Introduction
Chloral hydrate is a well-known sedative and
anesthetic that is used in pediatric procedures, including
echocardiograms and magnetic resonance imaging (1–5), and
in animal experiments (6–9). Its safety and pharmacological
mechanisms in clinical practice have been emphasized (10–12)
In a previous study, it was demonstrated that
therapeutic concentrations of chloral hydrate delayed and reduced
the magnitude of the inflammatory response and improved the
survival rate of mice following the induction of liver injury with
lipopolysaccharide and D-galactosamine. This altered inflammatory
response was associated with the inhibitory effects of chloral
hydrate on nuclear factor-κB (NF-κB) activity and the levels of
serum proinflammatory cytokines induced by lipopolysaccharide
(13).
Peptidoglycan (PGN) is a common and conserved
component of the cell walls of Gram-positive (G+) bacteria,
including Staphylococcus aureus, and has been used as a
toll-like receptor 2 (TLR2)-specific ligand (14). It has been reported that PGN is
detected in the blood of 80% of patients with serious bacterial
infections (15). The mononuclear
phagocyte system plays a crucial role against infection in the
innate immune response, and murine macrophages are an important
model for studying infection. Following stimulation with PGN, NK-κB
becomes activated and macrophages release large quantities of the
proinflammatory cytokines IL-6 and TNF-α. Simultaneously, the
levels of TLR2 expression become upregulated (16,17).
Activation of a murine macrophage cell line (RAW264.7) by PGN is
mediated through the TLR2 signaling cascade, which involves the
activation of a number of kinases, including p38 mitogen-associated
protein kinase (MAPK), extracellular signal-regulated kinase (ERK)
1/2, inhibitor of NF-κB α (IκBα) and Akt (18,19).
In the present study, the effect of chloral hydrate
on the production of the proinflammatory cytokines and the activity
of NF-κB in PGN-stimulated murine peritoneal macrophages and
RAW264.7, respectively, was investigated. The study includes an
exploration of the mechanisms and an investigation of the effects
of chloral hydrate treatment on the expression levels of TLR2 and
TLR2 signal transduction in murine peritoneal macrophages and
RAW264.7 cells stimulated with PGN.
Materials and methods
Reagents
PGN (S. aureus, strain DSM346), chloral
hydrate (cat. no. 302-17-0) and trypsin-EDTA solution (10X; cat.
no. T4174) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Iscove’s modified Dulbecco’s medium (IMDM) and fetal bovine serum
(FBS) were purchased from Gibco (Grand Island, NY, USA). The TNF-α
and IL-6 ELISA kits were obtained from R&D Systems, Inc.
(Minneapolis, MN, USA). The fluorescein isothiocyanate
(FITC)-conjugated anti-mouse TLR2 antibody (clone TL2.5),
FITC-conjugated mouse IgG1 (isotype control), anti-mouse TLR2
antibody (clone TL2.5), horseradish peroxidase (HRP)-conjugated
goat anti-mouse secondary antibody and Cell Staining Buffer were
purchased from BioLegend, Inc. (San Diego, CA, USA). The antibodies
against p38 MAPK, phospho-p38 MAPK, ERK 1/2, phospho-ERK1/2, IκBα,
phospho-IκBα, Akt, phospho-Akt and β-actin were obtained from Cell
Signaling Technology, Inc. (Waltham, MA, USA). The NF-κB-luciferase
and β-galactosidase reporter vectors and the dual luciferase
reporter assay system were obtained from Promega Corporation
(Madison, WI, USA). The Immobilon-P membrane was obtained from
Millipore UK Ltd. (Watford, UK) and the enhanced chemiluminescence
(ECL) kit was purchased from Amersham Life Science Ltd. (Little
Chalfont, UK).
Effects of chloral hydrate on
proinflammatory cytokine production by murine peritoneal
macrophages stimulated with PGN
The separation and cultivation of mouse peritoneal
macrophages were performed as previously described (20). The Institutional Review Board of
the Affiliated Hospital of Guangdong Medical College (Zhangjiang,
China) approved the removal of the macrophages from the
six-week-old BALB/c mice were purchased from Experimental Animal
Center of Southern Medical University in the present study. The
cells were seeded at a density of 1×105 cells/well in
12-well plates in IMDM supplemented with 5% heat-inactivated FBS in
a humidified 5% CO2 and air incubator at 37°C. The
supernatants were collected at 6, 12 and 24 h following stimulation
with PGN (1 μg/ml) or PGN (1 μg/ml) plus chloral hydrate (0.25 and
1 mg/ml) and saline-treated controls. The supernatants were then
stored at −80°C prior to the measurement of the levels of TNF-α and
IL-6 using the corresponding ELISA kits according to the
manufacturer’s instructions.
Effects of chloral hydrate on the levels
NF-κB activity in PGN-stimulated RAW264.7 cells transfected with a
NF-κB luciferase reporter plasmid
The RAW264.7 cells were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China) and cultured in IMDM (5% FBS) and maintained at 37°C in 5%
CO2. The cells were seeded in 25-mm dishes at a density
of 1×106 cells per well. These cells were harvested with
0.25 g/l trypsin-EDTA 48 h later, and 5×106 RAW264.7
cells were transiently transfected with NF-κB-luciferase (5 μg) and
β-galactosidase reporter (5 μg) vectors (for normalization of the
efficiency of the transfection) in a volume of 400 μl by
electroporation at 250 V, 960 μF capacitance pulse as previously
described (21). The cells were
subsequently washed once in IMDM and then split into 50 wells (100
μl/well) and cultured for 24 h in IMDM (5% FBS), prior to
stimulation with PGN (1 μg/ml) for 6 or 12 h in the presence or
absence of chloral hydrate (0.25 or 1 mg/ml). For the luciferase
activity assays, the transfected RAW264.7 cells were stimulated for
6 or 12 h and subsequently harvested and lysed, and the luciferase
activity in the extracts was assayed with the dual luciferase
reporter assay system according to the manufacturer’s instructions.
The luciferase activity of the cell extracts is expressed as the
fold of luciferase-induction over that of a saline-treated
control.
Effects of chloral hydrate on PGN-induced
upregulation of TLR2 expression levels in RAW264.7 cells
Flow cytometry was performed to investigate the
levels of TLR2 expression in the RAW264.7 cells. The RAW 264.7
cells were cultured in IMDM (5% FBS) for 12 h with PGN (1 μg/ml) in
the presence or absence of chloral hydrate (0.25 mg/ml). After
harvesting, the cells were incubated with FITC-conjugated
anti-mouse-TLR2 (1 μg) for 30 minutes at room temperature without
permeabilization. As the isotype control, FITC-conjugated mouse
IgG1 was used to detect nonspecific staining. The cells were washed
twice with Cell Staining Buffer. A six-parameter flow cytometer
(FACScan; BD Biosciences, San Jose, CA, USA) was used in the data
acquisition. The analysis of the acquired data was performed using
CellQuest software (BD Biosciences Immunocytometry Systems, San
Jose, CA, USA). The mean channel fluorescence intensity (MFI)
derived from the fluorescence histogram was used to study the
levels of TLR2 expression. The MFI was calculated as a ratio and
recorded as the MFI of the TLR2 antibody divided by the MFI of a
normal (saline) control.
The TLR2 expression levels in the extracts from the
RAW264.7 cells stimulated with PGN (1 μg/ml) for 12 h in the
presence or absence of chloral hydrate (0.25 mg/ml) were
semi-quantitatively analyzed by western blotting using the
anti-mouse TLR2 antibody (clone TL2.5). The extracts from the
RAW264.7 cells were analyzed by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The immunoblotting was
performed by a standard western blotting procedure onto the
Immobilon membrane. HRP-conjugated goat anti-mouse secondary
antibody followed by ECL was used for the detection of the TLR2
signal. The semi-quantitative analysis of the extracts from the
RAW264.7 cells was conducted using BandScan software, version 5.0
(Glyko Inc., Novato, CA, USA).
Effects of chloral hydrate on PGN-induced
TLR2 signal transduction in RAW264.7 cells
The TLR2 expression levels in the extracts from the
RAW264.7 cells stimulated with PGN (1 μg/ml) for 12 h in the
presence or absence of chloral hydrate (0.25 mg/ml) were
semi-quantitatively analyzed by western blotting using antibodies
against MAPK, phospho-p38 MAPK, ERK 1/2, phospho-ERK1/2, IκBα,
phospho-IκBα, Akt, phospho-Akt and β-actin. Western blot analysis
and detection were performed as previously described in this study.
The semi-quantitative analysis of the extracts from the RAW264.7
cells was conducted using BandScan software.
Statistical analysis
The data are presented as the mean ± standard
deviation, and the statistical analysis was performed with SPSS
statistical software, version 15.0 (SPSS, Inc., Chicago, IL, USA).
The statistical significance between the two groups was determined
by the unpaired Student’s t-test. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
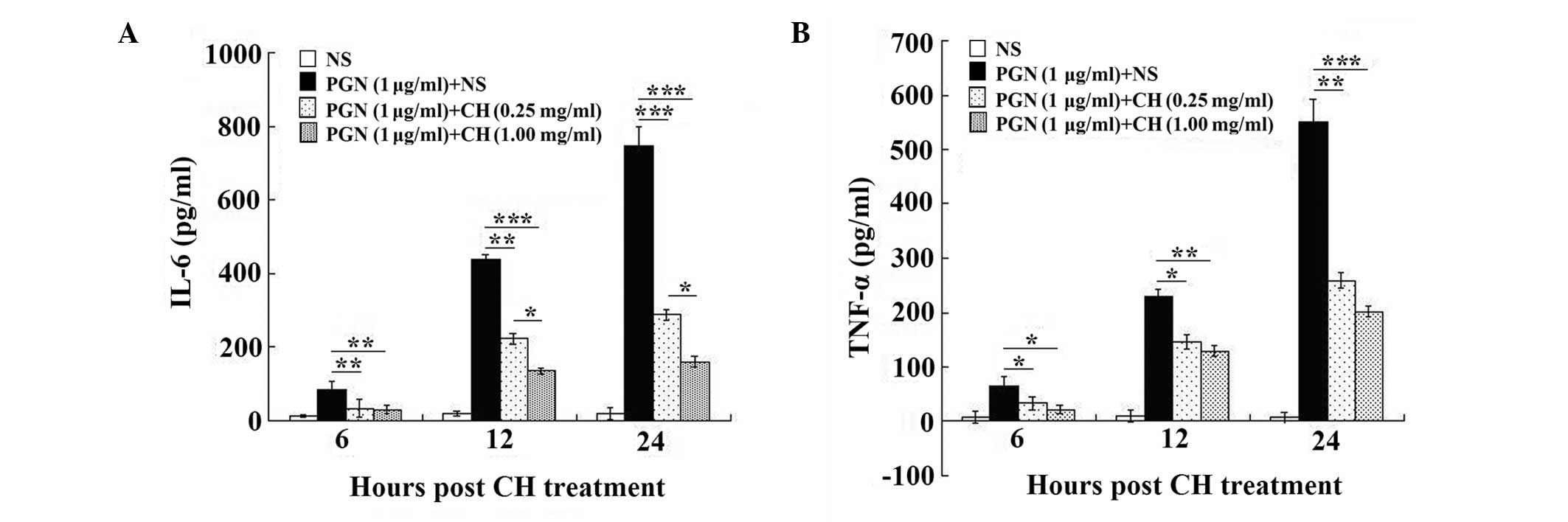
Chloral hydrate treatment reduces the
levels of IL-6 and TNF-α produced by PGN-stimulated peritoneal
macrophages
The results showed that the levels of IL-6 (Fig. 1A) and TNF-α (Fig. 1B) production increased sharply
post-stimulation with PGN (1 μg/ml) for 6, 12 and 24 h, compared
with those of the untreated cells, and decreased significantly
following the chloral hydrate treatment (0.25 and 1 mg/ml),
compared with those of the cells treated with PGN alone (all
P<0.01). In addition, the higher concentration of chloral
hydrate (1 mg/ml) significantly reduced the levels of IL-6
production following stimulation with PGN (1 μg/ml) for 12 and 24 h
compared with those of the cells treated with the lower
concentration of chloral hydrate (0.25 mg/ml; all P<0.05;
Fig. 1A).
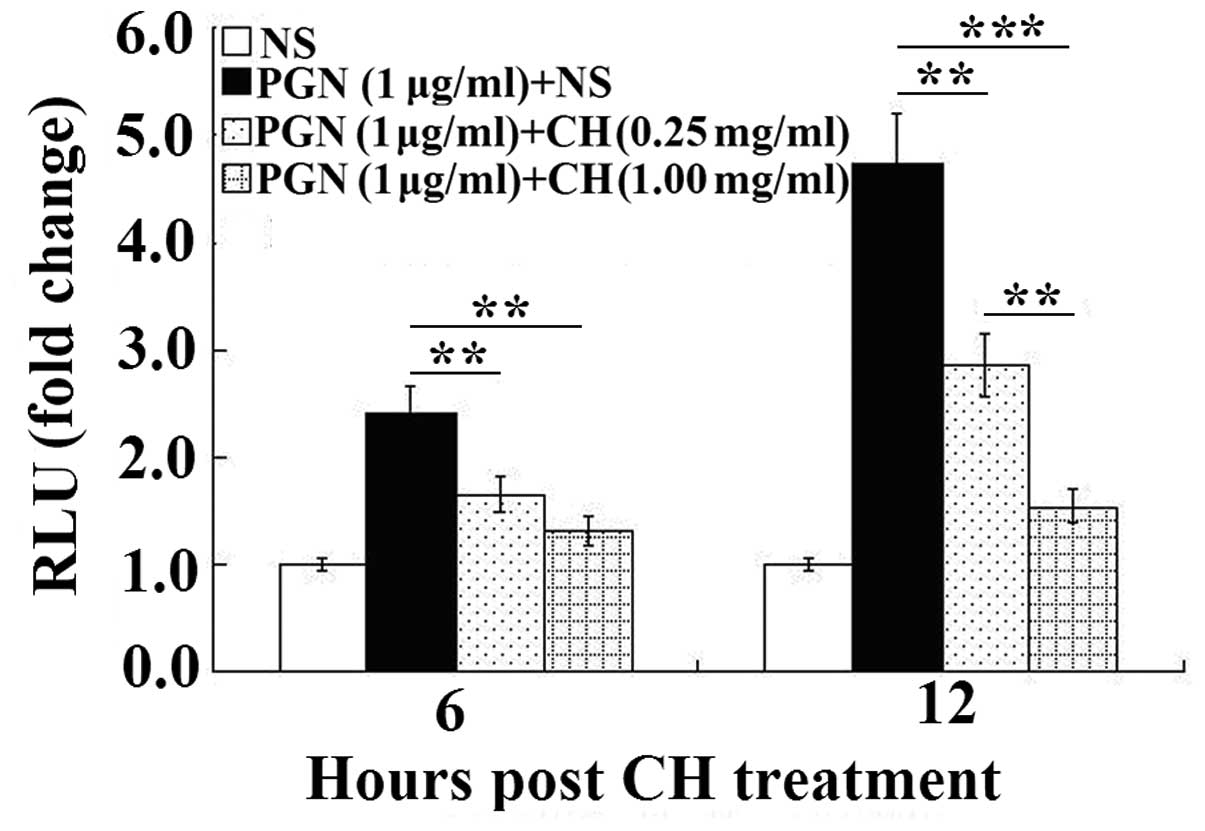
Chloral hydrate-treatment reduces the
levels of NF-κB activity in PGN-stimulated RAW264.7 cells
The levels of luciferase activities in the
transfected RAW264.7 cells were measured at 6 and 12 h after PGN
stimulation (Fig. 2). The results
are presented as a fold of induction over the values obtained in
the normal saline groups. As shown in Fig. 2, ~1.5- and 3.5-fold increases in
the luciferase activity were observed following the PGN stimulation
for 6 h and 12 h, respectively, compared with those of the
untreated cells. The effect of PGN-stimulation was significantly
reduced by the chloral hydrate treatment (0.25 and 1 mg/ml; all
P<0.01). A higher concentration of chloral hydrate (1 mg/ml) led
to a significantly larger reduction in the levels of luciferase
activity after 12 h than did the lower concentration of chloral
hydrate (0.25 mg/ml; P<0.01).
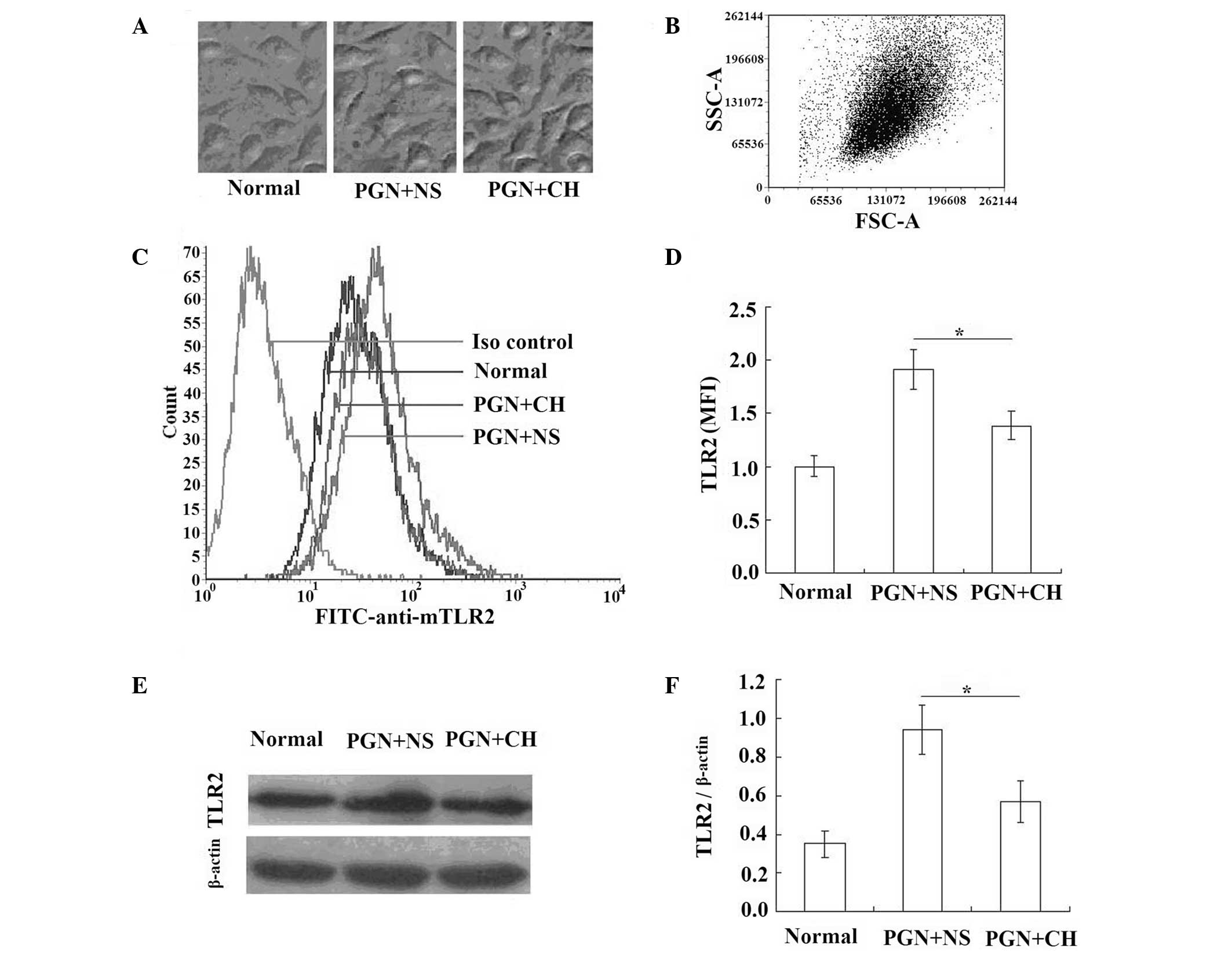
Chloral hydrate-treatment reduces the
increased TLR2 expression levels in PGN-treated RAW264.7 cells
The two concentrations (0.25 and 1 mg/ml) of chloral
hydrate treatment were shown to significantly reduce the
inflammatory response of the peritoneal macrophages and RAW264.7
cells following PGN-stimulation compared with that of the
macrophages and RAW264.7 cells treated with PGN alone, and the
effect of chloral hydrate treatment on the expression levels of the
receptor for PGN, TLR2, was tested using the lowest effective
concentration of chloral hydrate (0.25 mg/ml) in the RAW264.7
cells.
The cells were cultured in the IMDM supplemented
with 5% heat-inactivated FBS for 12 h with or without PGN (1 μg/ml)
in the presence or absence of chloral hydrate (0.25 mg/ml; Fig. 3A) and harvested, and the levels of
the MFI of TLR2 were measured. In the RAW264.7 cells stimulated by
PGN (1 μg/ml) for 12 h, the TLR2 expression levels were
significantly increased (Fig.
3B–D) compared with those in the unstimulated cells, and the
chloral hydrate treatment (0.25 mg/ml) significantly reduced the
upregulation of the levels of TLR2 stimulated by PGN (P<0.05;
Figs. 3B–D).
The corresponding semi-quantitative analysis of the
levels of TLR2 expression in the extracts of the RAW264.7 cells by
western blotting showed a similar trend (Fig. 3E and F). This shows that if the
amounts of TLR2 signal are considered in relation to the signal of
the household protein β-actin, the increase and the reduction of
the TLR2 signals are not caused by cell death or variations in the
number of cells in the sample.
Treatment of PGN-induced RAW264.7 cells
with chloral hydrate reduces the levels of TLR2 signal
transduction
Although chloral hydrate-treatment reduced the
increased TLR2 expression levels in the PGN-treated RAW264.7 cells,
whether the levels of TLR2-associated signal transduction
molecules, including phospho-p38 MAPK, phospho-ERK1/2, phospho-IκBα
and phospho-Akt (18,19), were reduced was unknown.
The effect of chloral hydrate on TLR2 signal
transduction was investigated by western blotting, and the lower
effective concentration (0.25 mg/ml) was selected to test the
effect of chloral hydrate treatment on TLR2 signal transduction in
RAW264.7 cells.
Following culture in IMDM supplemented with 5%
heat-inactivated FBS for 12 h with or without PGN (1 μg/ml) in the
presence or absence of chloral hydrate (0.25 mg/ml), the
upregulation of signal transduction of TLR2 in RAW264.7 stimulated
by PGN (1 μg/ml) for 12 h was tested for significance with western
blotting. The upregulation of TLR2 signal transduction stimulated
by PGN was reduced following chloral hydrate (0.25 mg/ml) treatment
(Fig 4).
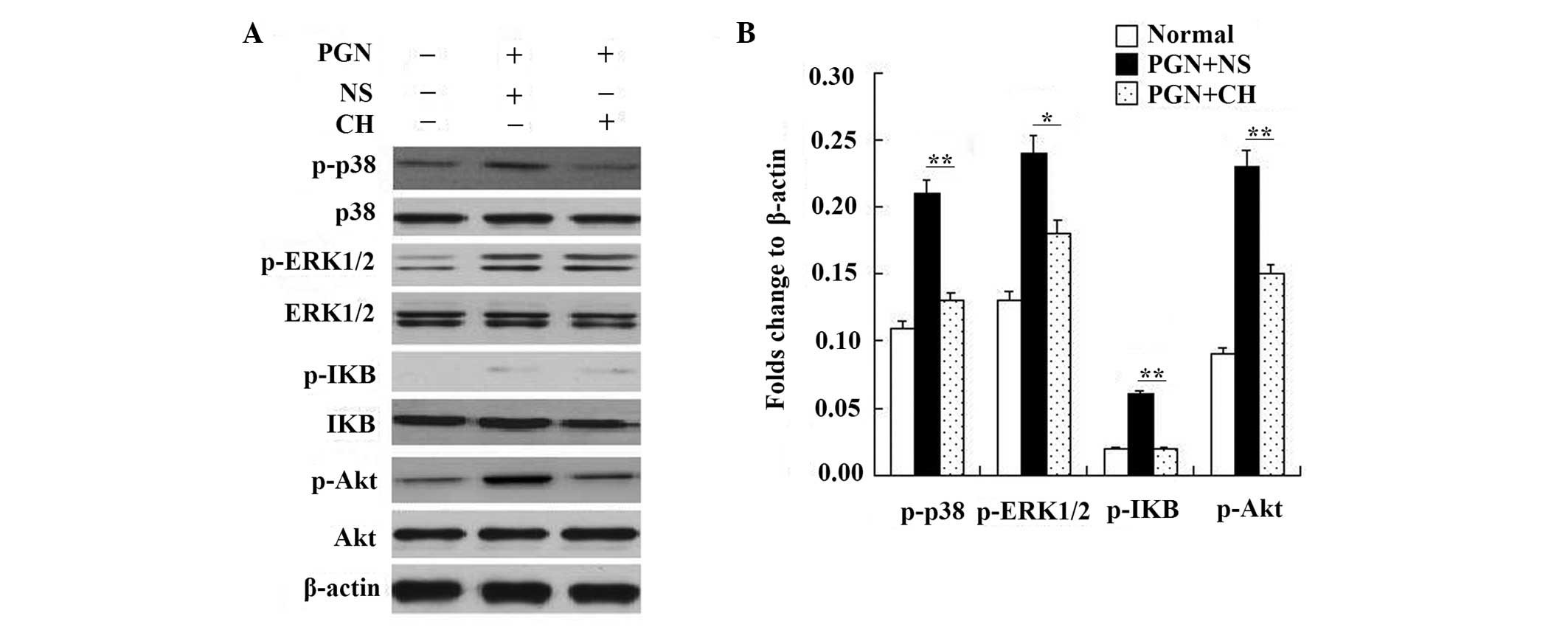 | Figure 4RAW264.7 cells were treated with PGN
(1 μg/ml) for 12 h in the presence or absence of chloral hydrate
(CH; 0.25 mg/ml). The cell lysates were (A) immunoblotted with
antibodies against MAPK, phospho-p38 MAPK, ERK 1/2, phospho-ERK1/2,
IκBα, phospho-IκBα, Akt, phospho-Akt or β-actin and (B)
semi-quantified to show the reduction in signal. The experiments
were repeated three times with similar results. PGN, peptidoglycan;
ERK, extracellular signal-regulated kinase; MAPK,
mitogen-associated protein kinase. |
Discussion
The mechanisms of bacteria-induced acute
inflammation and anti-cytokines as therapeutic agents have been
reevaluated. The majority of the reported studies on
sepsis-associated anti-cytokine therapies have been unpromising or
disappointing (22–24). Intensive investigative efforts have
been made with the aim of developing novel drugs and treatment
strategies for severe sepsis and acute inflammation.
The process for the development and approval of
novel drugs in therapeutic programs requires a large amount of time
and effort. Novel uses for traditional, old drugs may accelerate
the development and application of new therapies. For example, the
bisphosphonate zoledronic acid, which has been used to treat
osteoporosis and similar diseases, also decreases breast cancer
metastasis (25), and thalidomide,
which was originally used to alleviate nausea and morning sickness,
is able to treat multiple myeloma (26). The identification of the protective
effects of certain anesthetics and sedatives against infection or
inflammation (27–30) may yield novel insights into
anti-inflammatory therapies.
As a traditional anesthetic, chloral hydrate is
typically used in patients and animal models and may be
administered by mouth, injection and direct placement into the
intestine/small bowel. Chloral hydrate is unlike isoflurane, an
inhalation agent that must be inhaled continuously and is
expensive. Chloral hydrate is able to be used freely in a number of
countries, but other sedatives, for example ketamine, are under
strictly regulated conditions in China and are not approved for
patients under 16 years old by the US Food and Drug Administration
(1).
The present study in murine peritoneal macrophages
showed that chloral hydrate treatment reduced the rise of the
inflammatory cytokine levels induced by PGN stimulation (Fig. 1), indicating that the effect of
chloral hydrate on inflammation may be attributed to the inhibition
of the macrophage function. The TNF-α and IL-6 levels sharply
increased at 6, 12 and 24 h after the PGN-challenge. Similar
studies using PGN (10 or 25 μg/ml) have been performed by Shirasawa
et al (31) and Wang et
al (32), although they tested
the levels at 24 h after one challenge. The treatments with
different concentrations (0.25 and 1.0 mg/ml) of chloral hydrate
significantly reduced the rise of inflammatory cytokine levels at
the indicated time points compared with those of the cells treated
with PGN alone (Fig. 1).
In the present study, whether chloral hydrate
regulates PGN-induced NF-κB-dependent gene transcription was
investigated with a NF-κB-dependent luciferase reporter assay in
RAW264.7 cells co-transfected with a β-galactosidase control
plasmid. The results revealed that chloral hydrate treatment
reduces the levels of NF-κB activity (Fig. 2). As NF-κB plays a key role in the
transcriptional regulation of proinflammatory cytokine expression,
this result suggests that chloral hydrate may affect cytokine
expression by influencing the activity of NF-κB. After 6 and 12 h
of PGN challenge, the respective increases of the levels of
NF-κB-induced luciferase activity in the RAW264.7 cells were ~1.5-
and 3.5-fold compared with the basal level. A similar study using
PGN (5 μg/ml) was performed by Ito et al (33), and they observed the same
effect.
The present also study examined whether chloral
hydrate modulates the PGN-induced upregulation of TLR2 expression
levels. Flow cytometry was used to analyze the effect of chloral
hydrate on the expression levels of TLR2 in RAW264.7 cells
stimulated by PGN. The expression levels of TLR2 in the RAW264.7
cells were significantly upregulated following PGN stimulation
compared with those in the untreated cells. Similar results have
been demonstrated by Chen et al (16). Following the chloral hydrate
treatment, the marked upregulation of the TLR2 expression levels in
response to PGN exposure was significantly reduced (Fig. 3), which is consistent with the
effect of chloral hydrate on the levels of NF-κB activity and
inflammatory cytokine production by RAW264.7 cells stimulated with
PGN.
Having identified that the clear upregulation of
TLR2 expression levels in response to PGN exposure was markedly
diminished following treatment with chloral hydrate, the present
study evaluated the effects of PGN and chloral hydrate on TLR2
signal transduction by analyzing the levels of signaling species,
including p38 MAPK, ERK1/2, IκBα and Akt. In the RAW264.7 cells,
PGN markedly induced the activation by phosphorylation of p38 MAPK,
ERK1/2, IκBα and Akt, but this phosphorylation was reduced by the
chloral hydrate treatment (Fig.
4).
The present study showed, to the best of our
knowledge, for the first time that chloral hydrate reduced the
PGN-induced upregulation of the levels of NF-κB activity and TNF-α
and IL-6 production by macrophages in a time- and
concentration-dependent manner. It was demonstrated that this
reduction was associated with attenuation of the upregulation of
PGN-induced-TLR2 expression and TLR2 signal transduction levels.
Knowing the mechanisms of chloral hydrate treatment in inflammation
provides opportunities to design novel therapeutic strategies for
reducing the inflammation caused by G+ organisms.
Acknowledgements
The authors are grateful for the support of the
Natural Science Foundation of China (no. 81202346), the Natural
Science Foundation of Guangdong Province (no. S2012040006216), the
Zhanjiang Planning Project of Science and Technology (no.
2012C3101028/2013B01086), the Medical Scientific Research
Foundation of Guangdong Province (no. B2012284) and the Doctoral
Fund of Guangdong Medical College and Affiliated Hospital of
Guangdong Medical College to Qingjun Pan.
References
|
1
|
Mellon RD, Simone AF and Rappaport BA: Use
of anesthetic agents in neonates and young children. Anesth Analg.
104:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sury MR and Fairweather K: The effect of
melatonin on sedation of children undergoing magnetic resonance
imaging. Br J Anaesth. 97:220–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harrison D, Loughnan P, Manias E and
Johnston L: Utilization of analgesics, sedatives, and pain scores
in infants with a prolonged hospitalization: a prospective
descriptive cohort study. Int J Nurs Stud. 46:624–632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Layangool T, Sangtawesin C, Kirawittaya T,
et al: A comparison of oral chloral hydrate and sublingual
midazolam sedation for echocardiogram in children. J Med Assoc
Thai. 91(Suppl 3): S45–S52. 2008.PubMed/NCBI
|
|
5
|
Liu N, Fan G, Yu B and Guo Q: Effect of
slight sedation with chloral hydrate on the BOLD response of
children’s visual cortex: a self-control fMRI study. Chin J Med
Imaging Technol. 24:1901–1904. 2008.(In Chinese).
|
|
6
|
Yuksel BC, Serdar SE, Tuncel A, et al:
Effect of tempol, a membrane-permeable radical scavenger, on
mesenteric blood flow and organ injury in a murine cecal ligation
and puncture model of septic shock. Eur Surg Res. 43:219–227. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nazam Ansari M, Bhandari U, Islam F and
Tripathi CD: Evaluation of antioxidant and neuroprotective effect
of ethanolic extract of Embelia ribes Burm in focal cerebral
ischemia/reperfusion-induced oxidative stress in rats. Fundam Clin
Pharmacol. 22:305–314. 2008.PubMed/NCBI
|
|
8
|
Uematsu M, Takasawa M, Hosoi R and Inoue
O: Uncoupling of flow and metabolism by chloral hydrate: a rat
in-vivo autoradiographic study. Neuroreport. 20:219–222. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu M, Ackerman JJ and Yablonskiy DA: Body
and brain temperature coupling: the critical role of cerebral blood
flow. J Comp Physiol B. 179:701–710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinbiancho JK, Carvalho PR, Trotta Ede
A, et al: Evidence of safety of chloral hydrate for prolonged
sedation in PICU in a tertiary teaching hospital in southern
Brazil. Eur J Clin Pharmacol. 65:1253–1258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bronley-DeLancey A, McMillan DC, McMillan
JM, et al: Application of cryopreserved human hepatocytes in
trichloroethylene risk assessment: relative disposition of chloral
hydrate to trichloroacetate and trichloroethanol. Environ Health
Perspect. 114:1237–1242. 2006. View
Article : Google Scholar
|
|
12
|
Merdink JL, Robison LM, Stevens DK, et al:
Kinetics of chloral hydrate and its metabolites in male human
volunteers. Toxicology. 245:130–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan Q, Liu Y, Zheng J, et al: Protective
effect of chloral hydrate against
lipopolysaccharide/D-galactosamine-induced acute lethal liver
injury and zymosan-induced peritonitis in mice. Int
Immunopharmacol. 10:967–977. 2010. View Article : Google Scholar
|
|
14
|
Chang S, Dolganiuc A and Szabo G:
Toll-like receptors 1 and 6 are involved in TLR2-mediated
macrophage activation by hepatitis C virus core and NS3 proteins. J
Leukoc Biol. 82:479–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Merkel GJ and Scofield BA:
Characterization of a monoclonal antibody that binds to an epitope
on soluble bacterial peptidoglycan fragments. Clin Diagn Lab
Immunol. 8:647–651. 2001.
|
|
16
|
Chen BC, Kang JC, Lu YT, et al: Rac1
regulates peptidoglycan-induced nuclear factor-kappaB activation
and cyclooxygenase-2 expression in RAW 264.7 macrophages by
activating the phosphatidylinositol 3-kinase/Akt pathway. Mol
Immunol. 46:1179–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin HY, Tang CH, Chen JH, et al:
Peptidoglycan induces interleukin-6 expression through the TLR2
receptor, JNK, c-Jun, and AP-1 pathways in microglia. J Cell
Physiol. 226:1573–1582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Jiang S and Tapping RI: Toll-like
receptor signaling in cell proliferation and survival. Cytokine.
49:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee GL, Chang YW, Wu JY, et al: TLR 2
induces vascular smooth muscle cell migration through cAMP response
element-binding protein-mediated interleukin-6 production.
Arterioscler Thromb Vasc Biol. 32:2751–2760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin MZ, Li SP, Yuan L and Dai HL:
Separation, cultivation and identification of mouse peritoneal
macrophages. Med J Wuhan Univ. 27:203–205. 2006.(In Chinese).
|
|
21
|
Vabulas RM, Ahmad-Nejad P, Ghose S, et al:
HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor
signal pathway. J Biol Chem. 277:15107–15112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hall MW and Muszynski JA: Immune
modulation in sepsis. J Pediatr Infect Dis. 4:127–136. 2009.
|
|
23
|
Zeni F, Freeman B and Natanson C:
Anti-inflammatory therapies to treat sepsis and septic shock: a
reassessment. Crit Care Med. 25:1095–1100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ratsimandresy RA, Rappaport J and Zagury
JF: Anti-cytokine therapeutics: history and update. Curr Pharm Des.
15:1998–2025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coleman R and Gnant M: New results from
the use of bisphosphonates in cancer patients. Curr Opin Support
Palliat Care. 3:213–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cavo M, Di Raimondo F, Zamagni E, et al:
Short-term thalidomide incorporated into double autologous
stem-cell transplantation improves outcomes in comparison with
double autotransplantation for multiple myeloma. J Clin Oncol.
27:5001–5007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plachinta RV, Hayes JK, Cerilli LA and
Rich GF: Isoflurane pretreatment inhibits
lipopolysaccharide-induced inflammation in rats. Anesthesiology.
98:89–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gallos G, Jones DR, Nasr SH, et al: Local
anesthetics reduce mortality and protect against renal and hepatic
dysfunction in murine septic peritonitis. Anesthesiology.
101:902–911. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fuentes JM, Talamini MA, Fulton WB, et al:
General anesthesia delays the inflammatory response and increases
survival for mice with endotoxic shock. Clin Vaccine Immunol.
13:281–288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Klaver MJ, Manning L, Palmer LA and
Rich GF: Isoflurane pretreatment inhibits cytokine-induced cell
death in cultured rat smooth muscle cells and human endothelial
cells. Anesthesiology. 97:24–32. 2002.PubMed/NCBI
|
|
31
|
Shirasawa S, Sugiyama S, Baba I, et al:
Dermatitis due to epiregulin deficiency and a critical role of
epiregulin in immune-related responses of keratinocyte and
macrophage. Proc Natl Acad Sci USA. 101:13921–13926. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang N, Satoskar A, Faubion W, et al: The
cell surface receptor SLAM controls T cell and macrophage
functions. J Exp Med. 199:1255–1264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ito Y, Kawamura I, Kohda C, et al:
Seeligeriolysin O, a protein toxin of Listeria seeligeri,
stimulates macrophage cytokine production via Toll-like receptors
in a profile different from that induced by other bacterial
ligands. Int Immunol. 17:1597–1606. 2005.PubMed/NCBI
|