Introduction
Regardless of advances in their diagnosis and
treatment, gastric and colorectal cancer remain a common cause of
cancer-related mortality worldwide (1,2).
Acquiring curative therapy by surgery or radiotherapy is complex
for patients with advanced gastric cancer (AGC) or advanced
colorectal cancer (ACRC); therefore, systemic chemotherapy is
considered to be the primary effective treatment.
Traditional continuous infusion of 5-fluorouracil
(5-FU) in combination with folinic acid has been the primary
chemotherapeutic treatment for AGC and ACRC. However, continuous
infusion requires an indwelling central venous catheter; thus,
there is an increased risk of infection and thrombosis, in addition
to the requirement for regular hospital visits. Therefore,
administration of the traditional 5-FU-based chemotherapy (FBCT) is
time-consuming, uncomfortable and inconvenient for patients.
Recently, oral fluoropyrimidines have been developed
as a substitution for 5-FU infusion therapy. Capecitabine is an
oral fluoropyrimidine that was designed to simulate a continuous
intravenous infusion of 5-FU (3)
and has been approved for the treatment of patients with AGC and
ACRC. S-1 is another treatment, which is a combination of three
pharmacological compounds: Tegafur, gimeracil and oteracil
potassium at a molar ratio of 1:0.4:1, respectively (4), which has been identified as a
suitable alternative to 5-FU for the treatment of patients with
ACRC or AGC (5,6).
As the efficacy and safety of oral
fluoropyrimidines, capecitabine and S-1, have been confirmed, it is
necessary to identify which treatment exhibits a greater efficacy
and safety profile for AGC and ACRC patients. To date, there have
been a series of trials comparing S-1 with capecitabine as part of
a mono or combined therapeutic treatment (7–17);
however, a single study may not be sufficient to comprehensively
assess their efficacy and safety. Moreover, to the best of our
knowledge, a meta-analysis of S-1-based chemotherapy (SBCT) versus
capecitabine-based chemotherapy (CBCT) for ACRC or AGC has not been
conducted. Therefore, the present meta-analysis of eligible studies
was performed to compare the two treatment approaches and evaluate
their clinical efficacy and safety in patients with AGC and
ACRC.
Materials and methods
Literature search
A comprehensive search was conducted using PubMed
(http://www.ncbi.nlm.nih.gov/pubmed),
Embase (https://www.embase.com), the Cochrane
Library (http://www.thecochranelibrary.com) and the Chinese
Biological Medical Database (http://www.sinomed.ac.cn/zh/) to identify randomized
controlled trials (RCTs) from inception of the databases to August
31st 2013. Various combinations of different terms, such
as ‘gastric cancer’, ‘colorectal cancer’, ‘capecitabine’, ‘S-1’,
‘randomized’ and their synonyms served as search terms. The
following search strategy of Embase was used: i) ‘gastric
neoplasm’: ab, ti OR ‘gastric cancer’: ab, ti OR ‘gastric
carcinoma’: ab, ti OR ‘stomach cancer’: ab, ti OR ‘cancer of
stomach’: ab, ti OR ‘stomach neoplasm’: ab, ti OR ‘stomach
cancer’/exp OR ‘colorectal carcinoma’: ab, ti OR ‘colorectal
cancer’: ab, ti OR ‘colorectal neoplasm’: ab, ti OR ‘rectal
cancer’: ab, ti OR ‘colon cancer’: ab, ti OR ‘intestinal cancer’:
ab, ti OR ‘colorectal carcinoma’/exp. ii) ‘tegafur gimeracil
oteracil potassium’: ab, ti OR ‘S-1’: ab, ti OR ‘TS-1’: ab, ti OR
‘gimeracil plus oteracil potassium plus tegafur’/exp. iii)
‘capecitabine’: ab, ti OR ‘xeloda’: ab, ti OR ‘capecitabine’/exp.
iv) random*: ab, ti OR ‘randomized controlled trial’: ab, ti OR
‘controlled clinical trial’: ab, ti OR ‘randomized controlled
trial’/exp OR ‘randomized controlled trial (topic)’/exp. v) #1 AND
#2 AND #3 AND #4. This strategy was applied to the search of other
databases.
In addition, all of the abstracts from the American
Society of Clinical Oncology conferences (http://meetinglibrary.asco.org/) that were held
between 2003 and 2013 were searched to identify the relevant RCTs
and references that were cited in the identified articles were
searched manually. The search was conducted without any restriction
on language.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were delineated
prior to commencement of the literature search. The eligible
studies were included in the present meta-analysis if they met all
of the following criteria: i) Participants were patients with
histologically confirmed, advanced, recurrent or metastatic
colorectal or gastric cancer and did not present with severe, basic
diseases which may affect the treatment effect of patients
(including cardiovascular and cerebrovascular diseases); ii) only
RCTs were considered; iii) trials compared SBCT with CBCT,
particularly mono- or combined therapy of S-1 versus capecitabine,
without confusion resulting from the administration of additional
drugs or interventions (for example, experimental and control arms
exhibited differences between S-1 and capecitabine components alone
within a combination therapy). Accordingly, the following exclusion
criteria were used: i) Cross-over studies; ii) non-randomized or
single-arm phase II trials; iii) any review, letter, case report or
comment; iv) for repeated published articles or the same study of a
different follow-up period, the study with the strictest
methodology and most complete data was selected and the other
excluded.
Data extraction
The essential data was independently extracted from
the eligible studies by two investigators and any discrepancies
were resolved by a consensus between the two. Information was
collected from each study as follows: The first author’s name,
publishing year, country/region of origin, study design,
characteristics of the participants, interventions conducted and
outcomes. When the hazard ratio (HR) of overall survival (OS),
progression-free survival (PFS) and time to progression (TTP) could
not be directly extracted from the original reports, they were
extracted from Kaplan-Meier curves as reported by Tierney et
al (18).
Quality assessment of the included
studies
The quality of the eligible studies was assessed by
two investigators independently and any disagreements were resolved
by a third investigator. According to the Cochrane Collaboration’s
tool for assessing risk of bias of RCTs (5.1.0) (19), the following criteria were used to
appraise the RCTs included in full texts: Random sequence
generation, allocation concealment, binding of participants and
personnel, binding of outcome assessments, incomplete outcome data,
selective reporting and other bias. In all cases, high, low or
unclear risk was used to evaluate the risk of bias; when there was
insufficient detail included in the study, the judgment was that
the risk of bias was unclear.
Statistical analysis
Statistical analysis of the HR and 95% confidence
interval (CI) for OS, PFS and TTP, in addition to the odds ratio
(OR) and 95% CI for the overall response rate (ORR) and grade 3 or
4 adverse events (AEs) were calculated using RevMan 5.1.0 software.
The ORR was defined as the sum of the partial and complete response
rates according to the Response Evaluation Criteria in Solid Tumors
(20). A fixed-effects model was
initially used and the Q test and I2 statistical test
were subsequently performed to assess the heterogeneity between
studies; P<0.1 was considered to indicate a statistically
significant difference. When there was heterogeneity across the
trials, sensitivity analysis or a randomized-effect model was
applied to overcome this shortcoming, and the process of
sensitivity analysis excluding the study firstly according to
different inclusion criteria and then re-analyzing the remaining
studies. For the results mentioned above (not including the
heterogeneity test): P<0.05 was considered to indicate a
statistically significant difference. OR>1 indicated a favorable
outcome in the S-1-based group; however, it indicated a greater
level of toxicity and a HR>1 demonstrated a greater number of
fatalities or progression with S-1-based regimens for OS or TTP and
PFS, respectively.
Results
Literature search
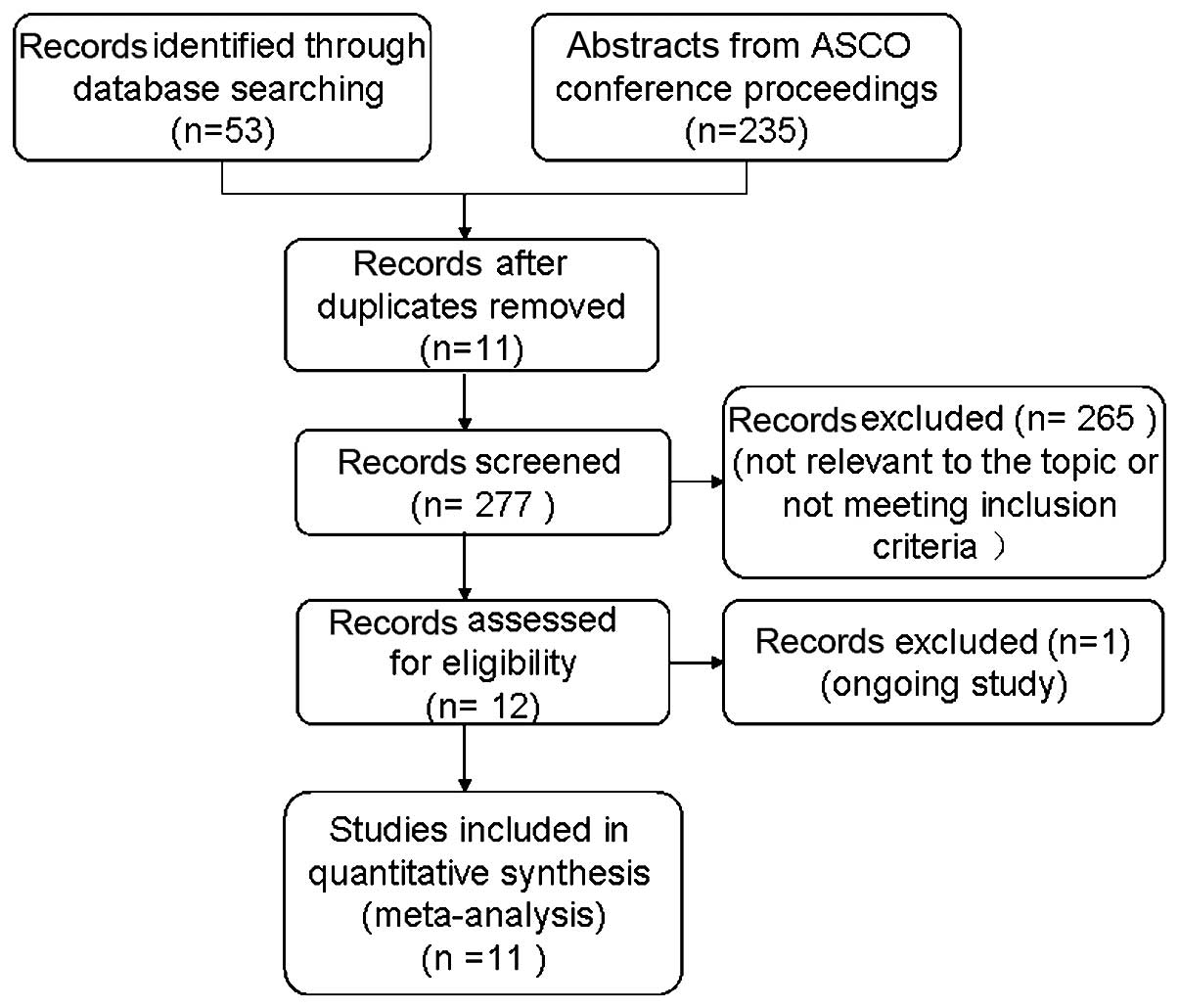
The search strategy yielded 288 records, of these,
11 duplicates were eliminated and 265 articles were excluded due to
irrelevancy or failing to meet the inclusion criteria, which was
determined by a review of the titles and abstracts. Further
evaluation of the remaining 12 studies revealed that one study was
ongoing (21). Thus, 11 studies
(7–17) qualified for inclusion in the
present meta-analysis (Fig. 1).
Table I displays the
characteristics of the 11 individual studies with respect to
author, year, country, demographic data, interventions and the
study outcomes.
 | Table IPredominant characteristics of the
studies included in the present meta-analysis. |
Table I
Predominant characteristics of the
studies included in the present meta-analysis.
| Study (ref.) | Country/tumor
type | No. of
patientsa | PS (score) | SBCT regimen | CBCT regimen | Outcome measures |
|---|
| Kim et al 2012
(8) | Korea/AGC | 65/64 | 0–2 | S-1: 80
mg/m2 d1–14, oxaliplatin: 130 mg/m2 d1,
q3w | Capecitabine: 2,000
mg/m2 d1–14
oxaliplatin: 130 mg/m2 d1, q3w | ORR, OS, TTP, QOL,
toxicities |
| Lee et al 2008
(9) | Korea/AGC | 45/46 | 0–2 | S-1: 40–60 mg, bid,
according to body surface area, d1–28, q6w | Capecitabine: 1,250
mg/m2, bid, d1–14, q3w | ORR, TTP, OS,
toxicities |
| Xiong et al
2013 (15) | China/AGC | 42/44 | >70b | Docetaxel: 25
mg/m2 d1 d8 d15, S-1: 80 mg/m2, d1–14,
q4w | Docetaxel: 25
mg/m2 d1 d8 d15, capecitabine: 1250 mg/m2
d1–14, q4w | ORR, MST,
toxicities |
| Zhang et al
2010 (17) | China/AGC | 17/19 | ≥60b | S-1: 80
mg/m2, d1–28, q6w | Capecitabine: 2500
mg/m2 d1–14, q3w | ORR,
toxicities |
| Ba et al
2012 (11) | China/AGC | 18/19 | 0–1 | S-1: 80
mg/m2 d1–14, cisplatin: 75 mg/m2 d1, q3w | Capecitabine: 2000
mg/m2 d1–14; cisplatin: 75 mg/m2 d1, q3w | ORR, TTP, OS,
toxicities |
| Yan et al
2012 (16) | China/AGC | 21/21 | - | S-1: 40
mg/m2, bid, d1–14, cisplatin: 75 mg/m2 d1,
q3w | Capecitabine: 1000
mg/m2, bid, d1–14; cisplatin: 75 mg/m2 d1,
q3w | ORR,
toxicities |
| Qiu et al
2011 (13) | China/AGC | 28/28 | ≥60b | S-1: 40
mg/m2, bid, d1–28, q5w | Capecitabine: 1,250
mg/m2, bid, d1–14, q3w | ORR,
toxicities |
| Hong et al
2012 (7) | Korea/ACRC | 168/172 | 0–2 | S-1: 40
mg/m2, bid, d1–14, oxaliplatin: 130 mg/m2 d1,
q3w | Capecitabine: 1000
mg/m2, bid, d1–14, oxaliplatin: 130 mg/m2 d1,
q3w | ORR, TTF, PFS, OS,
toxicities |
| Sun et al
2013 (14) | China/ACRC | 54/52 | >70b | S-1: 80
mg/m2, d1–14, q3w | Capecitabine: 2000
mg/m2, d1–14, q3w | ORR, MST,
toxicities |
| Lu et al
2012 (12) | China/ACRC | 26//27 | 0–2 | S-1: 80
mg/m2 d1–14; oxaliplatin: 130 mg/m2 d1,
q3w | Capecitabine: 2000
mg/m2 d1–14, oxaliplatin: 130 mg/m2 d1,
q3w | ORR,
toxicities |
| Zang et al
2012 (10) | Korea/ACRC | 43/45 | 0–2 | S-1: 80
mg/m2 d1–14, oxaliplatin: 130 mg/m2 d1,
q3w | Capecitabine: 2000
mg/m2 d1–14, oxaliplatin: 130 mg/m2 d1,
q3w | ORR, TTP, MST,
toxicities |
Quality of eligible studies
The present meta-analysis included 11 RCTs and all
of the studies included the term ‘random’, however, only three
studies (7,8,14)
specifically reported the methods that were utilized for random
sequence generation. Furthermore, only two studies (7,9)
adequately reported the reliability-determined allocation
concealment. Three trials (7,9,10)
were open-labeled and the other studies did not state whether a
blind method was adopted; however, these were unlikely to affect
the quality assessment results. One trial was an abstract and
included insufficient information regarding the outcome data,
selective reporting and other bias (10), additional trials satisfied the
criteria for complete outcome data and did not include selective
reporting or other bias (Table
II).
 | Table IIRisk of bias for each study. |
Table II
Risk of bias for each study.
| Risk of bias |
|---|
|
|
|---|
| Study (ref.) | A | B | C | D | E | F | G |
|---|
| Kim et al,
2012 (8) | Low | Unclear | Low | Low | Low | Low | Low |
| Lee et al,
2008 (9) | Unclear | Low | Low | Low | Low | Low | Low |
| Xiong et al,
2013 (15) | Unclear | Unclear | Low | Low | Low | Low | Low |
| Zhang et al,
2010 (17) | Unclear | Unclear | Low | Low | Low | Low | Low |
| Ba et al,
2012 (11) | Unclear | Unclear | Low | Low | Low | Low | Low |
| Yan et al,
2012 (16) | Unclear | Unclear | Low | Low | Low | Low | Low |
| Qiu et al,
2011 (13) | Unclear | Unclear | Low | Low | Low | Low | Low |
| Hong et al,
2012 (7) | Low | Low | Low | Low | Low | Low | Low |
| Sun et al,
2013 (14) | Low | Unclear | Low | Low | Low | Low | Low |
| Lu et al,
2012 (12) | Unclear | Unclear | Low | Low | Low | Low | Low |
| Zang et al,
2012 (10) | Unclear | Unclear | Low | Low | Unclear | Unclear | Unclear |
Tumor response
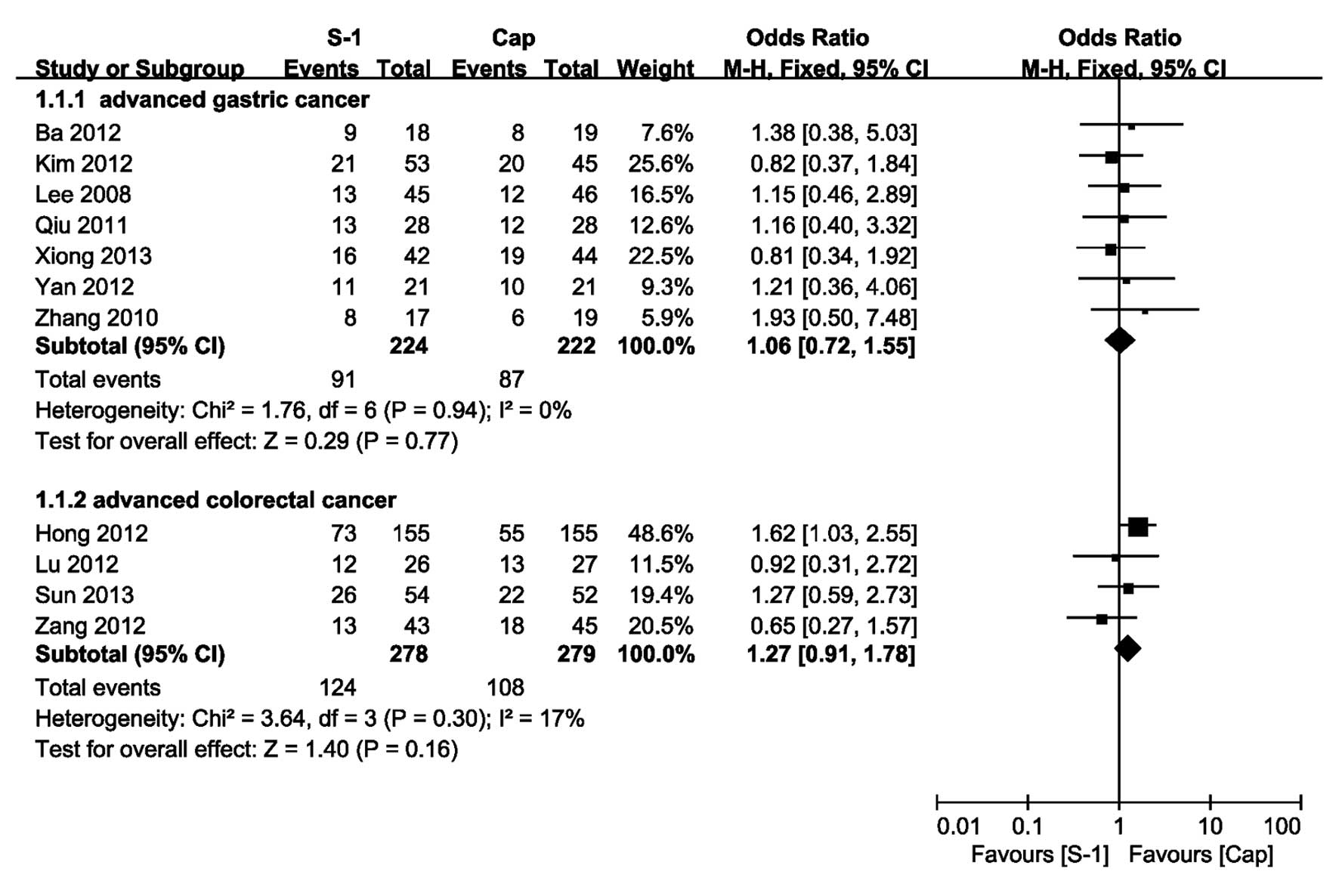
All of the included studies provided the information
on ORR and the pooled OR of ORR for AGC of the fixed-effect model
was 1.06 (95% CI, 0.72 and 1.55) with no heterogeneity (P=0.94,
I2=0%; Fig. 2). For
ACRC, there was no heterogeneity identified across the trials
(P=0.30, I2=17%) and the pooled OR of ORR using the
fixed-effect model was 1.27 (95% CI, 0.91 and 1.78). The OR
indicated that there was no significant difference between the SBCT
and CBCT group.
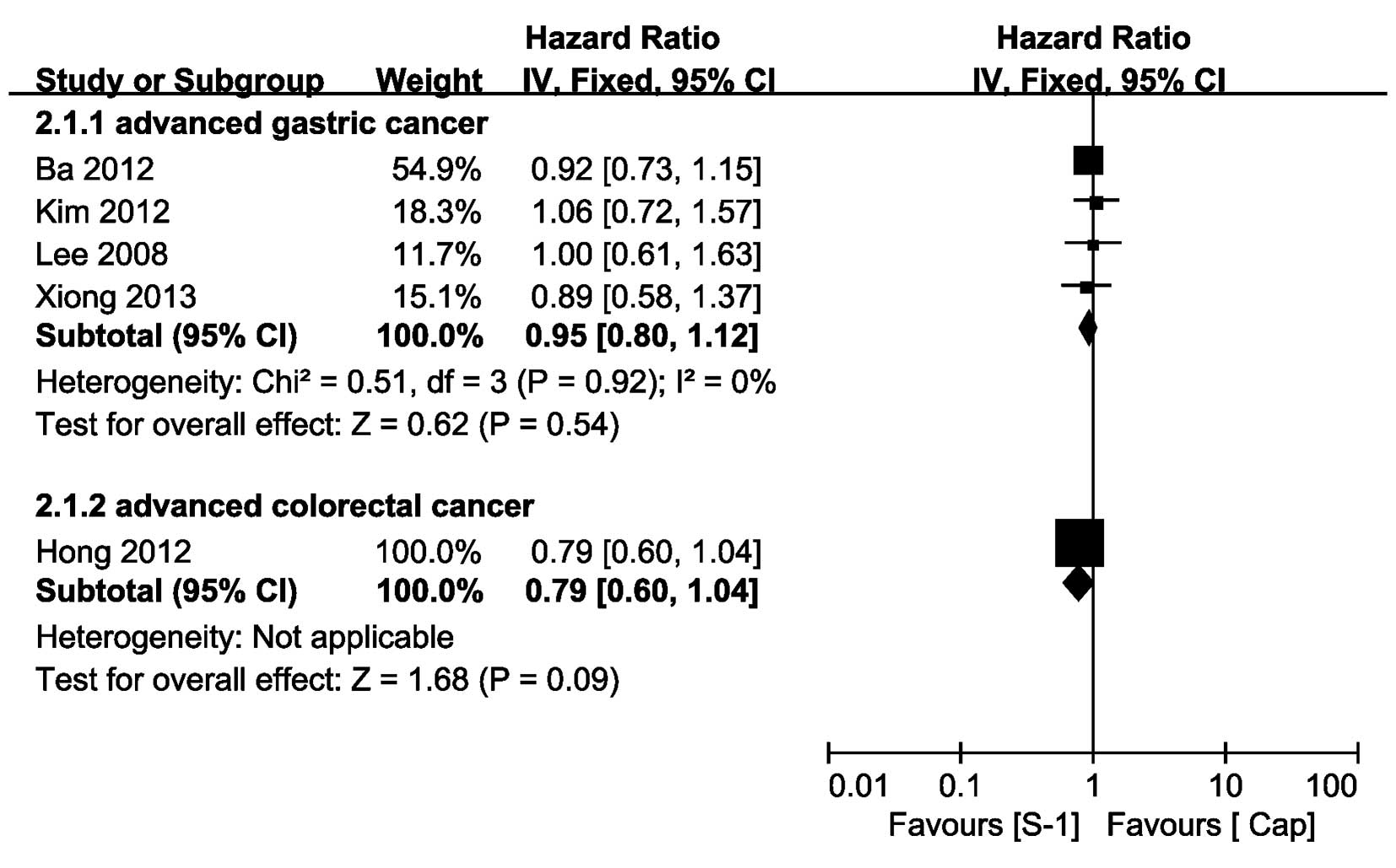
TTP or PFS
Regarding AGC, four trials provided TTP information
(8,9,11,15),
of which the TTP HR of the trial reported by Xiong et al
(15) was extracted from the
Kaplan-Meier curves that were presented in the study. The pooled HR
of TTP indicated that there was no significant difference between
the SBCT and CBCT group (HR=0.95, 95% CI, 0.80–1.12) and the pooled
HR was performed using a fixed-effect model, with no heterogeneity
observed (P=0.92, I2=0%). Concerning ACRC, no trials
provided the HR of TTP and only one trial provided PFS data
(7); moreover, the HR of PFS
identified no significant difference between the SBCT and CBCT
group (HR=0.79, 95% CI, 0.60–1.04; Fig. 3).
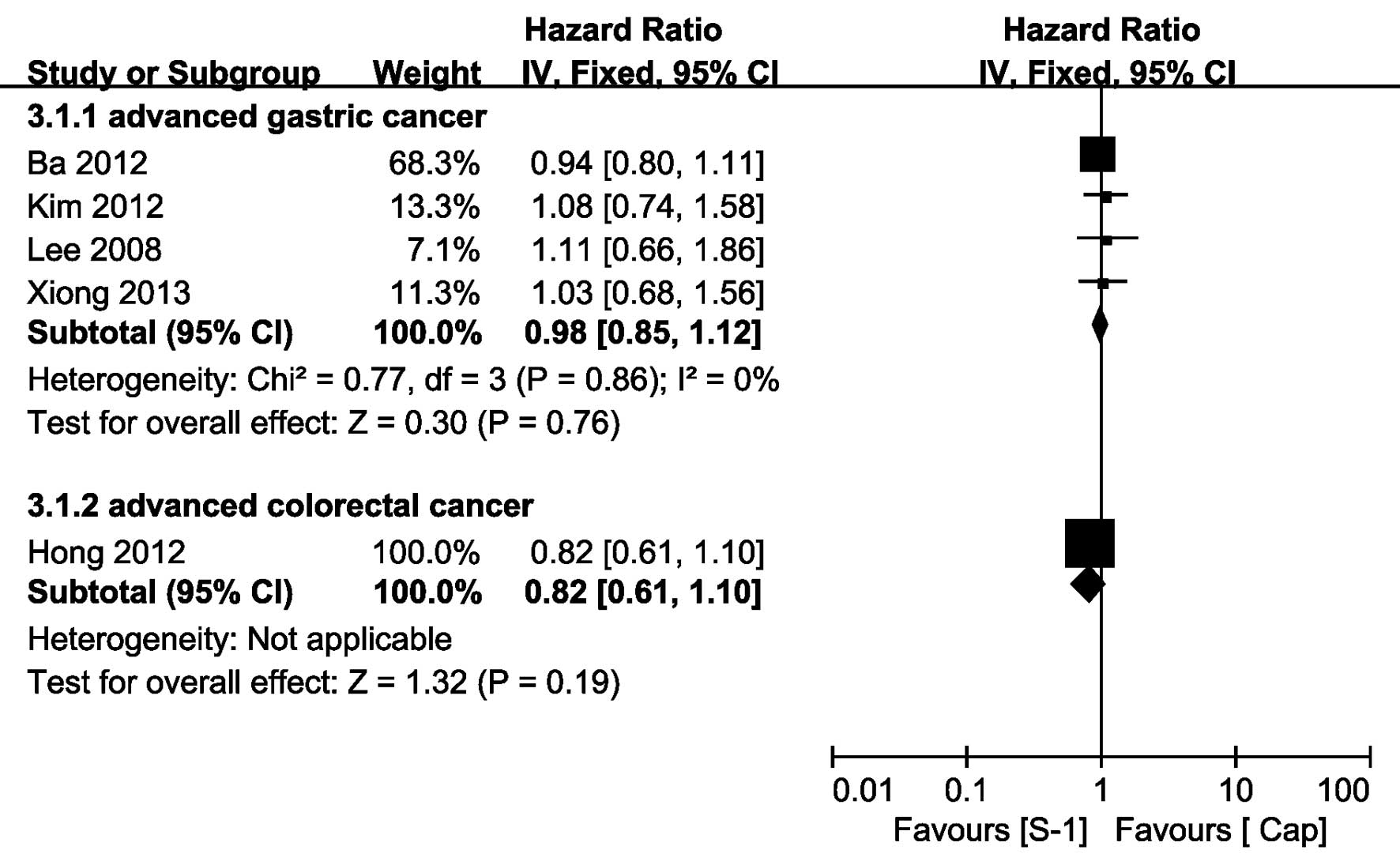
OS data
Five of the 12 trials provided OS data (7–9,11,15),
of which the OS HR of the trial, reported by Xiong et al
(15), was extracted from
the Kaplan-Meier curves that were presented in the study. Regarding
AGC, there was no significant heterogeneity observed between the
studies (P=0.86, I2=0%), the pooled HR of OS obtained
using a fixed-effect model showed no significant difference between
the SBCT and CBCT group, yielding a HR of 0.98 (95% CI, 0.85–1.12).
Concerning ACRC, only one trial provided the HR of OS (HR=0.82, 95%
CI, 0.61–1.10) (7), which
demonstrated that there was no significant difference between the
two groups (Fig. 4).
Safety profile
Neutropenia in hematologic
toxicities
A meta-analysis of seven trials (7–11,15,16)
regarding grade 3–4 neutropenia, which included 398 patients in the
SBCT group and 399 patients in the CBCT group, identified no
significant difference between the two groups (OR=1.17, 95%CI,
0.81–1.70), with significant heterogeneity observed across the
trials (P=0.02, I2=61%) and as the sensitivity analysis
did not identify the source of the heterogeneity, a random-effect
model was applied. The result of the random-effect model showed
that there was no significant difference between the two groups
(OR=0.79, 95% CI, 0.37–1.69).
Leucopenia
A meta-analysis of seven trials (7–9,12–14,17)
regarding grade 3–4 leucopenia, which included 401 patients in the
SBCT group and 400 patients in the CBCT group, showed no
significant difference between the two groups (OR=0.91, 95% CI,
0.37–2.24) and there was no significant heterogeneity identified
across the trials (P=0.72, I2=0%).
Thrombocytopenia
Nine trials (7–12,15–17)
reported grade 3–4 thrombocytopenia from the assessment of 889
participants (SBCT, n=441; CBCT, n=445). The pooled analysis showed
no significant difference between the two groups (OR=1.34, 95% CI,
0.89–2.02) and significant heterogeneity was demonstrated across
the trials (P=0.01, I2=62%). As the heterogeneity could
not be eliminated by conducting a sensitivity analysis, a
random-effect model was performed (OR=0.85, 95% CI, 0.36–1.97).
Anemia
Data concerning anemia was available from nine
trials (7–13,15,16),
which included 906 participants (SBCT, n=452; CBCT, n=454) in the
meta-analysis. The pooled OR of the nine trials indicated no
significant difference between the two groups (OR=1.61, 95% CI,
0.93–2.80) and there was no heterogeneity noted between the studies
(P=0.88, I2=0.0%).
Hand-foot syndrome (HFS) in
non-hematologic toxicities
Ten trials (7–13,15–17)
reported grade 3–4 HFS (SBCT, n=469; CBCT, n=473). The pooled OR of
grade 3–4 HFS (OR=0.15, 95% CI, 0.06–0.36) showed that there was a
statistically significant difference between the SBCT and CBCT
groups, with no significant heterogeneity observed across the
trials (P=0.99, I2=0%), which indicated that grade 3–4
HFS was less likely to occur in SBCT patients.
Diarrhea and nausea/vomiting
Ten studies (7–9,11–17)
that included 482 patients in the SBCT group and 484 patients in
the CBCT group, provided information on cases of grade 3–4 diarrhea
and nausea/vomiting. The meta-analysis of the 10 trials showed no
significant difference between the two groups (diarrhea: OR=1.00,
95% CI, 0.56–1.78; nausea/vomiting: OR=0.83, 95% CI, 0.48–1.43) and
there was no heterogeneity identified.
Stomatitis
Eight trials (7,9,11,13–17)
reported grade 3–4 stomatitis, these trials included 391 patients
in the SBCT group and 393 patients in the CBCT group. The
meta-analysis of the fixed-effects model indicated no significant
difference between the two groups (OR=1.23, 95% CI, 0.37–4.12;
heterogeneity: P=0.88, I2=0%).
The results of the grade 3 and 4 AE analyses are
displayed in Table III.
 | Table IIIOutcome of the toxicity meta-analysis
comparing SBCT with CBCT in advanced gastric and colorectal
cancer. |
Table III
Outcome of the toxicity meta-analysis
comparing SBCT with CBCT in advanced gastric and colorectal
cancer.
| | | | Heterogenity | | | |
|---|
| | | |
| | | |
|---|
| Toxicity | Trials | SBCT | CBCT | P-value | I2
(%) | OR (95% CI) | Model | References |
|---|
| Grade 3–4
anemia | 9 | 35/452 | 23/454 | 0.88 | 0 | 1.61 (0.93,
2.80) | Fixed | (7–13,15,16) |
| Grade 3–4
leucopenia | 7 | 9/401 | 10/400 | 0.72 | 0 | 0.91 (0.37,
2.24) | Fixed | (7–9,12–14,17) |
| Grade 3–4
neutropenia | 7 | 72/398 | 63/399 | 0.02 | 61 | 0.79 (0.37,
1.69) | Random | (7–11,15,16) |
| Grade 3–4
thrombocytopenia | 9 | 61/441 | 47/445 | 0.01 | 62 | 0.85 (0.36,
1.97) | Random | (7–12,15–17) |
| Grade 3–4
diarrhea | 10 | 21/482 | 21/484 | 0.42 | 2 | 1.00 (0.56,
1.78) | Fixed | (7–9,11–17) |
| Grade 3–4
nausea/vomiting | 10 | 23/482 | 28/484 | 0.69 | 0 | 0.83
(0.48,1.43) | Fixed | (7–9,11–17) |
| Grade 3–4
stomatitis | 8 | 5/391 | 4/393 | 0.88 | 0 | 1.23 (0.37,
4.12) | Fixed | (7,9,11,13–17) |
| Grade 3–4 HFS | 10 | 3/469 | 33/473 | 0.99 | 0 | 0.15 (0.06,
0.36) | Fixed | (7–13,15–17) |
Discussion
To the best of our knowledge, this was the first
meta-analysis to evaluate the efficacy and safety profile of SBCT
versus CBCT for AGC or ACRC. A total of 1,064 patients from 11
RCTs, including 527 patients in the SBCT group and 537 patients in
the CBCT group were analyzed. On the basis of intention-to-treat
analysis, with respect to ORR, TTP and OS, the present
meta-analysis showed no significant differences between the SBCT
and CBCT group for AGC, which suggested that SBCT was not inferior
to CBCT for patients with AGC. With regards to ACRC, the pooled
analysis indicated that there was no significant difference between
the SBCT and CBCT groups concerning the ORR, PFS and OS, which
indicated that SBCT exhibited similar efficacy to CBCT for patients
with ACRC.
With regard to the safety profile, although the
present meta-analysis indicated that grade 3–4 HFS was less likely
to occur in SBCT patients (OR=0.15, 95% CI, 0.06–0.36), grade 3–4
toxicities, such as anemia, leucopenia, neutropenia,
thrombocytopenia, nausea/vomiting, diarrhea and stomatitis were
identified to be similarly prevalent between the two groups. The
types of toxicities were deemed to be manageable, predictable and
tolerable. Therefore, compared with CBCT, SBCT exhibited an
approximately equivalent safety profile for patients with AGC and
ACRC.
Owing to the significant heterogeneity observed
between grade 3–4 neutropenia and grade 3–4 thrombocytopenia, a
sensitivity analysis was performed; however, the factors
contributing to the heterogeneity could not be identified,
therefore, they may be associated with variations in age,
performance status of patients, dose and the regimen of therapy
between the trials. Thus, a random-effects model was applied to
compensate for this and the conclusion did not alter relative to
the result of the fixed-effects model, which confirmed the
reliability of our outcome.
Of note, the patient population included in the
present meta-analysis was markedly heterogeneous, i.e. the patients
exhibited metastatic colorectal or advanced gastric cancer. Thus,
subgroup analysis by tumor type was performed on ORR, TTP and OS;
the findings of which indicated that the non-inferiority of SBCT
versus CBCT could be applied to all of the patient subgroups that
were considered within the present analysis. However, as the type
of toxicity was not specific to a certain type of tumor for the
same drug and AGC and ACRC are gastrointestinal cancers, a greater
significance was determined from the results of the overall
meta-analysis of grade 3–4 AEs for all of the patients, rather than
the results from the subgroup population analysis.
In 2009, a meta-analysis of individual patient data
was conducted by Okines et al (22) and demonstrated that OS was superior
in patients with AGC that were treated with CBCT compared with the
patients with AGC that were treated with FBCT (HR=0.87, 95% CI,
0.77–0.98). Similarly, a meta-analysis was performed by Huang et
al (23) comparing the use of
FBCT, which identified a significant OS benefit in favor of SBCT
for patients with AGC (HR=0.87, 95% CI, 0.79–0.96). The data from
these two meta-analyses indirectly showed that SBCT exhibited a
similar efficacy to CBCT for AGC patients, which was in line with
the present study.
In addition, S-1 and capecitabine possess different
optimal doses, efficacy and safety among patients that are from
different regions. Based on the metabolic pathway, capecitabine
exhibited no obvious racial difference concerning the dose and
efficacy regardless of a lower grade 3–4 gastrointestinal toxicity
when compared with FBCT in Asian patients (24). However, as the polymorphic variants
of the liver enzyme gene, cytochrome P450 (CYP) 2A6, which converts
tegafur to 5-FU, were more frequent in Asians than in Caucasians,
there were certain racial differences identified regarding S-1
tolerance, which results in varying optimal doses of S-1 for
different races (25). The study
by Kong et al showed that the CYP2A6 genotype was associated
with the treatment efficacy of SBCT in AGC patients (26) and it may result in certain racial
differences in S-1 efficacy. In 2009, the updated results of a
randomized phase III study, conducted by Fuse et al
(27), demonstrated that S-1 was
associated with an increased OS compared with 5-FU in Japanese
patients with AGC (HR=0.83, 95% CI, 0.68–1.00, P=0.02). However, in
2010 the findings of a RCT conducted by Ajani et al
(28) showed that SBCT did not
prolong the OS of Western patients with AGC when compared with FBCT
(HR=0.92, 95% CI, 0.80–1.05, P=0.20).
There were certain limitations in the present
meta-analysis. As all of the studies included were from Asia, the
present results require confirmation from studies conducted on
patients from a Western population. Moreover, the quantity of
included studies was small and there may have been publication
bias. The quality of the studies was not considered to be high,
with just three studies (7,8,14)
reporting the specific methods of random sequence generation and
two studies (7,9) reporting with adequate reliability the
method by which the allocation concealment was determined; thus, a
greater number of RCTs with improved methodologies are required to
update the present study. Furthermore, information was not obtained
from individual patients for each trial, which would have resulted
in a more comprehensive analysis and, finally, heterogeneous
results were included.
In conclusion, the present meta-analysis indicated
that SBCT exhibited a comparable efficacy and approximately
equivalent safety profile in patients with AGC and ACRC compared
with CBCT; therefore, it was considered to be an effective
alternative to CBCT. However, further investigation, which includes
large-scale prospective studies with an adequate methodological
quality and legitimate control measures that account for possible
confounding factors are required to confirm or update the present
study, particularly including patients from a Western
population.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyerhardt JA and Mayer RJ: Systemic
therapy for colorectal cancer. N Engl J Med. 352:476–487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miwa M, Ura M, Nishida M, et al: Design of
a novel oral fluoropyrimidine carbamate, capecitabine, which
generates 5-fluorouracil selectively in tumours by enzymes
concentrated in human liver and cancer tissue. Eur J Cancer.
34:1274–1281. 1998. View Article : Google Scholar
|
|
4
|
Shirasaka T, Shimamato Y, Ohshimo H, et
al: Development of a novel form of an oral 5-fluorouracil
derivative (S-1) directed to the potentiation of the tumor
selective cytotoxicity of 5-fluorouracil by two biochemical
modulators. Anticancer Drugs. 7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu RH, Sun GP, Lu HS, et al: A phase III
study of S-1 plus cisplatin versus fluorouracil plus cisplatin in
patients with advanced gastric or gastroesophageal junction
adenocarcinoma. Journal of Clinical Oncology. 31:2013.PubMed/NCBI
|
|
6
|
Muro K, Boku N, Shimada Y, et al:
Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid
plus irinotecan (FOLFIRI) as second-line chemotherapy for
metastatic colorectal cancer: a randomised phase 2/3
non-inferiority study (FIRIS study). Lancet Oncol. 11:853–860.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong YS, Park YS, Lim HY, et al: S-1 plus
oxaliplatin versus capecitabine plus oxaliplatin for first-line
treatment of patients with metastatic colorectal cancer: a
randomised, non-inferiority phase 3 trial. Lancet Oncol.
13:1125–1132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim GM, Jeung HC, Rha SY, et al: A
randomized phase II trial of S-1-oxaliplatin versus
capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer.
48:518–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JL, Kang YK, Kang HJ, et al: A
randomised multicentre phase II trial of capecitabine vs S-1 as
first-line treatment in elderly patients with metastatic or
recurrent unresectable gastric cancer. Br J Cancer. 99:584–590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zang DY, Chung IJ, Oh HS, Park KU, Lee KH,
Han B, Choi DR, Kim HS and Kim JH: Randomized phase II study of
oxaliplatin and S-1 (OS) versus oxaliplatin and capecitabine
(XELOX) in patients with metastatic or recurrent colorectal cancer.
J Clin Oncol. 30:5952012.
|
|
11
|
Ba Z, Wu M, Wang LJ and Zhang HQ: Curative
effect analysis of S-1 combined with cisplatin or capecitabine
combined with cisplatin in the treatment of advanced gastric
cancer. Chinese General Practice. 15:672–676. 2012.
|
|
12
|
Lu HM, Zhou LL, Liu JJ and Liu SH:
Clinical study of oxaliplatin plus TS-1 or capecitabine in the
treatment of advanced colon cancer. Cancer Research and Clinic.
24:613–615. 2012.
|
|
13
|
Qiu GQ, Xu LZ, Lin ZC and Chen YQ:
Clinical observation of S-1 in the treatment of old patients with
advanced gastric carcinoma. Chin J Clin Oncol Rehabil. 18:61–63.
2011.
|
|
14
|
Sun DS, Zhang GR, Ma LG and Zou YH: The
comparative effect of capecitabine and
tegafur/gimeracil/oteracil(S-1) on advanced colorectal cancer. Chin
J Prim Med Pharm. 20:826–827. 2013.
|
|
15
|
Xiong HL, Liu XQ, Sun AH, He Y, Li J and
Yuan X: Clinical comparison of docetaxel combined with S-1 or
Capicitabine in treating advanced gastric carcinoma. Modern
Oncology. 21:581–584. 2013.
|
|
16
|
Yan Z, Zhao TX, Xing Y, Chen D and He LL:
Curative effect analysis of S-1 combined with cisplatin in the
treatment of advanced gastric cancer. Progress in Modern
Biomedicine. 12:5324–5326. 2012.
|
|
17
|
Zhang HY and Sun LZ: The clinical study of
domestic compound Tegafur capsule first-line Therapy for patients
with elderly advanced gastric cancer. Medical Innovation of China.
7:94–96. 2010.
|
|
18
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins J and Green S: The Chochrane
Collaboration: Cochrane Handbook for Systematic Reviews of
Interventions. http://www.cochranehandbook.orguri.
Accessed August 31, 20132011
|
|
20
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuburaya A, Morita S, Kodera Y, et al: A
randomized phase II trial to elucidate the efficacy of capecitabine
plus cisplatin (XP) and S-1 plus cisplatin (SP) as a first-line
treatment for advanced gastric cancer: XP ascertainment vs. SP
randomized PII trial (XParTS II). BMC Cancer. 12:3072012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okines AFC, Norman AR, McCloud P, Kang YK
and Cunningham D: Meta-analysis of the REAL-2 and ML17032 trials:
evaluating capecitabine-based combination chemotherapy and infused
5-fluorouracil-based combination chemotherapy for the treatment of
advanced oesophago-gastric cancer. Ann Oncol. 20:1529–1534. 2009.
View Article : Google Scholar
|
|
23
|
Huang J, Cao Y, Wu L, Liao C, He Y and Gao
F: S-1-based therapy versus 5-FU-based therapy in advanced gastric
cancer: a meta-analysis. Med Oncol. 28:1004–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma Y, Tang L, Wang HX, Xu YC, Ma Y and
Zhang FC: Capecitabine for the treatment for advanced gastric
cancer: efficacy, safety and ethnicity. J Clin Pharm Ther.
37:266–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ajani JA, Faust J, Ikeda K, et al: Phase I
pharmacokinetic study of S-1 plus cisplatin in patients with
advanced gastric carcinoma. J Clin Oncol. 23:6957–6965. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kong SY, Lim HS, Nam BH, et al:
Association of CYP2A6 polymorphisms with S-1 plus docetaxel therapy
outcomes in metastatic gastric cancer. Pharmacogenomics.
10:1147–1155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuse N, Fukuda H, Yamada Y, et al: Updated
results of randomized phase III study of 5-fluorouracil (5-FU)
alone versus combination of irinotecan and cisplatin (CP) versus
S-1 alone in advanced gastric cancer (JCOG 9912). J Clin Oncol
(ASCO Annual Meeting abstracts). 27:45142009.
|
|
28
|
Ajani JA, Rodriguez W, Bodoky G, et al:
Multicenter phase III comparison of cisplatin/S-1 with
cisplatin/infusional fluorouracil in advanced gastric or
gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin
Oncol. 28:1547–1553. 2010. View Article : Google Scholar
|