Introduction
Human papillomavirus (HPV) causes various venereal
infections, including condylomata acuminata, which are frequently
asymptomatic but occasionally cause clinical symptoms of anogenital
pruritus and burning sensations (1,2). The
current methods for treating condyloma acuminata include
cryotherapy, immune stimulation with imiquimod and laser therapy
(3). However, HPV-mediated lesions
are complex to eradicate, thus, it is imperative to identify novel
and effective drugs for the treatment of condylomata acuminata.
Saikosaponin-d (Ssd) is one of the major
triterpenoid saponins derived from Bupleurum falcatum L.,
which is commonly prescribed for inflammatory and infectious
diseases in China, Japan and other Asian countries (4,5).
Previous studies have identified that Ssd exhibits
immunomodulatory, anti-inflammatory and antiviral activities, thus,
may be a promising chemotherapeutic drug candidate for condylomata
acuminata (6,7). Dendritic cells (DCs) have been
identified as the most potent antigen-presenting cells that are
effective initiators of the immune response to diseases resulting
from viral infection (8). Results
from previous investigations have indicated that DCs are critical
in the induction and regulation of immune responses (9,10).
Previous studies have identified that human peripheral blood
mononuclear cells (PBMCs) can be induced into DCs in the presence
of granulocyte-macrophage colony-stimulating factor (GM-CSF) and
interleukin (IL)-4, enabling the collection of a large quantity of
DCs for use in clinical application (11,12).
Following culturing for five to seven days, PBMCs can be induced to
differentiate into immature DCs, which may subsequently be induced
to differentiate into mature DCs using inflammatory factor
stimulators, including lipopolysaccharide (LPS) (13). As mature DCs alone are capable of
activating the immune system and protecting the body against
infected pathogens, it is critical to identify effective factors
that promote the maturation of DCs.
In the present study, monocyte-derived DCs obtained
from condylomata acuminata patients were used to evaluate the
effect of Ssd on the modulation of DC differentiation, maturation
and function. The aim of the present study was to identify novel
and effective drugs that are involved in immunomodulation and that
may be used to treat condylomata acuminata.
Materials and methods
Chemicals
Ssd (>95% purity, identified by high-performance
liquid chromatography), GM-CSF, IL-4 and LPS were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Ssd was dissolved in dimethyl
sulfoxide and stored at room temperature. RPMI 1640, fetal bovine
serum (FBS) and penicillin-streptomycin were obtained from
Gibco-BRL (Carlsbad, CA, USA). Fluorescein isothiocyanate
(FITC)-dextran, anti-human cluster of differentiation (CD)83, CD80,
CD86, CD14, CD1a, CD32, CD40 and mannose receptor (MR) were
purchased from BD Pharmingen (San Diego, CA, USA).
Isolation and generation of PBMCs
PBMCs were isolated using Ficoll density gradient
centrifugation, according to the manufacturer’s instructions.
Mononuclear cells were incubated for 3 h in six-well plates in RPMI
1640 buffer that was supplemented with 10% FBS at 37°C in a
humidified atmosphere with 5% CO2. The nonadherent cells
were removed by gentle washing and the remaining adherent monocytes
were cultured in RPMI 1640 with GM-CSF and IL-4 for five days to
generate immature DCs. Maturation was generated by stimulation of
the immature DCs with 10 ng/ml LPS for a further 48 h. Ssd at
varying concentrations (5, 10 and 20 μM) was added to the culture
for five days to investigate the effect on DC differentiation. Ssd
was subsequently added to the five-day cultured immature DCs to
evaluate the effect on the maturation of the DCs. The study was
approved by the ethics committee of Shanghai Jiao Tong University,
Shanghai, China. Written informed patient consent was obtained from
the patient.
Flow cytometry
Expression levels of cell surface markers were
detected via flow cytometry. Cells were harvested following a five
or seven-day culture and were resuspended in phosphate-buffered
saline. The expression levels of CD83, CD80, CD86, CD14, CD1a,
CD32, CD40 and MR markers were measured using flow cytometry; the
expression rate of the markers was determined by CellQuest software
(BD Biosciences, San Jose, CA, USA).
Detection of DC endocytic activity
Endocytic activity of the DCs was measured via
FITC-dextran uptake and determined using flow cytometry. Cells at a
density of 4×106 cells/sample were incubated in medium
containing FITC-dextran at 37°C for 2 h. The uptake of FITC-dextran
by the cells was calculated using flow cytometry and the mean
fluorescence density represented the uptake ability of the DCs.
Mixed lymphocyte reactions (MLRs)
Responder cells were purified with allogeneic
CD4+ T cells using a magnetic-activated cell sorting
CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, CA,
USA). The allostimulatory capacity of irradiated DCs (30 Gy) was
measured at different stimulator/responder cell ratios in 96-well
flat-bottom plates. Thymidine incorporation was measured via
standard liquid scintillation counting.
Determination of cytokine secretion by
DCs
Supernatants were collected and stored at −80°C,
until required for cytokine analysis. ELISA (R&D Systems,
Minneapolis, MN, USA) was used to determine the expression levels
of IL-12 and was performed according to the manufacturer’s
instructions. Absorbance was measured at 450 nm using a microplate
reader and IL-12 content was determined according to the standard
curve.
Statistical analysis
Data are expressed as the mean ± SEM and statistical
analysis was conducted using SPSS 10.0 software (SPSS, Inc.,
Chicago, IL, USA). Comparisons between groups were performed with
analysis of variance and P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of Ssd on the differentiation of
monocyte-derived DCs
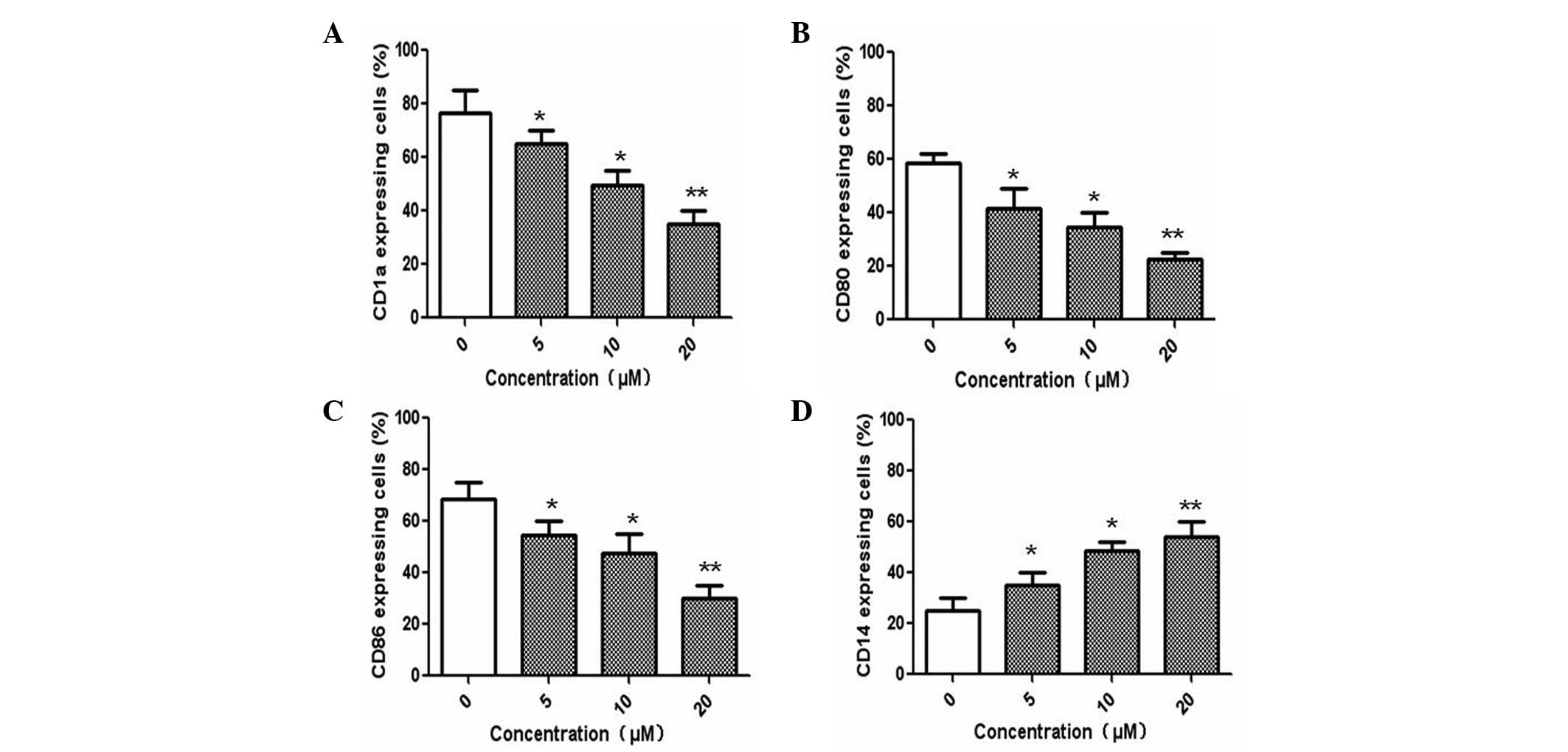
To investigate the effect of Ssd on DC
differentiation, monocytes were cultured in the presence of GM-CSF
and IL-4 with various concentrations of Ssd (5, 10 and 20 μM). The
expression levels of CD80, CD86, CD14 and CD1a were determined by
flow cytometry. As shown in Fig.
1, treatment of monocytes with Ssd resulted in decreased
expression levels of CD1a, CD80 and CD86 in a
concentration-dependent manner. However, the expression of CD14 was
significantly upregulated following Ssd stimulation, indicating
that Ssd inhibited the differentiation of DCs from monocytes in a
concentration-dependent manner.
Effects of Ssd on the endocytic activity
of immature DCs
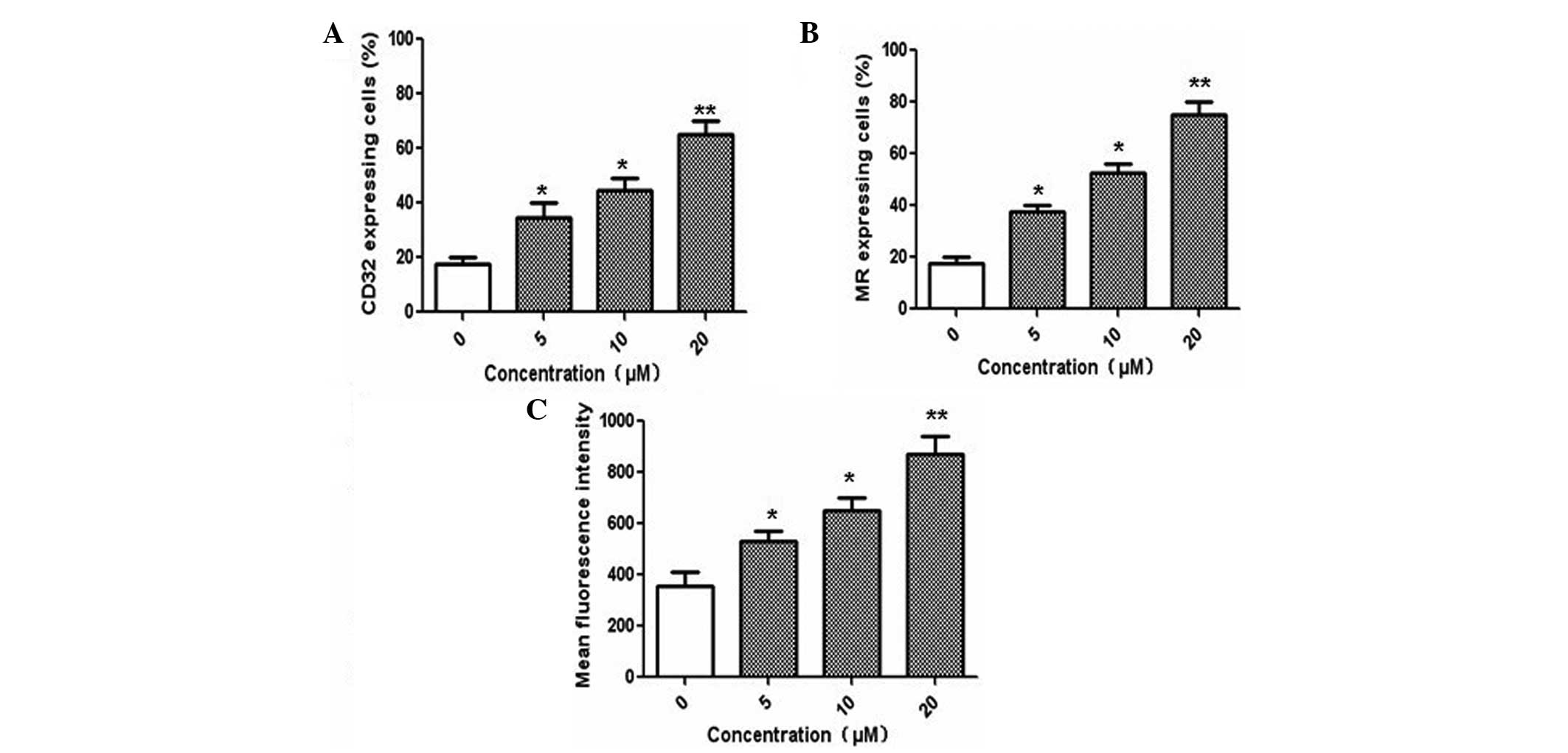
Immature DCs are capable of effectively capturing
and processing antigens. Therefore, the effects of Ssd on the
endocytic activity of immature DCs were examined. The results
indicated that Ssd treatment significantly enhanced the expression
levels of molecules involved in antigen uptake, including CD32 and
MR, which was consistent with the inhibition of Ssd on the
differentiation of monocyte-derived DCs (Fig. 2A and B). In addition, flow
cytometric analysis demonstrated that Ssd increased the uptake of
FITC-dextran, indicating the enhanced endocytic activity of the
immature DCs (Fig. 2C).
Effects of Ssd on the maturation of
monocyte-derived DCs
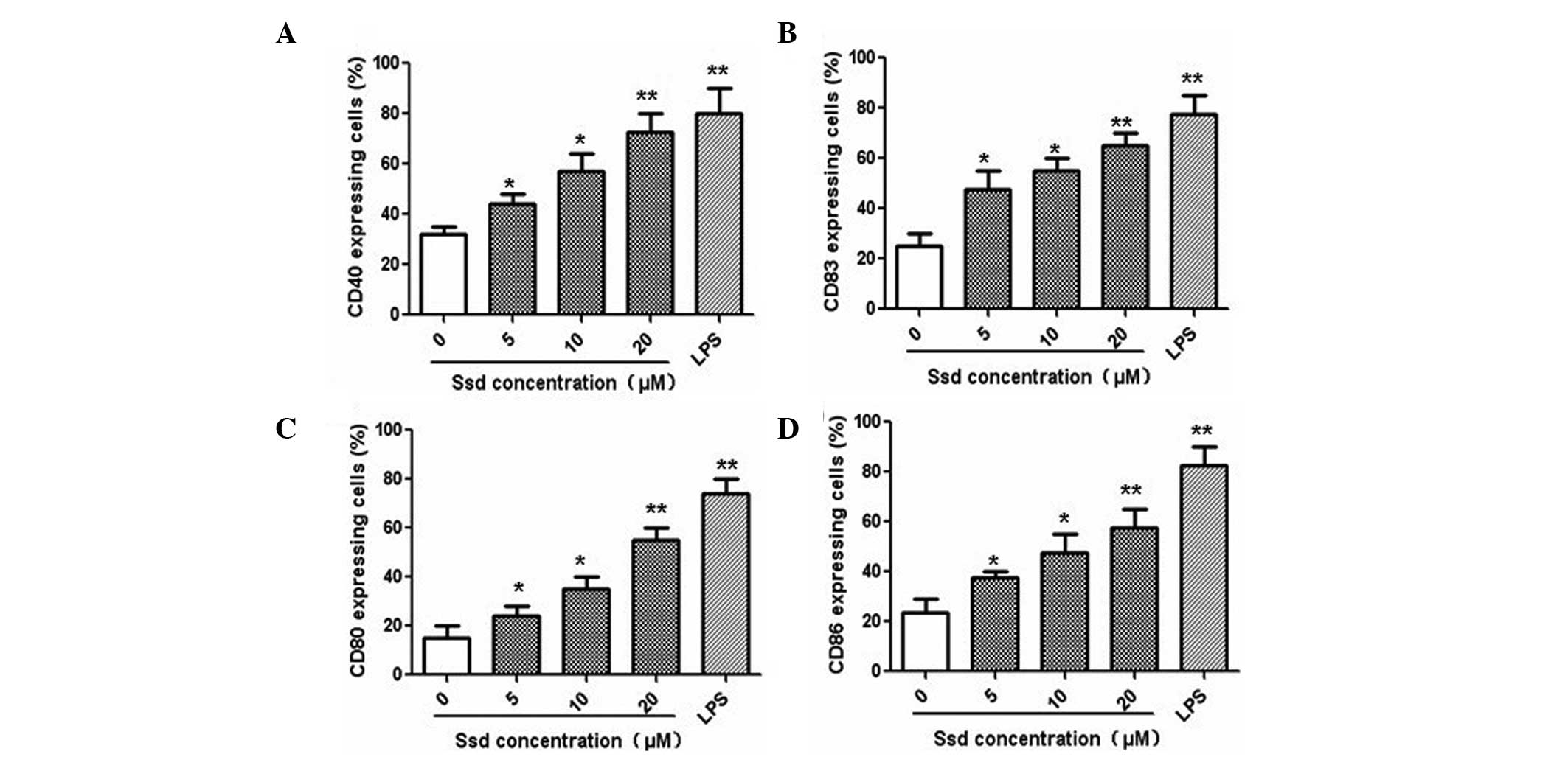
Following five days of culturing with GM-CSF and
IL-4, immature DCs were able to further differentiate into mature
DCs via stimulation with LPS. On day five, the immature DCs were
incubated with various concentrations of Ssd (5, 10 and 20 μM) or
10 ng/ml LPS for an additional 48 h. The results demonstrated that
Ssd promoted the maturation of the immature DCs in a dose-dependent
manner by increasing CD40 and CD83 expression, as well as by
upregulating CD80 and CD86 expression (Fig. 3). These results indicated that Ssd
promoted the maturation of immature DCs in a
concentration-dependent manner.
Effects of Ssd on the terminal function
of mature DCs
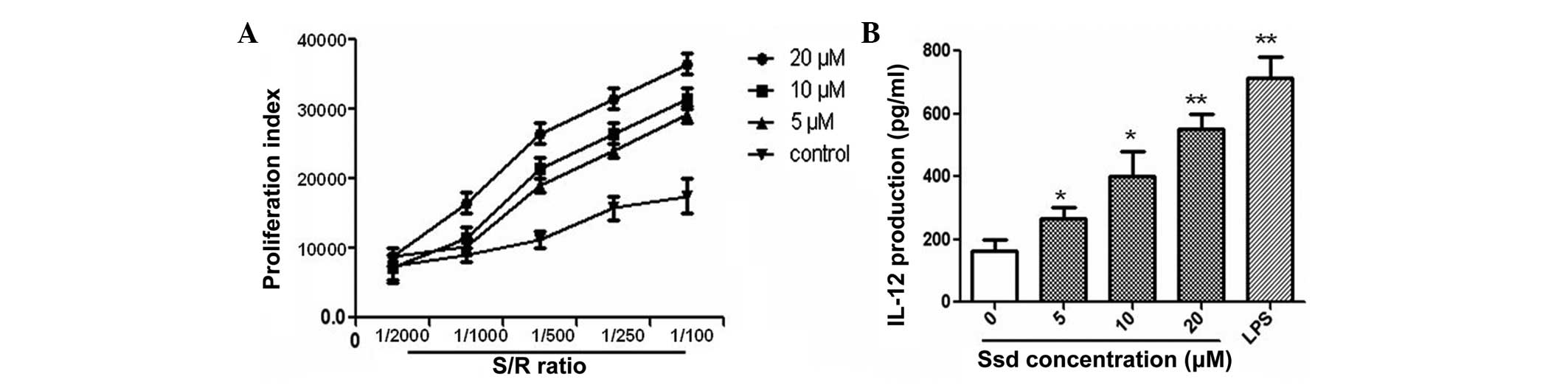
Mature DCs have been shown to play a critical role
in antigen presentation and the stimulation of lymphocyte
proliferation with MLRs. The present study demonstrated that
lymphocyte proliferation significantly increased in a
dose-dependent manner following Ssd stimulation (Fig. 4A). In addition, the effect of Ssd
on the secretion of IL-12 by mature DCs was measured using ELISA.
Compared with the control group, IL-12 expression in the mature DCs
treated with Ssd significantly increased in a dose-dependent manner
(Fig. 4B).
Discussion
HPV infection is prevalent around the world and can
lead to benign lesions, such as condyloma acuminata, and malignant
lesions, including cervical cancer (14). DCs are critical for antigen
presentation and defense of the antiviral host, thus, the infection
of immature DCs by numerous types of virus impairs maturation and
reduces the DC ability to stimulate lymphocyte proliferation
(15). A traditional drug, Ssd,
has been reported to possess immunoodulatory, anti-inflammatory and
antiviral properties; therefore, Ssd is commonly prescribed to
treat inflammatory and infectious diseases (4). In the present study, the effects of
Ssd on the differentiation, maturation and terminal function of
monocyte-derived DCs isolated from condyloma acuminata patients,
were investigated.
DCs have two stages, immature and mature. Immature
DCs possess a weak ability to stimulate lymphocyte proliferation,
but can effectively capture and process antigens (16). The present study demonstrated that
Ssd treatment reduced the differentiation of DCs, as shown by
decreased expression levels of CD1a, which is a characteristic
DC-associated molecule (17). The
expression of molecules involved in antigen presentation, including
CD80 and CD86, decreased while CD14 expression (a typical marker of
monocyte/macrophage not normally present on DCs) was markedly
increased (18). These
observations indicate that Ssd inhibits the differentiation of DCs
from monocytes in a concentration-dependent manner. In addition,
the immature DC is characterized by a high capacity for antigen
uptake and processing, thus, is associated with high expression
levels of molecules involved in antigen uptake, including CD32 and
MR (19). Flow cytometric analysis
showed that treatment with Ssd increased the uptake of
FITC-dextran, as well as the expression of CD32 and MR, indicating
the enhanced endocytic activity of immature DCs. These results are
consistent with the hypothesis that Ssd inhibits the
differentiation of DCs.
The unique ability of DCs to activate lymphocyte
proliferation is dependent on the stage of maturation, cytokine
secretion and expression of costimulatory molecules, including CD86
and CD80 (20,21). Viral and bacterial products, as
well as inflammatory cytokines, are able to initiate DC maturation
to increase the expression of costimulatory molecules and the
capacity of DCs to promote lymphocyte proliferation (22–24).
Mature DCs reduce their endocytic capacity, but enhance chemokine
and inflammatory cytokine production and become mobile to enable
the delivery of pathogen-derived antigens for lymphocyte activation
(25). Considering the importance
of mature DCs in primary immune responses, the induction of DC
maturation is critical to defend against viral infections. In the
present study, immature DCs were treated with Ssd or LPS for 48 h
to study the effect of Ssd on DC maturation. The expression of
CD83, a typical marker of DC maturation (26), was shown to significantly increase
in a dose-dependent manner. The expression levels of costimulatory
molecules, including CD80, CD86 and CD40, were also elevated
following Ssd treatment. Furthermore, consistent with the changes
of phenotype, Ssd stimulation significantly promoted lymphocyte
proliferation and IL-12 expression in mature DCs. Therefore, these
results indicate that Ssd promotes DC maturation in a
concentration-dependent manner.
In conclusion, the results of the present study
indicate that Ssd exhibits an immunomodulatory effect and therefore
may be a novel potent chemopreventive drug candidate for the
treatment of condylomata acuminata.
References
|
1
|
FUTURE I/II Study Group. Dillner J, Kjaer
SK, Wheeler CM, et al: Four year efficacy of prophylactic human
papillomavirus quadrivalent vaccine against low grade cervical,
vulvar, and vaginal intraepithelial neoplasia and anogenital warts:
randomised controlled trial. BMJ. 341:c34932010.
|
|
2
|
Viera MH, Amini S, Huo R, Konda S, Block S
and Berman B: Herpes simplex virus and human papillomavirus genital
infections: new and investigational therapeutic options. Int J
Dermatol. 49:733–749. 2010.
|
|
3
|
Le Poole C, Denman CJ and Arbiser JL:
Immunosuppression may be present within condyloma acuminata. J Am
Acad Dermatol. 59:967–974. 2008.PubMed/NCBI
|
|
4
|
Lu CN, Yuan ZG, Zhang XL, et al:
Saikosaponin a and its epimer saikosaponin d exhibit
anti-inflammatory activity by suppressing activation of NF-κB
signaling pathway. Int Immunopharmacol. 14:121–126. 2012.PubMed/NCBI
|
|
5
|
Wong VK, Zhang MM, Zhou H, et al:
Saikosaponin-d enhances the anticancer potency of TNF-α via
overcoming its undesirable response of activating NF-Kappa B
signalling in cancer cells. Evid Based Complement Alternat Med.
2013:7452952013.PubMed/NCBI
|
|
6
|
Lu XL, He SX, Ren MD, Wang YL, Zhang YX
and Liu EQ: Chemopreventive effect of saikosaponin-d on
diethylinitrosamine-induced hepatocarcinogenesis: involvement of
CCAAT/enhancer binding protein β and cyclooxygenase-2. Mol Med Rep.
5:637–644. 2012.PubMed/NCBI
|
|
7
|
Zhu J, Luo C, Wang P, He Q, Zhou J and
Peng H: Saikosaponin A mediates the inflammatory response by
inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7
cells. Exp Ther Med. 5:1345–1350. 2013.PubMed/NCBI
|
|
8
|
Li DY, Gu C, Min J, Chu ZH and Ou QJ:
Maturation induction of human peripheral blood mononuclear
cell-derived dendritic cells. Exp Ther Med. 4:131–134.
2012.PubMed/NCBI
|
|
9
|
Ghannam S, Pène J, Torcy-Moquet G,
Jorgensen C and Yssel H: Mesenchymal stem cells inhibit human Th17
cell differentiation and function and induce a T regulatory cell
phenotype. J Immunol. 185:302–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang XX, Zhang Y, Liu B, et al: Human
mesenchymal stem cells inhibit differentiation and function of
monocyte-derived dendritic cells. Blood. 105:4120–4126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu KJ, Shen QY, Cheng H, Mao XH, Lao LM
and Hao GL: Triptolide affects the differentiation, maturation and
function of human dendritic cells. Int Immunopharmacol.
5:1415–1426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chapuis F, Rosenzwajg M, Yagello M, Ekman
M, Biberfeld P and Gluckman JC: Differentiation of human dendritic
cells from monocytes in vitro. Eur J Immunol. 27:431–441. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dolganiuc A, Kodys K, Kopasz A, et al:
Hepatitis C virus core and nonstructural protein 3 proteins induce
pro- and anti-inflammatory cytokines and inhibit dendritic cell
differentiation. J Immunol. 170:5615–5624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Gao XH, Hong Y, Li X and Chen HD:
Local hyperthermia decreases the expression of CCL-20 in condyloma
acuminatum. Virol J. 7:3012010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abe M, Akbar SM, Horiike N and Onji M:
Induction of cytokine production and proliferation of memory
lymphocytes by murine liver dendritic cell progenitors: role of
these progenitors as immunogenic resident antigen-presenting cells
in the liver. J Hepatol. 34:61–67. 2001. View Article : Google Scholar
|
|
16
|
Banchereau J, Briere F, Caux C, et al:
Immunobiology of dendritic cells. Annu Rev Immunol. 18:767–811.
2000. View Article : Google Scholar
|
|
17
|
Zhu KJ, Cen JP, Lou JX, et al: Imiquimod
inhibits the differentiation but enhances the maturation of human
monocyte-derived dendritic cells. Int Immunopharmacol. 9:412–417.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piemonti L, Monti P, Sironi M, et al:
Vitamin D3 affects differentiation, maturation, and function of
human monocyte-derived dendritic cells. J Immunol. 164:4443–4451.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frison N, Taylor ME, Soilleux E, et al:
Oligolysine-based oligosaccharide clusters: selective recognition
and endocytosis by the mannose receptor and dendritic cell-specific
intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin. J
Biol Chem. 278:23922–23929. 2003. View Article : Google Scholar
|
|
20
|
Horváth R, Budinský V, Kayserová J, et al:
Kinetics of dendritic cells reconstitution and costimulatory
molecules expression after myeloablative allogeneic haematopoetic
stem cell transplantation: implications for the development of
acute graft-versus host disease. Clin Immunol. 131:60–69. 2009.
|
|
21
|
Ferrer IR, Liu D, Pinelli DF, Koehn BH,
Stempora LL and Ford ML: CD40/CD154 blockade inhibits dendritic
cell expression of inflammatory cytokines but not costimulatory
molecules. J Immunol. 189:4387–4395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wakim LM and Bevan MJ: Cross-dressed
dendritic cells drive memory CD8+ T-cell activation
after viral infection. Nature. 471:629–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cervantes-Barragan L, Lewis KL, Firner S,
et al: Plasmacytoid dendritic cells control T-cell response to
chronic viral infection. Proc Natl Acad Sci USA. 109:3012–3017.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schreiber HA, Hulseberg PD, Lee J, et al:
Dendritic cells in chronic mycobacterial granulomas restrict local
anti-bacterial T cell response in a murine model. PLoS One.
5:e114532010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dullaers M and Thielemans K: From pathogen
to medicine: HIV-1-derived lentiviral vectors as vehicles for
dendritic cell based cancer immunotherapy. J Gene Med. 8:3–17.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koski GK, Lyakh LA, Cohen PA and Rice NR:
CD14+ monocytes as dendritic cell precursors: diverse
maturation-inducing pathways lead to common activation of
NF-kappab/RelB. Crit Rev Immunol. 21:179–189. 2001.
|