Introduction
Chronic obstructive pulmonary disease (COPD) is a
systemic inflammatory disease that primarily affects the lungs. It
is an important disease with high incidence and mortality rates
worldwide. Chronic inflammation is its basic pathology and the key
cause of morbidity (1). At
present, global guidelines for the management of COPD using Western
medicine include treatment with bronchodilators and oral or inhaled
corticosteroids to improve ventilation and alleviate airway
inflammation. Antibiotic treatment is recommended for infection
control in the acute phase, as well as the administration of
Pneumococcal vaccine or influenza vaccine, such as the H1N1
vaccine, in remission to reduce the recurrence of the disease
(2). However, these methods are
associated with several problems, particularly in relation to the
systemic effects of COPD, including poor symptom control, frequent
relapse and adverse drug reactions (3). Furthermore, there has been a lack of
focus on the resolution of these problems. For patients with COPD,
the systemic effects of the disease cause progressive
deterioration, including loss of appetite, severe malnutrition and
osteoporosis (4), and there are
few effective treatments available. Thus, the quality of life of
the patients becomes seriously diminished and disease prognosis
worsens.
Quanzhenyiqitang has been used to treat the systemic
effects of COPD. A study demonstrated that Quanzhenyiqitang was
capable of improving clinical signs and symptoms of patients with
chronic obstructive pulmonary emphysema (5). An experiment using a rat model of
COPD also indicated that Quanzhenyiqitang was capable of
significantly improving the cell morphology of damaged lung,
kidney, adrenal gland and testicular tissue (6). However, the mechanism by which
Quanzhenyiqitang improves damaged tissue has yet to be elucidated.
In this study, rat alveolar macrophages (AMs) were treated with
Quanzhenyiqitang-treated serum, and the ability of Quanzhenyiqitang
to induce apoptosis of inflammatory cells and to modulate the
expression of histone deacetylase 2 (HDAC2) was evaluated. The
anti-inflammatory effect and therapeutic mechanisms of
Quanzhenyiqitang in the treatment of COPD are discussed.
Materials and methods
Animals
Male 42-month-old Sprague Dawley rats (weight, 200±5
g) were provided by the Experimental Animal Center of B&K
Universal Group Limited (Shanghai, China). The rats were reared in
the animal housing facility of the Fujian Research Academy of
Traditional Chinese Medicine (Fuzhou, China) and were fed with
standard grain in the clean facilities of the animal room in
Pingshan College of the Fujian University of Traditional Chinese
Medicine (Fuzhou, China). Acclimation was performed for seven days
under the following conditions: free water and food, room
temperature of 20°C and natural light. Among the rats, 30 (of a
total of 40) were used for treated serum preparation; the others
were used as rat models of COPD.
Rat model of COPD
The rat model of COPD was prepared according to the
methodology of the Respiratory Department of Shuguang Hospital
Affiliated to Shanghai University of Traditional Chinese Medicine
(Shanghai, China) and combined with kidney deficiency (7). Rats were fed daily with 1% adenine
granulated feed (obtained from the Medical Research Institute of
Fujian Province, Fuzhou, China), provided with normal drinking
water, and subjected to smoke treatment with 250 ppm SO2
(Tianjin Specialty Gases Co., Ltd., Tianjin, China) for 5 h/day, 5
days/week for 7 weeks. This study was conducted in accordance with
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol was approved
by the Institutional Animal Care and Use Committee of the Second
Affiliated People’s Hospital of Fujian University of Traditional
Chinese Medicine (Fuzhou, China).
Grouping
Cultured AMs were randomly divided into four groups
as follows: Blank (group A), untreated serum (group B), serum
treated with Traditional Chinese Medicine (Quanzhenyiqitang; group
C), and serum treated with Western medicine (aminophylline; group
D).
Cell culture
Rats were anesthetized by intraperitoneal injection
of pentobarbital sodium (30 mg/kg body weight). The chest of each
rat was opened, and the right side of the main bronchus was tied.
The left lung was then lavaged four times with 5 ml saline solution
at 37°C, and the alveolar lavage fluid was collected and
centrifuged at 106 × g for 10 min at 4°C. The supernatant was
subsequently discarded, and the pellet was washed twice with
phosphate-buffered saline (PBS) for 10 min each time. The cells
were resuspended in low-sugar Dulbecco’s modified Eagle medium
(DMEM) with fetal bovine serum and counted. Cell viability was
measured to be >98%. The cells were then replated in six-well
plates (1 ml/well; 1×109 cells/l) and cultured at 37°C
in 5% CO2 for 2 h. Non-adherent cells were removed by
washing with PBS. Low-sugar, serum-free DMEM (1 ml) and macrophage
stimulating protein (50 μl) were added, prior to the cells being
incubated for 24 h and centrifuged at 45 × g for 10 min. The cell
culture supernatant was then collected.
Preparation of treated serum
A total of 30 rats were randomly and equally divided
into three groups. The untreated serum group was lavaged four times
with 5 ml saline solution. The Traditional Chinese Medicine group
was lavaged with Quanzhenyiqitang (15 g stewed, sun-cured ginseng,
15 g radix ophiopogonis, 15 g cooked rehmannia, 6 g light monkshood
that had been decocted for 20 min, 6 g Atractylodes, 15 g
Achyranthes and 6 g Schisandra; Fuzhou Tongchun Medicine Co., Ltd.,
Fuzhou, China). The Western medicine group was lavaged with
aminophylline (0.25 g/2 ml; batch no. 110402; Shanghai Xinyi Jinzhu
Pharmaceutical Co., Ltd., Shanghai, China) twice every day. All
groups were lavaged twice every day.
The rats were lavaged for seven days. On the seventh
day, the rats underwent a 12-h fast and were then lavaged once with
a full daily dose. At 1 h post-dose, the rats were anesthetized by
intraperitoneal injection of pentobarbital sodium (0.2 ml/100 g).
Following routine disinfection, 6 ml abdominal aortic blood was
collected from each rat and transferred to a negative-pressure
vessel using a puncture needle in aseptic conditions. The blood
samples were then placed in a water bath at 37°C for 15 min and
centrifuged at 402 × g for 15 min. The serum was filtered through a
0.22-μm microporous membrane and the cells were moved to new
Eppendorf tubes and stored at −20°C. The treated serum and
serum-free culture medium were combined in a proportion of 1:4 to
produce a new culture medium containing 20% treated serum.
Observation of apoptosis
The AM culture medium was purified and the cells
were cultured for 4 h. Fresh cell culture fluid was added and the
cell culture wells were divided into four experimental groups,
comprising a blank group and groups treated with untreated serum,
100 μl Quanzhenyiqitang-treated serum and 100 μl
aminophylline-treated serum, respectively. Following culture at
37°C with 5% CO2, the cells were harvested at 2, 4 and 6
h. Upon staining with an Annexin V-fluorescein
isothiocyanate/propidium iodide kit (Viaud Co. Ltd., Shanghai,
China), in accordance with the manufacturer’s instructions,
apoptotic cell morphology was observed under a fluorescence
microscope (Olypus, Tokyo, Japan). Flow cytometry was performed to
determine the rate of apoptosis of AMs at the 2-, 4- and 6-h
time-points.
Fluorescence quantitative polymerase
chain reaction (PCR)
In the previous experiment, apoptosis was observed
at the 6-h time-point. Therefore, cells harvested at the 6-h
time-point were used to assess gene expression. The cells were
washed twice with PBS, centrifuged and resuspended in
TRIzol® (Invitrogen Life Technologies, Carlsbad, CA,
USA), prior to being left to rest for 5 min at room temperature.
Chloroform (~200 μl) was added and the suspension was then rested
again at room temperature for 5 min. The cells were subsequently
centrifuged at 8,765 × g for 20 min at 4°C, the supernatant was
collected and an equal volume of isopropyl alcohol was added,
blended and precipitated at 80°C for 1 h.
Following centrifugation at 8,765 × g for 20 min at
4°C, the supernatant was discarded, 75% ethanol (~800 μl) was added
and the pellet was washed and precipitated. A further round of
centrifugation (8,765 × g for 20 min at 4°C) was performed, 75%
ethanol (800–1,000 μl) was added and the pellet was washed and
precipitated. Following a final round of centrifugation, the
supernatant was discarded, the pellet was air-dried and
diethylpyrocarbonate (DEPC)-water was added at an appropriate
volume until the precipitate was completely dissolved. The
suspension was cryopreserved at −70°C, and the samples were used
for electrophoretic and ultraviolet analyses.
A total of 1–4 μg total RNA was transferred to a
0.2-ml PCR tube, and 1 μl random primers and 12 μl DEPC-water were
added. The solution was vortexed and placed in a −70°C ice bath for
3–5 min. Approximately 4 μl 5X reaction buffer was added to make a
final volume of 20 μl. Following mixing, the solution was placed in
a 42°C water bath for 60 min, a 70°C water bath for 10 min and then
stored at 20°C. The reverse transcription products were subjected
to fluorescence quantitative PCR. The 96-well PCR plate was covered
with a sealing membrane (exclusively used in fluorescence
quantitative PCR; Bio-Rad, Hercules, CA, USA), centrifuged and
placed in a quantitative PCR machine (Bio-Rad). Data were then
collected and analyzed.
Western blotting
Confluent AMs were taken from the serum-free M199
culture solution and cultivated for 24 h. The cells were added to
their respective culture media, blank serum and treated serum, and
cultured for 24 h until the cells were synchronized at
G0. The supernatant was discarded, and the pellet was
washed three times with cold PBS and air-dried. A specific volume
of cell lysates was added to the cell pellet for a few minutes.
Using a cell scraper, the cells were transferred to a centrifuge
tube along with the cellular debris and pyrolysis liquid.
Centrifugation was performed at 6,440 × g for 10 min at 4°C. The
precipitate was discarded, and the supernatant was immediately
placed in a new centrifuge tube and stored at −20°C.
A sample of the supernatant protein was assessed
using a bicinchoninic acid protein assay kit (Viaud Co. Ltd.).
According to the optical density value and the standard
concentration of protein, the standard curve was drawn and the
total protein concentration of the samples was calculated.
The supernatant was combined with an epoxy potting
compound, and electrophoresis, protein transfer and immune
detection were performed. The supernatant was sensitized, developed
and fixed via X-ray film photography in the darkroom. All reagents
were provided by Shanghai Weiao Co., Ltd (Shanghai, China).
Statistical analysis
Data were analyzed using the SPSS 17.0 statistical
software package (SPSS, Inc., Chicago, IL, USA). Results are
expressed as the mean ± standard deviation, and one-way analysis of
variance was performed once variance was confirmed to be equal
(P<0.05) using Levene’s test. Multiple comparisons between
groups were analyzed by the least significant difference t-test.
Comparisons between two means were analyzed using the
Student-Newman-Keuls q-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell apoptosis
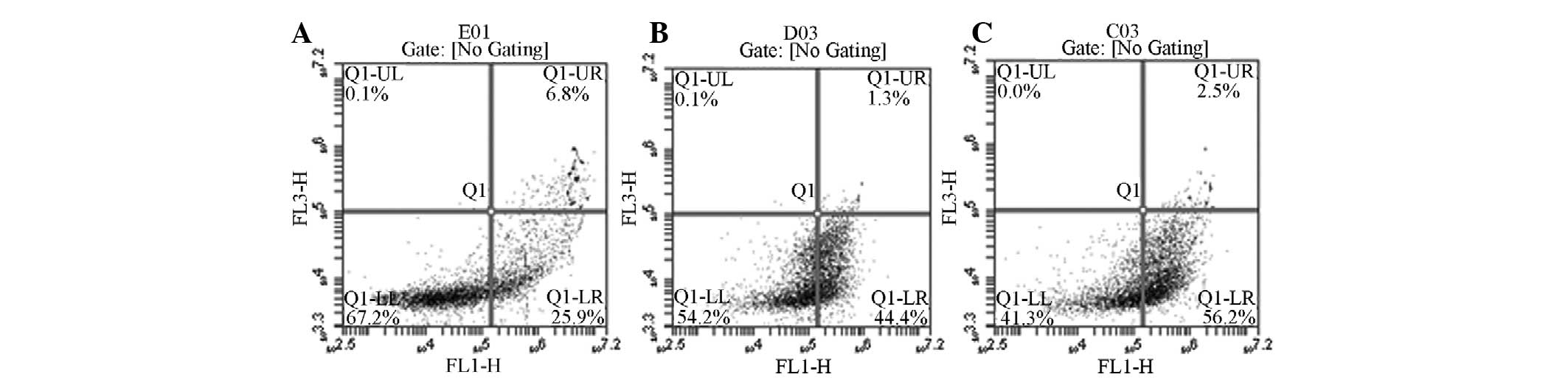
Microscopic observations and the analysis of the
rate of apoptosis using flow cytometry demonstrated that
Quanzhenyiqitang-treated serum resulted in a significantly higher
rate of AM apoptosis compared with the other three experimental
groups in the rat model of COPD (Figs.
1–3).
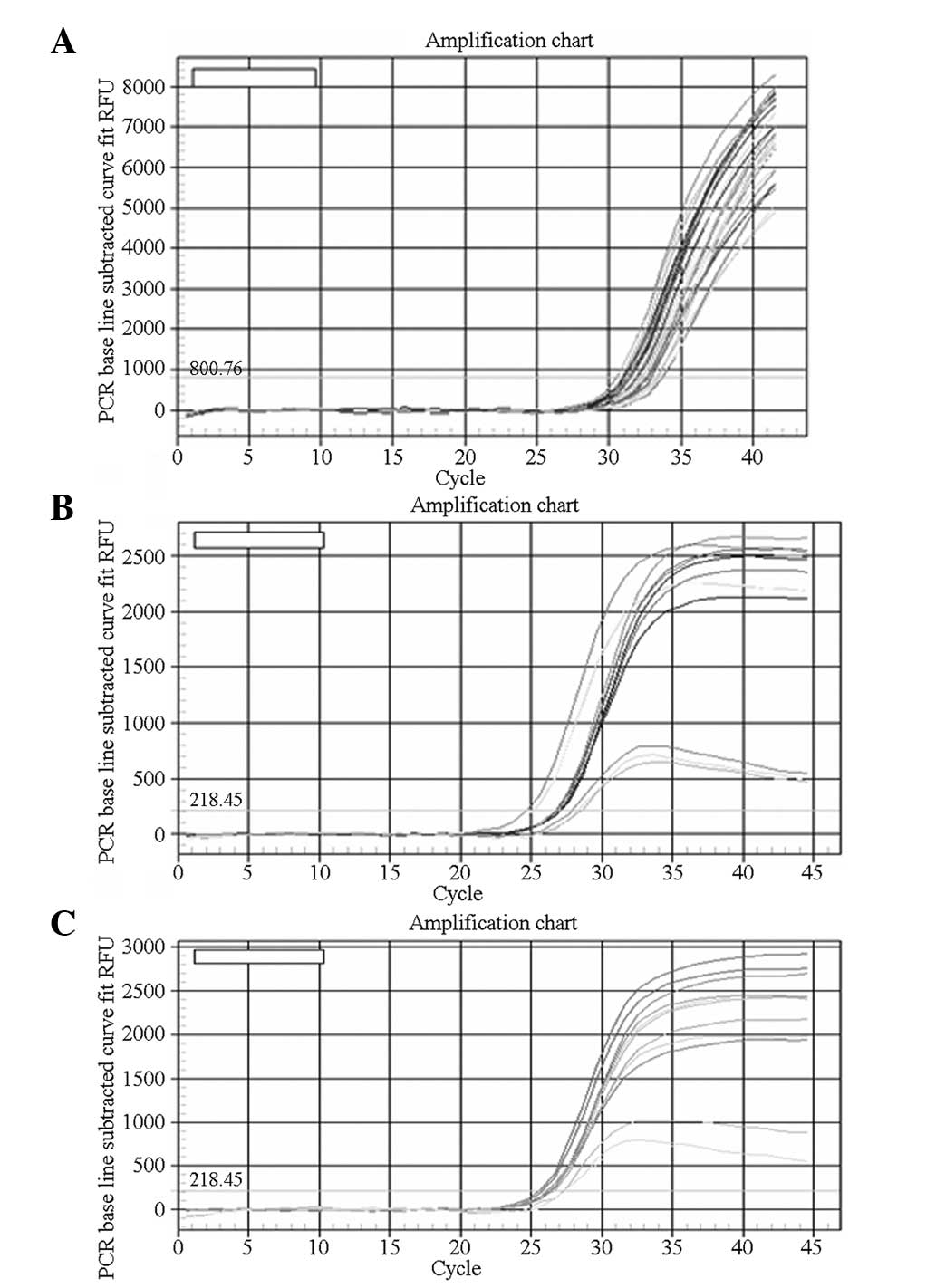
Fluorescence quantitative PCR
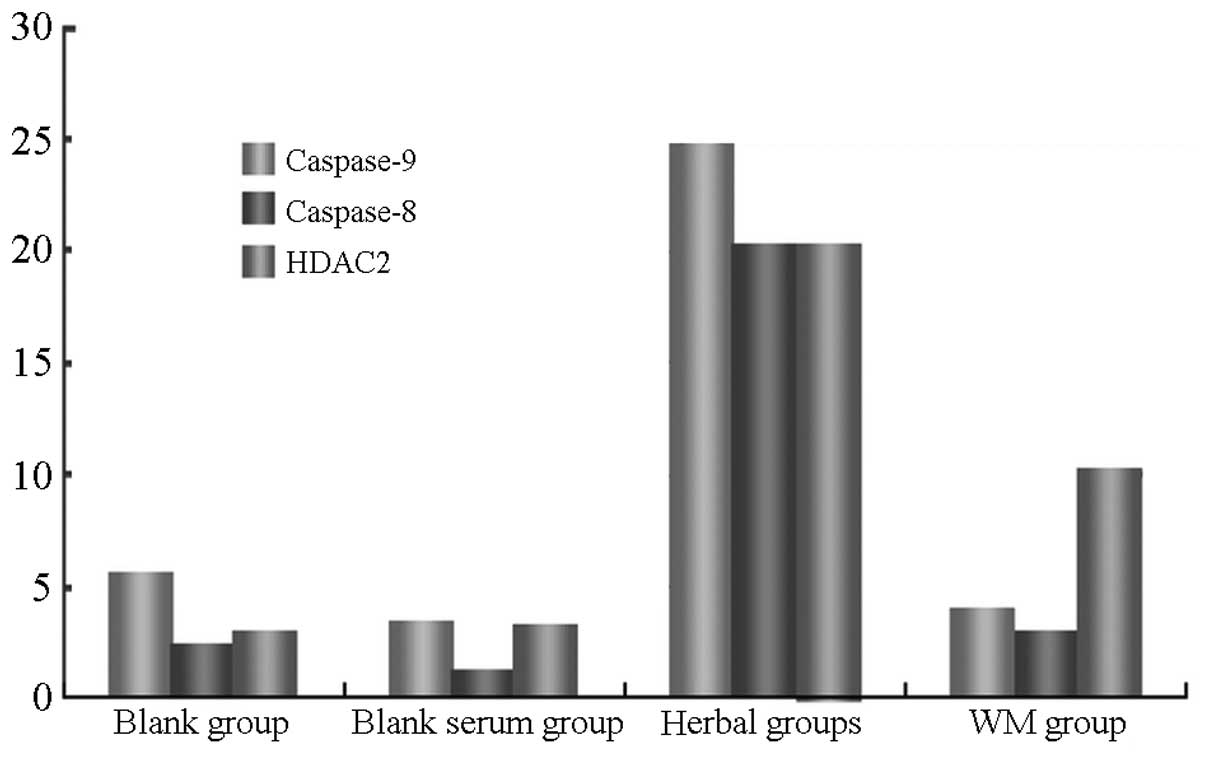
Caspase-8 gene expression was significantly higher
in AMs of the rats of the Quanzhenyiqitang-treated serum group
compared with the other three experimental groups. Levels of
caspase-9 gene expression were similar in the Quanzhenyiqitang- and
aminophylline-treated serum groups, which were higher than in the
control and blank serum groups. Fluorescence quantitative PCR also
demonstrated that HDAC2 gene expression was significantly higher in
AMs in the rats of the Quanzhenyiqitang-treated serum group
compared with the other three groups (Fig. 4).
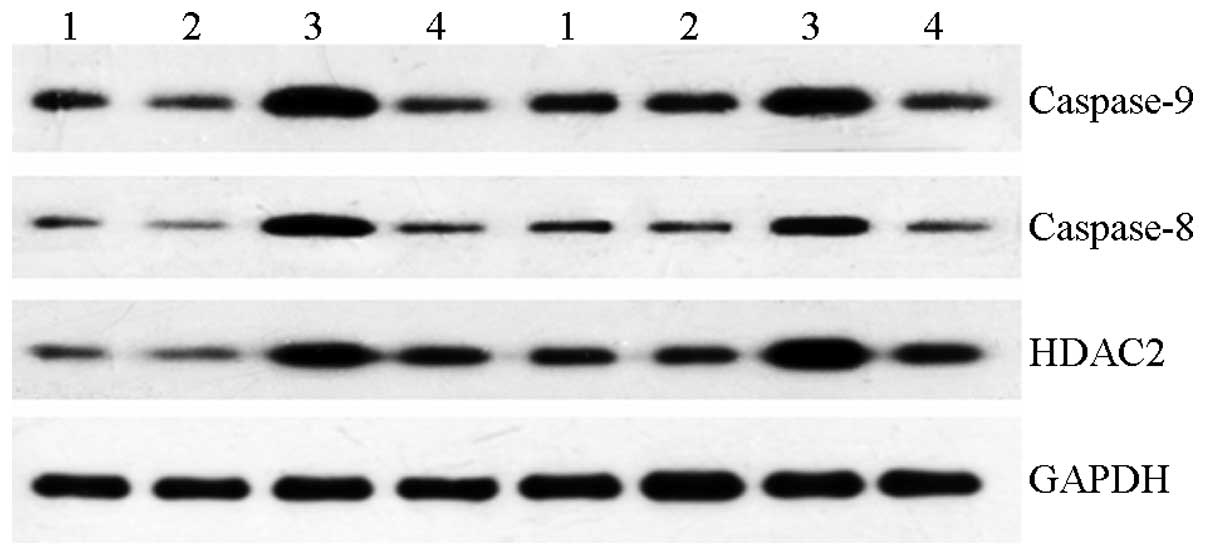
Western blotting
Levels of caspase-8, caspase-9 and HDAC2 protein
expression were significantly higher in AMs in the
Quanzhenyiqitang-treated serum group compared with the other three
groups (Figs. 5 and 6). The results indicated that
Quanzhenyiqitang was capable of inducing apoptosis of AMs derived
from rats with COPD by regulating the expression of caspase-8 and
caspase-9, and that Quanzhenyiqitang improved the status of the
inflammation in COPD by regulating HDAC2.
Discussion
The pathogenesis of COPD is complicated and usually
associated with apoptosis, oxidative stress and imbalances between
protease and antiprotease activity, as well as tissue destruction
and reconstruction (8). One of the
most important clinical manifestations of COPD is chronic
inflammation. As a chronic and systemic inflammatory disease, the
inflammatory reaction in the lungs may be either the cause or the
result of the systemic effects associated with COPD (9). Systemic inflammation may worsen the
clinical symptoms of patients, decreasing their activity levels and
reducing quality of life, thereby resulting in disease progression
and worsening disease prognosis (10).
A laboratory experiment investigating
Quanzhenyiqitang indicated that it is capable of improving the
tissue structure of damaged organs of multiple systems, including
the lungs of rats with COPD (6).
In addition, a clinical study demonstrated that Quanzhenyiqitang
alleviates the symptoms of COPD and significantly improves the
quality of life of patients (11).
In the present study, it was hypothesized that Quanzhenyiqitang has
therapeutic effects in COPD by controlling inflammation.
The primary characteristics of inflammation in COPD
are infiltration of AMs and neutrophils (12). Senior (8) studied lung tissue in patients with
COPD and observed that only ~0.3% of AMs underwent apoptosis,
demonstrating that the level of apoptosis of AMs in the lungs of
patients with COPD was significantly reduced. Furthermore, the
reduced rate of apoptosis contributed to the persistence and
progression of inflammation in the COPD airway (13).
The HDAC protein is capable of causing DNA
condensation, reducing gene transcription and controlling
inflammation (14). HDAC2 has an
important function in the process of inflammatory reactions in COPD
(15). Studies have indicated that
the low levels of HDAC activity in bronchial biopsies and AMs of
patients with COPD are closely associated with HDAC2 (16), and the degree of decline in HDAC2
activity is closely correlated with the intensity of inflammation
(17). Furthermore, the reduction
in HDAC2 expression and activity is specific to COPD. In a study of
AMs derived from bronchoalveolar lavage fluid of patients with
bronchial asthma reduced HDAC2 activity was not observed (18).
Quanzhenyiqitang-treated serum from rats is capable
of significantly increasing caspase-8 and caspase-9 expression,
inducing apoptosis of AMs in rats with COPD and reducing the number
and activity of AMs through the mitochondrial and death receptor
pathways on the cell surface (19,20).
Thus, it has a significant function in the prevention and control
of COPD. Quanzhenyiqitang-treated serum significantly increased
HDAC2 activity in AMs from rats with COPD; thus, the dynamic
balance between histone acetyltransferases (HATs) and HDAC was
adjusted and inflammation was ameliorated.
Quanzhenyiqitang has been demonstrated to be a
promising therapeutic agent for COPD. The ability of
Quanzhenyiqitang to induce apoptosis of inflammatory cells and
regulate the dynamic balance of inflammatory factors may represent
only one aspect of its therapeutic actions. The mechanisms of its
other effects, including improvement of diaphragm muscle function,
digestion and immune status, merit further study.
References
|
1
|
Global Initiative for Chronic Obstructive
Lung Disease (GOLD). Global Strategy for the Diagnosis, Management,
and Prevention of Chronic Obstructive Pulmonary Disease. (Revised
2011). http://www.goldcopd.org/uri.
Accessed December, 2011
|
|
2
|
Fujii T, Hayashi S, Hogg JC, et al:
Interaction of AMs and airway epithelial cells following exposure
to particulate matter produces mediators that stimulate the bone
marrow. Am J Respir Cell Mol Biol. 27:34–41. 2002. View Article : Google Scholar
|
|
3
|
Buhl R and Farmer SG: Future directions in
the pharmacologic therapy of chronic obstructive pulmonary disease.
Proc Am Thorac Soc. 2:83–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schleimer RP: Innate immune response and
chronic obstructive pulmonary disease: ‘Terminator’ or ‘Terminator
2’? Proc Am Thorac Soc. 2:342–346; discussion 371–372. 2005.
|
|
5
|
Zhang ZM: The curative effect of
QuanzhenYiqi decoction on78 cases of chronic obstructive emphysema
patients. Jiangxi Journal of Traditional Chinese Medicine.
36:27–28. 2005.(In Chinese).
|
|
6
|
Li DZ, Ruan SW, Huang HQ, Chen ZB and Lin
RH: Effect of Quanzhenyiqitang decoction on morphologic change of
lung, kidney, adrenal glands and testis from COPD-rats cast of
kidney failing to promote inspiration. China Journal of Traditional
Chinese Medicine and Pharmacy. 8:2073–2075. 2012.(In Chinese).
|
|
7
|
Zhang W and Bi XL: How to make rat models
of chronic obstructive pulmonary disease combined with kidney
deficiency. Laboratory Animal and Comparative Medicine. 25:157–161.
2005.
|
|
8
|
Senior RM: Mechanisms of COPD: conference
summary. Chest. 117(5 Suppl 1): 3205–3235. 2000. View Article : Google Scholar
|
|
9
|
Chung KF: Inflammatory mediators in
chronic obstructive pulmonary disease. Curr Drug Targets Inflamm
Allergy. 4:619–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rennard SI: Inflammation in COPD: a link
to systemic comorbidities. Eur Respir Rev. 16:91–97. 2007.
View Article : Google Scholar
|
|
11
|
Li DZ and Wang C: The curative effect of
QuanzhenYiqi decoction combined with western medicine on 38 cases
of COPD patients. Fujian Journal of Traditional Chinese Medicine.
42:20–21. 2011.
|
|
12
|
Di Stefano A, Caramori G, Ricciardolo FL,
Capelli A, Adcock IM and Donner CF: Cellular and molecular
mechanisms in chronic obstructive pulmonary disease: an overview.
Clin Exp Allergy. 34:1156–1167. 2004.PubMed/NCBI
|
|
13
|
Rytilä P, Plataki M, Bucchieri F, et al:
Airway neutrophilia in COPD is not associated with increased
neutrophil survival. Eur Respir J. 28:1163–1169. 2006.
|
|
14
|
Ito K, Barnes PJ and Adcock IM:
Glucocorticoid receptor recruitment of histone deacetylase 2
inhibits interleukin-1beta-induced histone H4 acetylation on
lysines 8 and 12. Mol Cell Biol. 20:6891–6903. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao R, de Zoeten EF, Ozkaynak E, et al:
Deacetylase inhibition promotes the generation and function of
regulatory T cells. Nat Med. 13:1299–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito K, Ito M, Elliot WM, et al: Decreased
histone deacetylase activity in chronic obstructive pulmonary
disease. N Engl J Med. 352:1967–1976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito K, Hanazawa T, Tomita K, Barnes PJ and
Adcock IM: Oxidative stress reduces histonedeacetylase 2 activity
and enhances IL-8 gene expression: role of tyrosine nitration.
Biochem Biophys Res Commun. 315:240–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higashimoto Y, Iwata T, Okada M, Satoh H,
Fukuda K and Tohda Y: Serum biomarkers as predictors of lung
function decline in chronic obstructive pulmonary disease. Respir
Med. 103:1231–1238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuida K: Caspase-9. Int J Biochem Cell
Biol. 32:121–124. 2000. View Article : Google Scholar
|
|
20
|
Mandal D, Mazumder A, Das P, Kundu M and
Basu J: Fas-, caspase 8-, and caspase 3-dependent signaling
regulates the activity of the aminophospholipid translocase and
phosphatidylserine externalization in human erythrocytes. J Biol
Chem. 280:39460–39467. 2005. View Article : Google Scholar
|