Introduction
Ischemia/reperfusion (I/R) is a predominant cause of
hepatic injury, which is of clinical significance following liver
surgery, hemorrhagic shock and liver transplantation (1). Therefore, developing effective
preventive and therapeutic strategies for I/R-induced hepatic
injury is required.
It has been demonstrated that I/R-induced hepatic
injury is initially triggered by reactive oxygen species (ROS),
which induce oxidative damage and apoptosis, an important mechanism
for cell death following hepatic I/R injury (2). In addition, certain pro-inflammatory
chemokines and cytokines are key during the initial period of
reperfusion, whereas the late period of hepatic injury is
neutrophil-mediated (3).
Gynostemma pentaphyllum is a traditional
Chinese medicine, which has previously been used for the treatment
of chronic inflammation, hyperlipidemia and liver diseases
(4). Gypenoside (GP) is the
predominant component of Gynostemma pentaphyllum and has
been shown to possess anti-inflammatory, antitumor and anti-oxidant
capabilities (5–7). Furthermore, GP exhibits a therapeutic
effect on chronic hepatic injury, fibrosis, as well as fatty liver
disease, which were induced by a high fat, high cholesterol diet
and alcohol in mice (5,8). Qi et al (9) reported that GP protected against DNA
damage in neurons in I/R-induced cerebral injury. To the best of
our knowledge, the role of GP in hepatic I/R injury and the
underlying molecular mechanism, have not yet been investigated.
Therefore, in the present study the protective
effects of GP against I/R-induced hepatic injury in mice was
studied. In addition, the molecular mechanisms involved were
investigated, particularly regarding oxidative stress and
apoptosis.
Materials and methods
Hepatic I/R procedure
All of the methods in the present study were
approved by the Ethics Committee of Xiangya Hospital of Central
South University (Changsha, China) and were performed according to
the Guidelines for Care and Use of Experimental Animals published
by the Chinese Association for Laboratory Animal Sciences. Male
C57BL/6 mice (age, 8–12 weeks; weight, 20–25 g) were housed in a
laminar flow, temperature-controlled, pathogen-free environment
under a 12 h light/dark cycle, at Xiangya Medical Experimental
Animal Center of Central South University (Changsha, China).
Prior to the experiments, the mice were fasted for
24 h and provided with tap water ad libitum. During the
procedure, xylazine (7 mg/kg body weight) and ketamine (55 mg/kg
body weight) were administered as an anesthetic. To induce ischemia
of the median and left lobes of the liver, a micro clip was used to
clamp the left branches of the portal vein and hepatic artery
following a transverse incision, which was made to the abdomen. The
clamp was removed after 1 h and the wound was closed. Six hours
following reperfusion, the mice were sacrificed by cervical
dislocation under anesthesia, and the ischemic liver tissues and
blood were collected immediately. The same procedure was performed
in the sham group but without vessel occlusion.
Administration of GP
GP was purchased from the China National
Pharmaceutical Group Corporation (Beijing, China) and dissolved in
saline according to the manufacturer’s instructions. Thirty minutes
prior to ischemia, 50 mg/kg GP was administered orally. In the
present study, the mice were divided into four groups as follows:
i) Vehicle-treated sham; ii) GP-treated sham; iii) vehicle-treated
I/R; and iv) GP-treated I/R. As there were no differences
identified in any of the parameters between the vehicle-treated and
GP-treated sham groups, these two groups were pooled and referred
to as the sham group.
Hepatic lipid peroxidation and
glutathione (GSH) content
The level of thiobarbituric acid reactive substances
in the liver homogenates was measured spectrophotometrically
(Shimadzu, Kyoto, Japan) at a wavelength of 535 nm to determine the
steady-state level of malondialdehyde (MDA), which is the
end-product of lipid peroxidation. 1,1,3,3-tetraethoxypropane
(Sigma-Aldrich, St. Louis, MO, USA) served as the standard.
Following precipitation with 1% picric acid, the GSH level was
determined in the liver homogenates using yeast-GSH reductase,
5,5′-dithio-bis (2-nitrobenzoic acid) and nicotinamide adenine
dinucleotide phosphate, at a wavelength of 412 nm. The same method
was used to measure the oxidized GSH (GSSG) level in the presence
of 2-vinylpyridine; the GSH:GSSG ratio was subsequently
calculated.
Serum aminotransferase activity
The blood was collected and alanine aminotransferase
(ALT) and aspartate aminotransferase (AST) assay kits (Biovision,
Milpitas, CA, USA) were used to determine the activity of serum ALT
and AST, respectively, in accordance with the manufacturer’s
instructions.
Activity of caspase-3 and -8
In accordance with the manufacturer’s instruction,
caspase-3 and-8 colorimetric assay kits (Biovision, San Francisco
Bay Area, CA, USA) were used to determine the activity of caspase-3
and -8, respectively. Briefly, 20 μg liver cytosolic protein was
extracted and incubated in a solution buffer at room temperature
for 30 min. The caspase reaction was initiated by adding 200 μM
N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarine and
incubated at 37°C for 2 h. The change in fluorescence (excitation
at 400 nm) was detected.
Western blot analysis
Cytoplasmic extraction reagents (Pierce
Biotechnology, Rockford, IL, USA) were used to obtain cytosolic
proteins from the mice liver tissues, in accordance with the
manufacturer’s instructions. Following the determination of the
concentration of protein using the protein assay reagents, 20 μg
protein was separated using 10% SDS-PAGE and transferred to
nitrocellulose membranes, which was maintained at room temperature
for 1 h in a buffer solution containing 5% dried skimmed milk. The
membrane was incubated at room temperature for 3 h with mouse
anti-heme oxygenase-1 (HO-1) monoclonal antibody (1:400; Abcam,
Cambridge, UK), mouse anti-cytochrome c monoclonal antibody
(1:200; Abcam), mouse anti-Bcl-2 monoclonal antibody (1:200;
Abcam), mouse anti-Bax monoclonal antibody (1:200; Abcam) or mouse
anti-GAPDH monoclonal antibody (1:200; Abcam), respectively, and
with rabbit anti-mouse secondary antibody conjugated to horseradish
peroxidase (1:10,000; Abcam) for 1 h. The signals on the membranes
were detected using an enhanced chemiluminescence reagent
(PerkinElmer, Waltham, MA, USA) and the densitometry was analyzed
using Image-Pro plus software 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
All data are indicated as the mean ± standard error
of the mean and were analyzed by one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
GP treatment attenuates the increase in
hepatic lipid peroxidation and GSH content in hepatic tissue of an
I/R mouse model
To evaluate the degree of hepatic injury in each
group, the hepatic lipid peroxidation and GSH content were
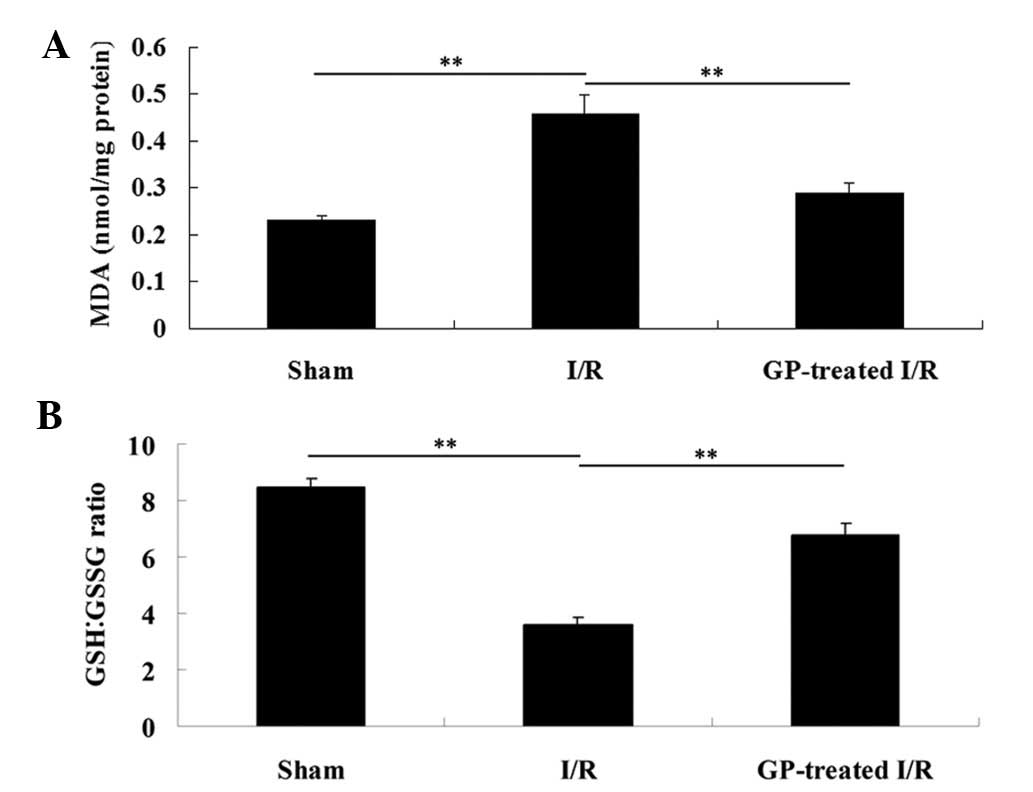
investigated in the liver tissue. As shown in Fig. 1A, the MDA level in the
sham-operated liver tissues was 0.23±0.01 nmol/mg. However, the MDA
level in the I/R group significantly increased to 0.46±0.04
nmol/mg, compared with that in the sham group (P<0.01). In the
GP-treated I/R group, the MDA level was significantly reduced
compared with the I/R group (P<0.01). In addition, as shown in
Fig. 1B, the GSH:GSSG ratio in the
I/R group was markedly decreased (from 8.44 to 3.61), when compared
with that observed in the sham group (P<0.01), which was
attenuated by pre-treatment with GP (P<0.01).
GP treatment attenuates the increase in
activity of serum aminotransferases in the hepatic tissue of an I/R
mouse model
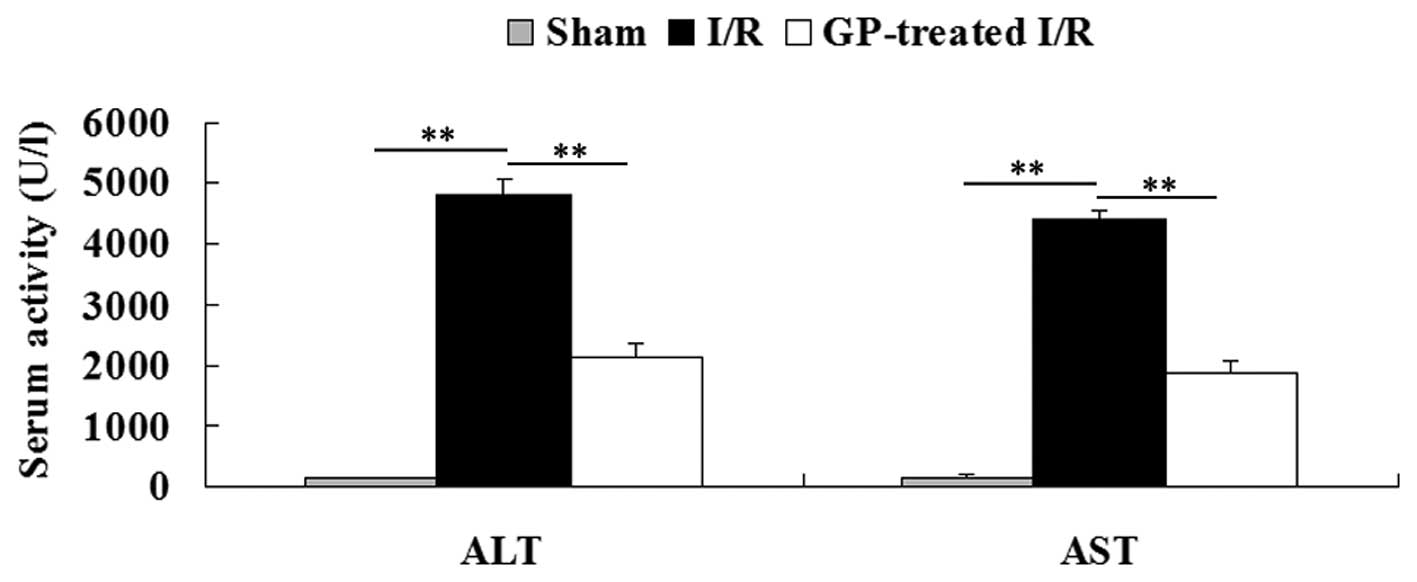
The activity of serum ALT and AST was determined in
each group. As shown in Fig. 2,
the activity of serum ALT and AST was 97.4±9.9 U/l and 107.9±12.1
U/l, respectively, in the sham group. However, the activity of
serum ALT and AST in the I/R group was significantly increased
(4,833±242 U/l and 4,426±142 U/l, respectively) when compared with
those in the sham group (P<0.01). Furthermore, these increases
were significantly attenuated by pre-treatment with GP prior to I/R
(P<0.01).
GP treatment attenuates the upregulation
of HO-1 protein expression in the hepatic tissue of an I/R mouse
model
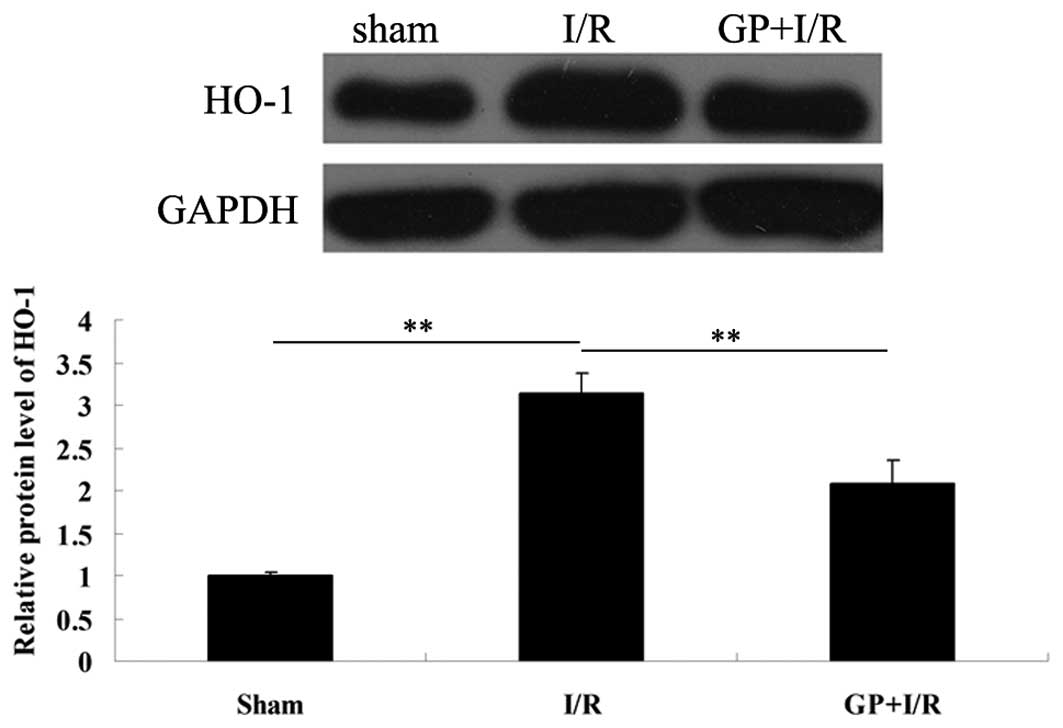
HO-1, a protein that is ubiquitously expressed in
various cells, may be markedly induced by numerous stress stimuli,
including I/R (10). Therefore,
the protein expression of HO-1 was determined in each group. As
demonstrated in Fig. 3, the
protein expression level of HO-1 notably increased following 6 h of
reperfusion compared with the sham group (P<0.01); however, the
increase was attenuated by pre-treatment with GP prior to I/R
(P<0.01).
GP treatment attenuates I/R-induced
hepatic cell apoptosis by modulating key apoptosis-related
proteins
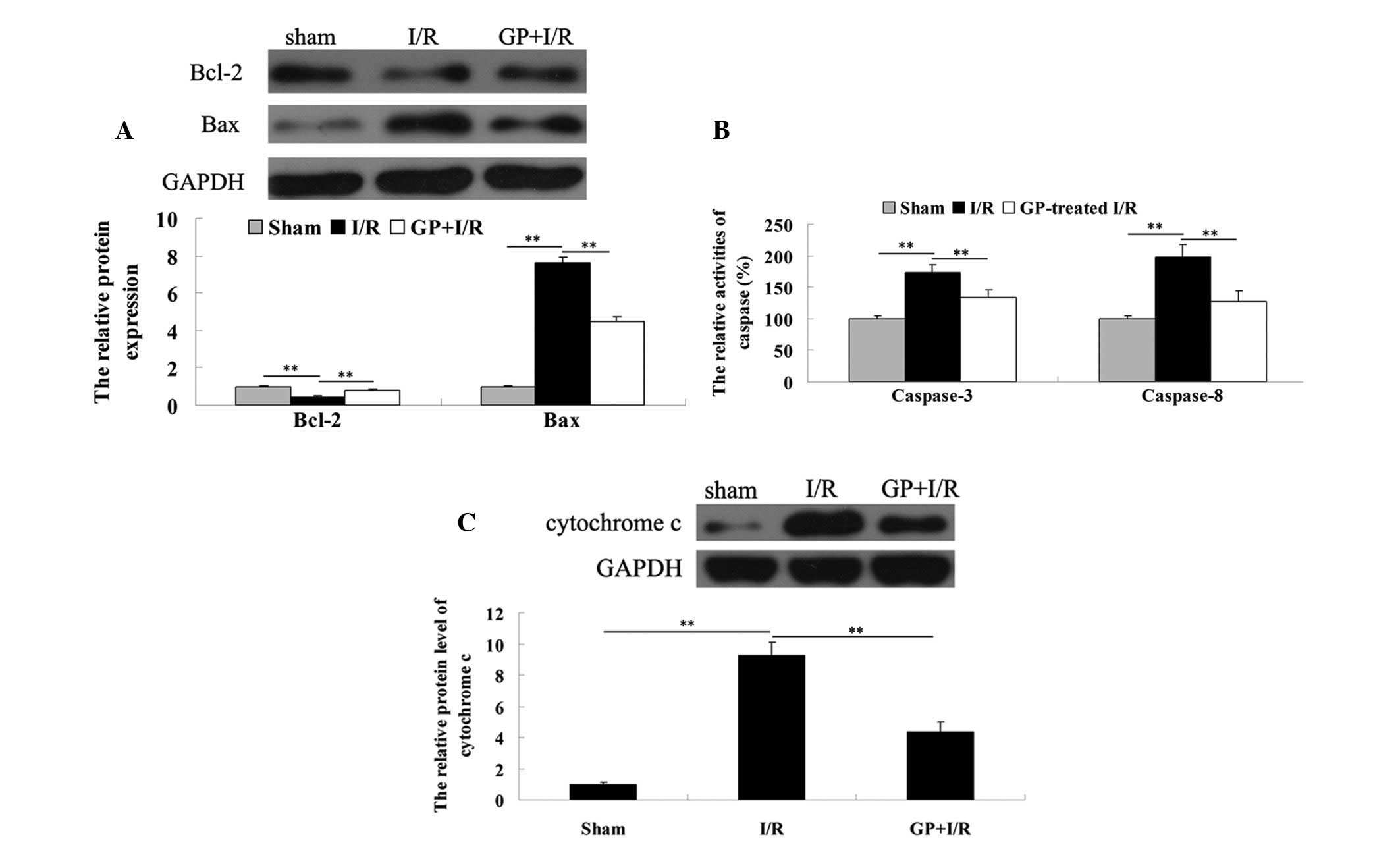
To determine the apoptosis level in the liver
tissues in each group, the expression levels of two
apoptotic-related proteins, Bcl-2 and Bax, were determined via a
western blot assay. As demonstrated in Fig. 4A, the protein expression level of
anti-apoptotic Bcl-2 was lower in the I/R group compared with the
sham group, which was attenuated by pre-treatment with GP.
Consistently, the protein level of pro-apoptotic Bax was
upregulated in the I/R group, when compared with that observed in
the sham group, which was markedly attenuated by the administration
of GP.
In addition, the activity of caspase-3 and -8, two
important indicators for cell apoptosis, was determined. As shown
in Fig. 4B, the caspase-3 and -8
activities in the I/R group was significantly increased when
compared with that observed in the sham group; however, this
increase was attenuated by pre-treatment with GP.
In the I/R group, the protein level of cytochrome
c in the cytosol was significantly higher following 6 h of
reperfusion, when compared with that observed in the sham group.
However, these increases were significantly attenuated by
pre-treatment with GP (Fig.
4C).
Discussion
The bioactivity of GP, including anti-inflammatory,
anti-oxidative and anti-tumor activity, has been investigated in
numerous studies (6,11,12).
However, its anti-oxidative capacity is the significant function.
It has been demonstrated that reactive oxygen species (ROS) are
important in I/R injury, predominantly as ROS initiate lipid
peroxidation, leading to structural and functional damage to the
organelles (13–15). Furthermore, as endogenous
antioxidant levels decrease significantly during I/R, exogenous
antioxidants may decrease the severity of I/R damage.
GP has been shown to have a protective role in liver
fibrosis, chronic hepatic injury and fatty liver disease (5,16).
However, whether GP protects against I/R-induced hepatic injury has
not previously been investigated. In the present study, it was
hypothesized that GP may attenuate hepatic I/R injury via its
anti-oxidative and anti-apoptotic functions.
Reduced GSH has been demonstrated to act as a free
radical scavenger and counteract the deleterious effect of ROS
(17). In the present study, the
administration of GP was shown to effectively attenuate I/R-induced
increases in the GSH:GSSG ratio, indicating that GP acts against
ROS via reducing GSH during I/R. Furthermore, treatment with GP
suppressed the I/R-induced increase in lipid peroxidation. In
addition, as HO-1 exhibits a crucial role in the defense against
oxidative damage, and overexpression of HO-1 may protect against
hepatic I/R injury by modulating oxidative stress (18), the protein level of HO-1 was
examined in each group. It was found that GP treatment upregulated
the protein expression of HO-1 following I/R. Furthermore, the
activity of aminotransferase is an important indicator of oxidative
damage (19) and the upregulation
of the activity of aminotransferase following I/R was demonstrated
to be attenuated by the administration of GP in mice. Therefore, it
was hypothesized that GP effectively protects against I/R-induced
hepatic injury via suppression of oxidative damage.
Previous studies have indicated that ROS are key in
the regulation of cell apoptosis and abundant ROS, resulting from
I/R, induce cell apoptosis (2,20).
Numerous key factors have been demonstrated to be involved in
ROS-mediated cell apoptosis, including the Bcl-2 family,
caspase-3/8 and cytochrome c. Bcl-2, an anti-apoptotic
protein member of the Bcl-2 family inhibits cell endoplasmic
reticulum Ca2+ release, lipid peroxide formation and
free radical production (21). By
contrast, Bax, another member of Bcl-2 family, promotes cell
apoptosis via a mitochondrial-mediated apoptosis pathway (22). Furthermore, Bax is an endogenous
antagonist of Bcl-2, as Bax is able to bind to Bcl-2 via an
associated protein that is homologous to Bcl-2, which further
inhibits the function of Bcl-2 (23). Notably, Bcl-2 and Bcl-xL form a
heterodimer, which is able to suppress the pro-apoptotic effect of
Bax (24). In physiological
conditions, the protein levels of Bcl-2 and Bax are maintained in
equilibrium (22). In the present
study, it was hypothesized that GP inhibited I/R-induced cell
apoptosis in the liver via a Bcl-2 family-dependent mechanism.
As key members of the cysteine-aspartate-specific
protease family, caspase-3 and -8 are ubiquitously expressed in
various mammalian cells, and have been demonstrated to be critical
in cell apoptosis (25). The
activation of caspase-3 and -8 has been observed in various
apoptotic cells (26) and
upregulation of caspases-3 and -8 has been identified in
I/R-induced hepatic injury, indicating that caspase-mediated cell
apoptosis is crucial in I/R-induced organ damage (27). Furthermore, an interaction
mechanism exists between caspase-3 and Bcl-2; the expression of
caspase-3 is negatively regulated by Bcl-2, caspase-3 subsequently
hydrolyzes Bcl-2 and the hydrolyzed fragment of Bcl-2 exerts a
pro-apoptotic effect (28,29). In the present study, the expression
levels of caspase-3 and -8 were investigated and it was identified
that GP significantly inhibited the upregulation of these two
caspase family members in the I/R-liver tissues in mice. These
findings indicated that GP inhibits I/R-induced hepatic cell
apoptosis, potentially via the downregulation of caspase-3 and -8.
In addition, GP may maintain the balance between caspase-3 and
Bcl-2.
Furthermore, during mitochondria-mediated apoptosis,
the mitochondrial membrane potential collapses, which leads to
further mitochondrial depolarization and cytochrome c moves
into the cytosol, thus activating an apoptotic cascade (30). Therefore, the cytochrome c
level in the cytosol was examined in the present study and it was
identified that the administration of GP attenuated the increase of
cytochrome c in the cytosol within I/R-liver tissues.
In conclusion, the present study showed that oral
administration of GP effectively protected against I/R-induced
hepatic injury, predominantly via the attenuation of oxidative
damage as well as by inhibiting cell apoptosis. Therefore, GP may
serve as a promising agent for the treatment of hepatic I/R
injury.
Acknowledgements
The present study was supported by the Scientific
and Technological Brainstorm Project of Wuhan City (grant no.
201161038344-01), the Natural Science Fund of Hubei Province (grant
no. 2012FFA044) and the Public Service Platform Construction
Projects of Wuhan Technology Bureau (grant no.
2013060705010326).
References
|
1
|
Mendes-Braz M, Elias-Miró M,
Jiménez-Castro MB, Casillas-Ramírez A, Ramalho FS and Peralta C:
The current state of knowledge of hepatic ischemia-reperfusion
injury based on its study in experimental models. J Biomed
Biotechnol. 2012:2986572012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu HC, Bai L, Yue SQ, et al: Notch signal
protects non-parenchymal cells from ischemia/reperfusion injury in
vitro by repressing ROS. Ann Hepatol. 12:815–821. 2013.PubMed/NCBI
|
|
3
|
Zhang A, Chi X, Luo G, et al: Mast cell
stabilization alleviates acute lung injury after orthotopic
autologous liver transplantation in rats by downregulating
inflammation. PLoS One. 8:e752622013. View Article : Google Scholar
|
|
4
|
Niu Y, Yan W, Lv J, Yao W and Yu LL:
Characterization of a novel polysaccharide from tetraploid
Gynostemma pentaphyllum makino. J Agric Food Chem.
61:4882–4889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qin R, Zhang J, Li C, et al: Protective
effects of gypenosides against fatty liver disease induced by high
fat and cholesterol diet and alcohol in rats. Arch Pharm Res.
35:1241–1250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang G, Zhao Z, Gao L, et al: Gypenoside
attenuates white matter lesions induced by chronic cerebral
hypoperfusion in rats. Pharmacol Biochem Behav. 99:42–51. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang GL, Deng JP, Wang BH, et al:
Gypenosides improve cognitive impairment induced by chronic
cerebral hypoperfusion in rats by suppressing oxidative stress and
astrocytic activation. Behav Pharmacol. 22:633–644. 2011.
View Article : Google Scholar
|
|
8
|
Chen JC, Tsai CC, Chen LD, Chen HH and
Wang WC: Therapeutic effect of gypenoside on chronic liver injury
and fibrosis induced by CCl4 in rats. Am J Chin Med. 28:175–185.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi G, Zhang L, Xie WL, Chen XY and Li JS:
Protective effect of gypenosides on DNA and RNA of rat neurons in
cerebral ischemia-reperfusion injury. Acta Pharmacol Sin.
21:1193–1196. 2000.PubMed/NCBI
|
|
10
|
Zhang SC, Shi Q, Feng YN and Fang J:
Tissue-protective effect of glutamine on hepatic
ischemia-reperfusion injury via induction of heme oxygenase-1.
Pharmacology. 91:59–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aktan F, Henness S, Roufogalis BD and
Ammit AJ: Gypenosides derived from Gynostemma pentaphyllum
suppress NO synthesis in murine macrophages by inhibiting iNOS
enzymatic activity and attenuating NF-kappaB-mediated iNOS protein
expression. Nitric Oxide. 8:235–242. 2003.
|
|
12
|
Chen JC, Chung JG and Chen LD: Gypenoside
induces apoptosis in human Hep3B and HA22T tumour cells. Cytobios.
100:37–48. 1999.PubMed/NCBI
|
|
13
|
Farmer EE and Mueller MJ: ROS-mediated
lipid peroxidation and RES-activated signaling. Annu Rev Plant
Biol. 64:429–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi Y, Pulliam DA, Liu Y, et al: Reduced
mitochondrial ROS, enhanced antioxidant defense, and distinct
age-related changes in oxidative damage in muscles of long-lived
Peromyscus leucopus. Am J Physiol Regul Integr Comp Physiol.
304:R343–R355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amaral S, Redmann K, Sanchez V, Mallidis
C, Ramalho-Santos J and Schlatt S: UVB irradiation as a tool to
assess ROS-induced damage in human spermatozoa. Andrology.
1:707–714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng Q, Li X, Peng J, Duan X, Fu Q and Hu
Y: Effect of gypenosides on DMN-induced liver fibrosis in rats.
Zhongguo Zhong Yao Za Zhi. 37:505–508. 2012.(In Chinese).
|
|
17
|
Kim J, Kim HY and Lee SM: Protective
effects of geniposide and genipin against hepatic
ischemia/reperfusion injury in mice. Biomol Ther (Seoul).
21:132–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sass G, Barikbin R and Tiegs G: The
multiple functions of heme oxygenase-1 in the liver. Z
Gastroenterol. 50:34–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong Z and Lemasters JJ: Role of free
radicals in failure of fatty liver grafts caused by ethanol.
Alcohol. 34:49–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dinnen RD, Mao Y, Qiu W, et al:
Redirecting apoptosis to aponecrosis induces selective cytotoxicity
to pancreatic cancer cells through increased ROS, decline in ATP
levels, and VDAC. Mol Cancer Ther. 12:2792–2803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shamas-Din A, Kale J, Leber B and Andrews
DW: Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb
Perspect Biol. 5:a0087142013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Renault TT and Manon S: Bax: Addressed to
kill. Biochimie. 93:1379–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou F, Yang Y and Xing D: Bcl-2 and
Bcl-xL play important roles in the crosstalk between autophagy and
apoptosis. FEBS J. 278:403–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Appelqvist H, Wäster P, Eriksson I,
Rosdahl I and Ollinger K: Lysosomal exocytosis and caspase-8
mediated apoptosis in UVA-irradiated keratinocytes. J Cell Sci.
126:5578–5584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin Y, Vanden Hoek TL, Wojcik K, et al:
Caspase-dependent cytochrome c release and cell death in chick
cardiomyocytes after simulated ischemia-reperfusion. Am J Physiol
Heart Circ Physiol. 286:H2280–H2286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kale J, Liu Q, Leber B and Andrews DW:
Shedding light on apoptosis at subcellular membranes. Cell.
151:1179–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zakeri Z and Lockshin RA: Cell death:
history and future. Adv Exp Med Biol. 615:1–11. 2008. View Article : Google Scholar
|
|
30
|
Bernardi P and Rasola A: Calcium and cell
death: the mitochondrial connection. Subcell Biochem. 45:481–506.
2007. View Article : Google Scholar : PubMed/NCBI
|