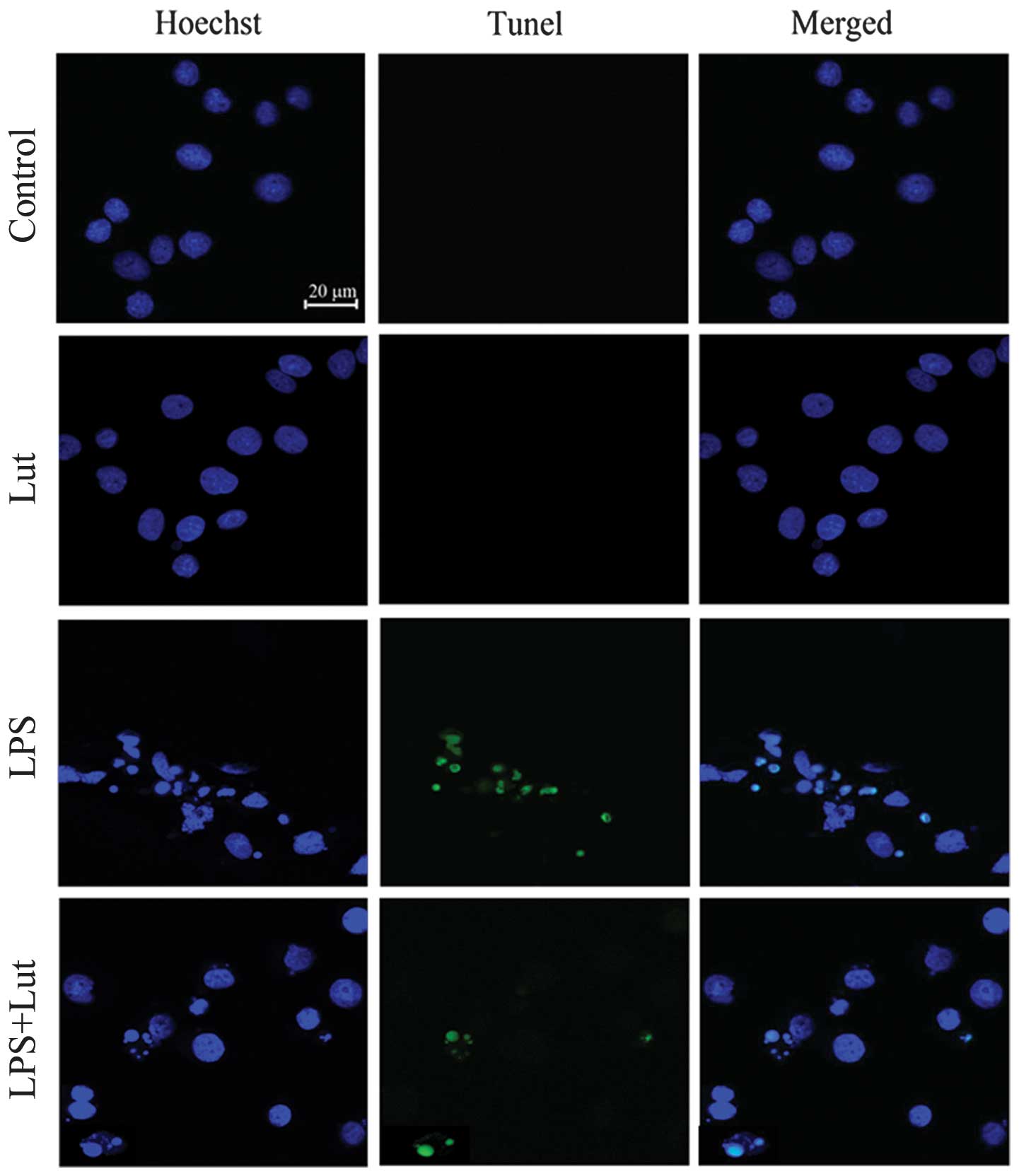
As shown in Fig. 7,
SH-SY5Y nuclei were stained with Hoechst 33342 (blue) and apoptotic
neurons were stained using the TUNEL technique (green). Coculture
with BV2 microglia exposed to LPS alone resulted in a significant
increase in the number of apoptotic SH-SY5Y cells compared with the
number of untreated cells. The administration of luteolin reduced
the number of apoptotic SH-SY5Y cells (Fig. 8). These results clearly demonstrate
that luteolin protected neurons from microglial-mediated LPS
neurotoxicity, supporting its potential role in the prevention of
neurodegenerative diseases.
Discussion
The flavonoid luteolin has been shown to inhibit
LPS-induced IL-6 production in the brain by inhibiting the JNK
signaling pathway and the activation of AP-1 in microglia (17). Luteolin also suppressed microglial
TNF-α and IL-6 production stimulated by IFN-γ in the presence of
CD40 ligation, and markedly inhibited the IFN-γ-induced
phosphorylation of STAT1 (18).
These data suggest that this flavonoid is a potent modulator of
microglial activation and affects several signaling pathways,
leading to a unique phenotype with anti-inflammatory,
anti-oxidative and neuroprotective characteristics (19). Previous findings suggest dietary
luteolin enhances spatial working memory by mitigating
microglial-associated inflammation in the hippocampus (20). Our previous observations confirm
the inhibitory effects of luteolin on pro-inflammatory cytokine
expression in microglia (15).
However, the mechanism by which luteolin mediates these
anti-inflammatory effects on microglia is not completely understood
and few studies have explored the impact of luteolin on
neuroprotection.
In vitro, microglia may be activated
experimentally with the bacterial cell wall component LPS.
Internalization of TLR-4, rendering microglial cells less sensitive
to activation by LPS, plays a role in inducing a reduction in TNF-α
production by BV-2 microglial cells (21). TLR-4 is a member of the TLR family
of pattern recognition receptors that generate innate immune
responses to pathogens by activating a cascade of pro-inflammatory
events (22). Therefore,
treatments that attenuate TLR-4-associated inflammatory cascades
may prove beneficial in ameliorating microglial activation and
preventing neurodegenerative processes. The results of the present
study indicate that luteolin pretreatment inhibited the
upregulation of TLR-4 expression induced by LPS at the
transcriptional and translational levels. It was therefore
hypothesized that the underlying molecular mechanisms involved
include interference with the LPS-triggered increase in TLR-4
expression. The results of the present study indicate that luteolin
may inhibit NF-κB, p38, JNK, MAPK and Akt activation through the
suppression of TLR-4 expression.
Activation of NF-κB leads to its translocation to
the nucleus where it mediates the transcriptional regulation of
pro-inflammatory genes (23). The
activation and nuclear translation of NF-κB is a key step in
LPS-stimulated microglial activation (24). In the current study, treatment with
20 μM luteolin significantly blocked NF-κB p65 nuclear
translocation in LPS-stimulated BV-2 cells. In our previous study,
a luciferase reporter assay was performed to investigate the
possibility that luteolin inhibits NF-κB transcriptional activity,
and the possibility that luteolin blocks the phosphorylation and
subsequent degradation of IκB in LPS-induced BV2 cells was
investigated; it was observed that luteolin causes a marked
inhibition of NF-κB p65 nuclear translocation (15). These results, also confirmed by the
current study, suggest that luteolin suppresses pro-inflammatory
enzymes and pro-inflammatory cytokines through the inhibition of
NF-κB activation.
There is evidence that MAPKs play a key role in the
regulation of the synthesis and release of pro-inflammatory
substances by activated microglia (25). LPS is known to activate various
MAPKs, including p38, JNK and ERK. The MAPKs tested in this study
(p38, JNK and ERK1/2) were activated in glia and neurons following
LPS treatment, suggesting their involvement in glial activation and
the neuronal response to diffusible, glia-derived neurotoxic
molecules (26). To investigate
whether the inhibition of NF-κB activation by luteolin is mediated
via the MAPK pathway, the phosphorylation of three MAPK molecules,
p38 MAPK, JNK and ERK1/2 in LPS-stimulated BV-2 cells was examined.
LPS rapidly activated MAPKs within 15 min of LPS stimulation, while
luteolin at 20 μM markedly inhibited the LPS-induced
phosphorylation of p38 and JNK, but had no effect on ERK. However,
further studies are necessary to support this conclusion.
Multiple signaling pathways, such as those involving
MAPKs and Akt, are involved in LPS-stimulated signal transduction
and lead to the activation of NF-κB and the subsequent induction of
pro-inflammatory gene expression (27,28).
Akt is activated via the phosphoinositide-3-OH kinase (PI3K)
pathway, an important pathway regulating inflammation and immunity
(29). The results of the present
study indicate that luteolin also significantly inhibited the
LPS-induced activation of Akt. The data suggest that inhibition of
Akt by luteolin may contribute to the suppression of LPS-induced
NF-κB activation and the expression of inflammatory mediators in
BV2 cells.
Neurotoxic microglial-neuronal interactions have
been implicated in the pathogenesis of various neurodegenerative
diseases (30). Microglial
activation has been shown to promote the production of inflammatory
cytokines leading to neuronal apoptosis (31,32).
In order to investigate whether luteolin is able to rescue neurons
from death induced by microglial activation, SH-SY5Y cells and BV2
microglia in a coculture system were used in the present study. The
results clearly indicate that when SH-SY5Y cells were cocultured
with LPS-stimulated BV2 microglia, neuronal cell death increased by
60.2% and the number of apoptotic neurons increased by 57.0%.
However, treatment with luteolin in this LPS-induced coculture
system increased cell viability by 28.6% and reduced the apoptotic
cell number by 27.0%. These data suggest that luteolin inhibited
SH-SY5Y cell apoptosis via inhibition of microglial activation in
the microglial-neuronal coculture system. These results provide
strong evidence that luteolin protects neurons from
microglial-mediated LPS neurotoxicity. However, further in
vivo investigation of this activity is necessary in order to
clarify the molecular mechanisms involved and assess the full
medicinal potential of luteolin.
In conclusion, the present study demonstrated that
luteolin inhibited the LPS-stimulated expression of TLR-4. Luteolin
also blocked LPS-induced NF-κB, p38, JNK and Akt activation, but
had no effect on ERK. When SH-SY5Y cells were cocultured with
LPS-stimulated BV2 microglia, pretreatment with luteolin increased
neuronal viability and reduced the number of apoptotic cells. These
observations suggest that luteolin has a therapeutic application in
the treatment of neurodegenerative diseases.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (Nos. 81102449 and 81200930),
the Natural Science Foundation of Guangdong Province (Nos.
S2011040003038 and S2012040007768) and the Fundamental Research
Funds for the Central Universities (No. 21612423).
References
|
1
|
Bernardi A, Frozza RL, Meneghetti A, Hoppe
JB, Battastini AM, Pohlmann AR, Guterres SS and Salbego CG:
Indomethacin-loaded lipid-core nanocapsules reduce the damage
triggered by Aβ1–42 in Alzheimer’s disease models. Int J
Nanomedicine. 7:4927–4942. 2012.PubMed/NCBI
|
|
2
|
Yokoyama H, Uchida H, Kuroiwa H, Kasahara
J and Araki T: Role of glial cells in neurotoxin-induced animal
models of Parkinson’s disease. Neurol Sci. 32:1–7. 2011.PubMed/NCBI
|
|
3
|
Dibaj P, Zschüntzsch J, Steffens H,
Scheffel J, Göricke B, Weishaupt JH, Le Meur K, Kirchhoff F,
Hanisch UK, Schomburg ED and Neusch C: Influence of methylene blue
on microglia-induced inflammation and motor neuron degeneration in
the SOD1(G93A) model for ALS. PLoS One. 7:e439632012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McGeer EG and McGeer PL: Neuroinflammation
in Alzheimer’s disease and mild cognitive impairment: a field in
its infancy. J Alzheimers Dis. 19:355–361. 2010.
|
|
5
|
Mrak RE and Griffin WS: Glia and their
cytokines in progression of neurodegeneration. Neurobiol Aging.
26:349–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anisman H: Cascading effects of stressors
and inflammatory immune system activation: implications for major
depressive disorder. J Psychiatry Neurosci. 34:4–20.
2009.PubMed/NCBI
|
|
7
|
Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park
JH, et al: Apigenin inhibits the production of NO and PGE2 in
microglia and inhibits neuronal cell death in a middle cerebral
artery occlusion-induced focal ischemia mice model. Neurochem Int.
52:878–886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lyons A, Downer EJ, Crotty S, Nolan YM,
Mills KH and Lynch MA: CD 200 ligand receptor interaction modulates
microglial activation in vivo and in vitro: a role for IL-4. J
Neurosci. 27:8309–8313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian L, Flood PM and Hong JS:
Neuroinflammation is a key player in Parkinson’s disease and a
prime target for therapy. J Neural Transm. 117:971–979. 2010.
|
|
10
|
Krause DL and Müller N: Neuroinflammation,
microglia and implications for anti-inflammatory treatment in
Alzheimer’s disease. Int J Alzheimers Dis. 2010:7328062010.
|
|
11
|
Park E, Kum S, Wang C, Park SY, Kim BS and
Schuller-Levis G: Anti-inflammatory activity of herbal medicines:
Inhibition of nitric oxide production and tumor necrosis
factor-alpha secretion in an activated macrophage-like cell line.
Am J Chin Med. 33:415–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JS and Jobin C: The flavonoid luteolin
prevents lipopolysaccharide-induced NF-κB signaling and gene
expression by blocking IκB kinase activity in intestinal epithelial
cells and bone-marrow derived dendritic cells. Immunology.
115:375–387. 2005.PubMed/NCBI
|
|
13
|
Kim SH, Shin KJ, Kim D, Kim YH, Han MS,
Lee TG, et al: Luteolin inhibits the nuclear factor-κB
transcriptional activity in Rat-1 fibroblasts. Biochem Pharmacol.
66:955–963. 2003.
|
|
14
|
Gutiérrez-Venegas G, Kawasaki-Cárdenas P,
Arroyo-Cruz SR and Maldonado-Frías S: Luteolin inhibits
lipopolysaccharide actions on human gingival fibroblasts. Eur J
Pharmacol. 541:95–105. 2006.PubMed/NCBI
|
|
15
|
Zhu LH, Bi W, Qi RB, Wang HD and Lu DX:
Luteolin inhibits microglial inflammation and improves neuron
survival against inflammation. Int J Neurosci. 121:329–336. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bureau G, Longpré F and Martinoli MG:
Resveratrol and quercetin, two natural polyphenols, reduce
apoptotic neuronal cell death induced by neuroinflammation. J
Neurosci Res. 86:403–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jang S, Kelley KW and Johnson RW: Luteolin
reduces IL-6 production in microglia by inhibiting JNK
phosphorylation and activation of AP-1. Proc Natl Acad Sci USA.
105:7534–7539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rezai-Zadeh K, Ehrhart J, Bai Y, Sanberg
PR, Bickford P, Tan J and Shytle RD: Apigenin and luteolin modulate
microglial activation via inhibition of STAT1-induced CD40
expression. J Neuroinflammation. 5:412008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dirscherl K, Karlstetter M, Ebert S, Kraus
D, Hlawatsch J, Walczak Y, Moehle C, Fuchshofer R and Langmann T:
Luteolin triggers global changes in the microglial transcriptome
leading to a unique anti-inflammatory and neuroprotective
phenotype. J Neuroinflammation. 7:32010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jang S, Dilger RN and Johnson RW: Luteolin
inhibits microglia and alters hippocampal-dependent spatial working
memory in aged mice. J Nutr. 140:1892–1898. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Willis LM, Bielinski DF, Fisher DR,
Matthan NR and Joseph JA: Walnut extract inhibits LPS-induced
activation of BV-2 microglia via internalization of TLR4: Possible
involvement of phospholipase D2. Inflammation. 33:325–333. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ozato K, Tsujimura H and Tamura T:
Toll-like receptor signaling and regulation of cytokine gene
expression in the immune system. Biotechniques. 33(4 Suppl):
S66–S75. 2002.PubMed/NCBI
|
|
24
|
Bi W, Jing X, Zhu L, Liang Y, Liu J, Yang
L, Xiao S, Xu A, Shi Q and Tao E: Inhibition of 26S protease
regulatory subunit 7 (MSS1) suppresses neuroinflammation. Plos One.
7:e361422012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bi W, Zhu L, Wang C, Liang Y, Liu J, Shi Q
and Tao E: Rifampicin inhibits microglial inflammation and improves
neuron survival against inflammation. Brain Res. 1395:12–20. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie Z, Smith CJ and Van Eldik LJ:
Activated glia induce neuron death via MAP kinase signaling
pathways involving JNK and p38. Glia. 45:170–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhat NR, Zhang P, Lee JC and Hogan EL:
Extracellular signal-regulated kinase and p38 subgroups of
mitogen-activated protein kinases regulate inducible nitric oxide
synthase and tumor necrosis factor-α gene expression in
endotoxin-stimulated primary glial cultures. J Neurosci.
18:1633–1641. 1998.PubMed/NCBI
|
|
28
|
Madrid LV, Wang CY, Guttridge DC,
Schottelius AJ, Baldwin AS Jr and Mayo MW: Akt suppresses apoptosis
by stimulating the transactivation potential of the Rel A/p65
subunit of NF-κB. Mol Cell Biol. 20:1626–1638. 2000.PubMed/NCBI
|
|
29
|
Downward J: Lipid-regulated kinases: some
common themes at last. Science. 279:673–674. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Liu L, Barger SW, Mrak RE and
Griffin WS: Vitamin E suppression of microglial activation is
neuroprotective. J Neurosci Res. 15:163–170. 2001. View Article : Google Scholar
|
|
31
|
Brown GC and Neher JJ: Inflammatory
neurodegeneration and mechanisms of microglial killing of neurons.
Mol Neurobiol. 41:242–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bi W, Zhu L, Jing X, Liang Y and Tao E:
Rifampicin and Parkinson’s disease. Neurol Sci. 34:137–141.
2013.
|