Introduction
Varicella-zoster virus (VZV) belongs to the
α-herpesvirus family and causes varicella (chickenpox) with primary
infection and zoster (shingles) during reactivation from a latent
state. VZV causes chronic pain or postherpetic neuralgia in
patients, but may also cause zoster paresis in the arm, leg,
diaphragm or abdominal muscles (1,2). The
life cycle of VZV relies on its tropism for T cells, skin and
neurons (3–5). VZV replication is an important part
of the life cycle; however, the replication mechanisms remain
largely unknown.
MicroRNAs (miRNAs) consist of ~22 nucleotides and
are small, noncoding and functional RNAs. Due to the numerous
functions of miRNAs, they play crucial roles in the pathogenesis of
cancers and infections and in particular viral infections (6–8). It
has been shown that miRNAs function to activate interferon
(IFN)-mediated antiviral activity, suppress the viral
counter-responses to cell restriction and affect the viral
replication and thus, the pathogenesis of viral infections
(7). miRNAs have been documented
to have diverse effects in terms of viral replication. Human
immunodeficiency virus-1 mRNA is suppressed by a cluster of miRNAs,
including miR-28, miR-125b, miR-150, miR-223 and miR-382 (9). Numerous other miRNAs function as
inhibiting factors and interfere with viral replication. For
example, miR-181 suppresses the respiratory syndrome virus and
miR-17-29 suppresses hepatitis B virus (HBV) (10,11).
Conversely, miR-501 increases HBV production via the same mechanism
by which miR-126 affects coxsackievirus (12,13).
Therefore, miRNAs may affect viral replication as suppressors or
promoters. However, to date no miRNAs that impact the replication
of VZV have been identified.
A major miRNA that plays a crucial role in cancer,
cardiovascular diseases, inflammation and virus infection is
miRNA-21 (miR-21) (14). It has
been shown to affect immunity and viral replication. With respect
to viral replication, upregulated miR-21 suppresses hepatitis C
virus (HCV)-triggered type I IFN production, which promotes HCV
replication. However, dysexpression of miR-21 has been revealed to
be an antiviral factor in specific viruses, including Epstein Barr
virus (EBV) and HBV (15–17). With regard to immunity, miR-21 is
important in maintaining the effector phase of T cells and
regulating the Th1 immune response and immune cell functions
(14). In addition, miR-21 has
been shown to directly target signal transducer and activator of
transcription 3 (STAT3) and markedly activate it, subsequently
modulating immune functions (18).
STAT3 is one of seven STAT transcription factors
that comprehensively affects cell survival, cell cycle progression
and homeostasis (19,20). Moreover, STAT3 is important in the
pathogenesis of a number of viruses, including γ-herpesviruses,
Kaposi’s sarcoma herpesvirus, EBV and herpesvirus saimiri (21,22).
A study has found that VZV triggers the STAT3 signaling pathway and
activates STAT3, which increases VZV replication via a mechanism
dependent on the anti-apoptotic protein survivin (23). Considering the numerous mechanisms
and associations among miRNA, virus infection and STAT3, it
possible that certain miRNAs may interact with STAT3 and VZV
replication.
In the present study, by ectopically increasing
miR-21 and knocking down STAT3, the effect of miR-21 expression and
STAT3 on the replication of VZV was investigated.
Materials and methods
Cell lines and virus
Human malignant melanoma cells (MeWo) and human
embryonic lung fibroblasts (HELF) were HELF were purchased from
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and grown in Dulbecco’s minimal essential medium
(Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum in a humidified incubator containing 5% CO2 at
37°C. The parental Oka strain of VZV was acquired from Wuhan
Institute of Virology, Chinese Academy of Sciences (Wuhan, China).
MeWo cells were used to propagate VZV by co-cultivating infected
cells with uninfected cells at a ratio of 1/5.
Cell-free virus preparation and virus
infection
Cell-free VZV was provided by Beijing Wantai
Biological Pharmacy Enterprise Co., Ltd. (Beijing, China).
VZV-infected cells were suspended in a cryoprotective solution
(Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.). Cells
were lysed by shaking vigorously with 1-mm glass beads. Next, the
lysates were centrifuged at 1,240 × g for 10 min at 4°C. The
supernatant was stored at −70°C. Viral titers were determined using
a standard VZV plaque assay with MeWo cells. The titer of the VZV
stock was 7.9×106 plaque forming units (PFU)/ml. The
cells were infected with VZV at a multiplicity of infection (MOI)
of 1 × 10−3.
RNA/DNA extraction and quantitative
polymerase chain reaction (qPCR)
DNA and RNA were extracted from cells using DNeasy
and RNeasy kits (Qiagen, Valencia, CA, USA). The expression levels
of cellular miR-21 were detected with a mirVana miRNA
detection kit (Ambion, Inc., Austin, TX, USA) and qPCR primer sets.
U6 small nuclear RNA was used as an internal control. The mRNA
expression levels of STAT3 and survivin were assayed using GAPDH as
an internal control. The ΔΔCt method was used for relative
quantification. The primers were as follows: Survivin, forward, CAT
GGG TGC CCC GAC GTT G; and reverse, GCT CCG GCC AGA GGC CTCA A;
STAT3, forward, GGG TGG AGA AGG ACA TCA GCG GTA A; and reverse, GCC
GAC AAT ACT TTC CGA ATC C; and GAPDH, forward, GGA GTC AAC GGA TTT
GGT C; and reverse, GGA ATC ATT GGA ACA TGT AAA C.
Western blot analysis
Following seeding or transfection, western blotting
of the cells were performed by a standard protocol. Briefly, cells
were washed with PBS and lysed. Next, the lysates were centrifuged
at 13,000 × g at 4°C for 30 min. The supernatants were subjected to
10% SDS-PAGE. Following electrophoresis, proteins were transferred
onto nitrocellulose membranes and detected using human monoclonal
STAT3 antibodies (R&D Systems, Minneapolis, MN, USA) or β-actin
antibodies (Sigma-Aldrich, St. Louis, MO, USA). Secondary
antibodies conjugated to horseradish peroxidase were goat
anti-mouse IgG or goat anti-rabbit IgG (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Enhanced chemiluminescent detection systems
(SuperSignal West Femto; Pierce Biotechnology, Inc.) were used for
detection.
Gene knockdown and transfection
The expression vector, pSUPERneo, was acquired from
OligoEngine (Seattle, WA, USA). The pSUPERSTAT3si plasmid was
constructed with a STAT3-specific RNAi sequence (GAT CCC CTT CAG
ACC CGT CAA CAA ATT CAA GAG ATT TGT TGA CGG GTC TGA AGT TTT T)
which was cloned to the pSUPERneo vector with
EcoRI/HindIII (Promega Corporation, Madison, WI,
USA). pSUPERSTAT3si was used to conduct the knockdown of the human
STAT3 gene (24). STAT3 depletion
was validated by western blot analysis. Cells were transfected with
an miR-21-mimic or miR-control (Shanghai GenePharma Co., Ltd.,
Shanghai, China) at a concentration of 100 nM using Lipofectamine
2000 (Invitrogen Life Technologies, Carlsbad, CA, USA).
Statistical analysis
The data are normalized as mean ± standard
deviation. Student’s t-tests were conducted to compare differences
in two groups. Comparisons among multiple samples were made by
ANOVA. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was
utilized to perform the statistical analysis. P<0.05 was
considered to indicate a statistically significant result.
Results
miR-21 expression increases in HELF and
MeWo cell lines following VZV infection
miR-21 has a multifunctional role in virus
infection. To demonstrate the effect that miR-21 has on VZV
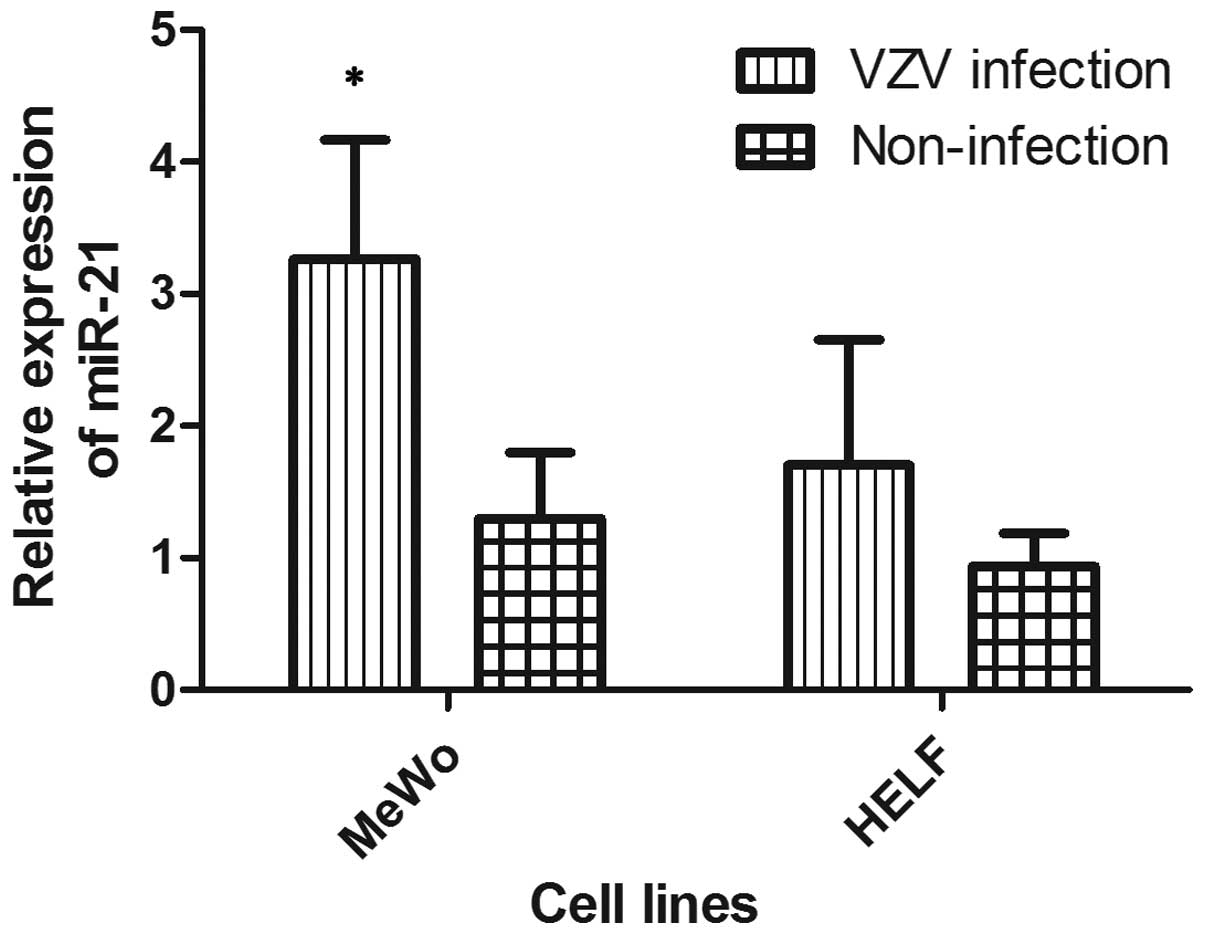
infection, the expression of miR-21 in HELF and MeWo was examined
by relative qPCR following VZV infection. The results showed that
miR-21 was significantly upregulated in MeWo cells following VZV
infection, compared with that in MeWo cells without viral infection
(P=0.03). However, the results indicated that the expression level
of miR-21 was not significantly higher in VZV-infected HELF cells
compared with that in HELF cells without VZV infection. The mean
expression level of miR-21 in HELF cells infected with VZV was
higher than that without VZV infection (1.7 and 0.93,
respectively), as shown in Fig. 1.
This indicates that the expression of miR-21 is increased in
vitro following VZV infection.
Overexpression of miR-21 is associated
with the activation of the STAT3 signaling pathway in vitro
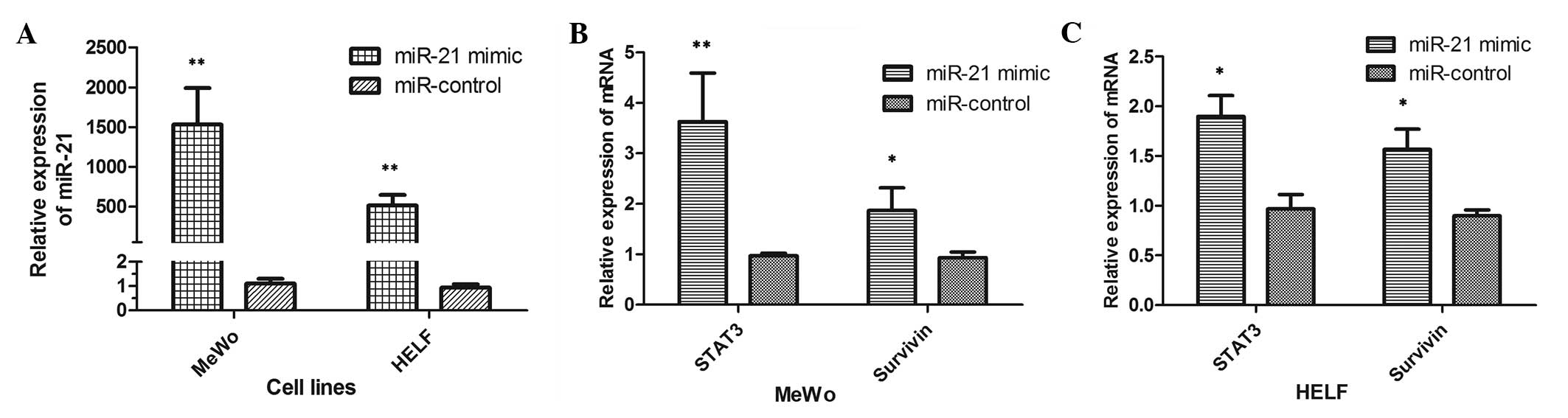
The ectopic expression of miR-21 was increased by
transfection with an miR-21-mimic to investigate whether or not
there was an association between miR-21 and STAT3. The expression
of miR-21 was confirmed, which certified the efficiency of
transfection, as shown in Fig. 1A.
The results showed the cells had been successfully transfected with
the miR-21-mimic. Next, the differences in the mRNA expression
levels of STAT3 and survivin were compared. The mRNA expression
levels of STAT3 (P=0.009) and survivin (P=0.026) were significantly
enhanced in the MeWo cells transfected with miR-21-mimic (Fig. 2B). Comparable results were obtained
for HELF cells (STAT3, P=0.020; survivin, P=0.034), as shown in
Fig. 2C. The results showed that
the overexpression of miR-21 stimulated the expression of STAT3 and
survivin in vitro. These observations indicate that the
upregulation of miR-21 is associated with activation of the STAT3
signaling pathway in vitro.
Overexpression of miR-21 promotes VZV
replication in vitro
Since miR-21 overexpression was identified in MeWo
cell lines, whether changes in miR-21 expression were associated
with changes in VZV replication was investigated. Transfection of
MeWo cells with miR-21-mimic or miR-control was performed in order
to detect any differences in replication following VZV infection at
a MOI of 1 × 10−3. Detection of miR-21 expression
confirmed the efficiency of transfection, as shown in Fig. 3A. Furthermore, the results
indicated that the higher the expression levels of miR-21, the
higher the virus titer was. However, the virus titer did not
increase significantly when miR-21 was transfected at a
concentration of 200 nM, as shown in Fig. 3B. The virus titer in HELF cells
transfected with 100 nM miR-21-mimic was also detected. The results
showed the mean virus titer to be 1.29×106 PFU/ml in
HELF cells transfected with miR-21-mimic, compared with
3.55×103 PFU/ml in cells transfected with the
miR-control (Fig. 3C). Therefore,
this indicates that the ectopic expression of miR-21 contributes to
VZV replication.
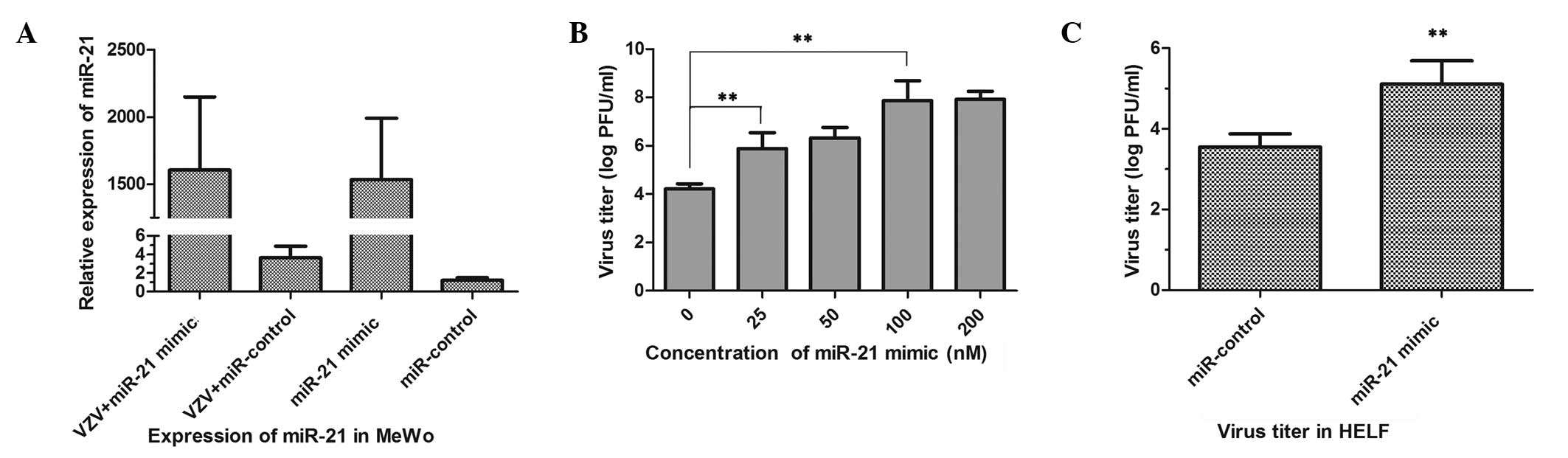 | Figure 3Upregulation of miR-21 stimulates VZV
replication. (A) Expression of miR-21 in MeWo cells transfected
with 100 nM miR-control or 100 nM miR-21-mimic, with and without
VZV infection at a MOI of 1 × 10−3 for 24 h, to
investigate the interaction of VZV and upstream miR-21. (B) Virus
titers, performed by standard VZV plaque assays, were compared
among MeWo cells transfected with miR-21-mimic at concentrations of
0, 25, 50, 100 and 200 nM following VZV infection for 24 h. (C)
Comparison between the virus titers in HELF cells transfected with
100 nM miR-21-mimic and 100 nM miR-control following VZV infection
for 24 h. *P<0.05 and **P<0.01 compared
with control. miR-21, microRNA-21; MeWo, human malignant melanoma
cells; HELF, human embryonic lung fibroblasts; VZV,
varicella-zoster virus; MOI, multiplicity of infection. |
Overexpression of miR-21 promotes VZV
replication by STAT3 activation in vitro
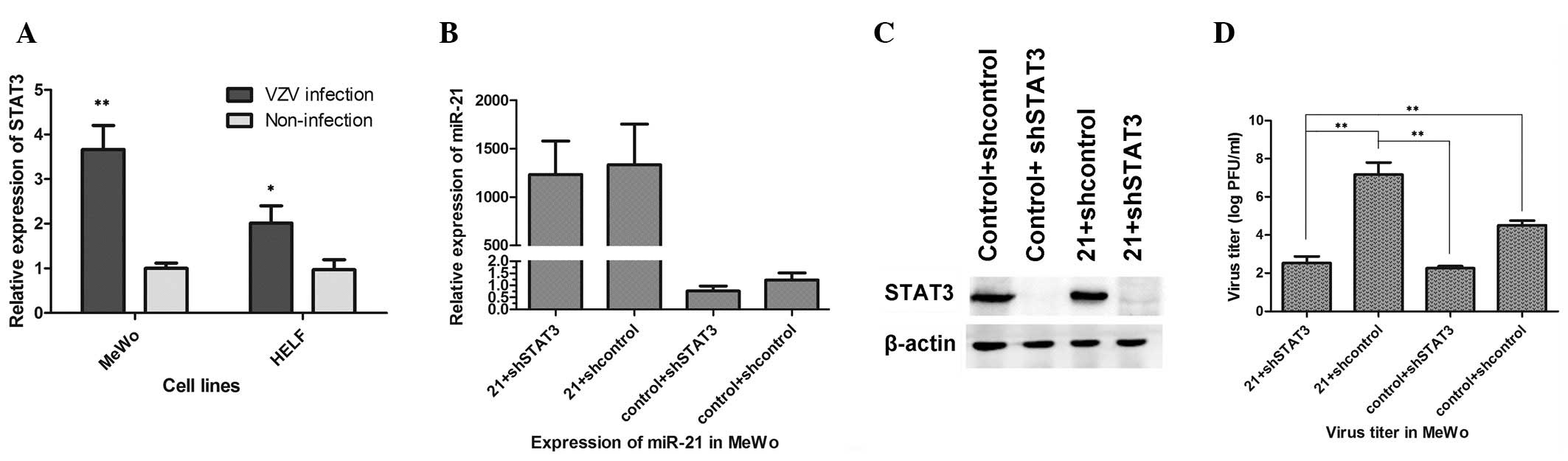
The relative mRNA expression levels of STAT3 were
assayed following VZV infection to confirm the association between
VZV infection and the STAT3 signaling pathway. The results
indicated that the relative mRNA expression level of STAT3 in MeWo
cells infected with VZV was significantly higher compared with that
in MeWo cells not infected with VZV (P=0.001). Comparable results
were obtained in HELF cells (P=0.016; Fig. 4A).
To further investigate the associations among
miR-21, STAT3 and VZV infection, the expression levels of miR-21
were increased by transfection with miR-21-mimic and STAT3 genes
were silenced following VZV infection in MeWo cells. The four
groups were designated as follows: 21 + shSTAT3, cells
co-transfected with miR-21-mimic and STAT3 knockdown; 21 +
shcontrol, cells co-transfected with miR-21-mimic and control
knockdown; control + shSTAT3, cells co-transfected with miR-control
and STAT3 knockdown; and control + shcontrol, cells co-transfected
with miR-control and control knockdown. The expression of miR-21
was confirmed in the in MeWo cells of the four groups. Higher
miR-21 expression levels were observed in the 21 + shSTAT3 and 21 +
shcontrol groups compared with those in the other two groups, as
shown in Fig. 4B. The expression
of STAT3 protein was detected by western blot analysis. The results
showed that the expression level of STAT3 protein was extremely low
in the 21 + shSTAT3 and control + shSTAT3 groups, as shown in
Fig. 4C. This demonstrated that
the STAT3 gene was knocked down effectively. Finally, the virus
titers were assayed and the mean titers were 3.47×102
PFU/ml (21 + shSTAT3), 1.48×107 PFU/ml (21 + shcontrol),
1.82×102 PFU/ml (control + shSTAT3) and
3.23×104 PFU/ml (control + shcontrol), as shown in
Fig. 4D. These results indicate
that VZV replication was significantly decreased with STAT3
knockdown. In addition, the overexpression of miR-21 stimulated VZV
replication, but not when the STAT3 gene was silenced in MeWo cell
lines. The results indicate that the ectopic overexpression of
miR-21 increased VZV replication by activating STAT3 in
vitro.
Discussion
MiRNAs, including miR-21, have important effects on
viral replication, and function as inhibiting or enhancing factors
(7). Moreover, it has been
documented that miR-21 may be highly involved in certain
cell-signaling pathways that modulate the immune system, including
the IFN, nuclear factor κB (NF-κB), extracellular signal-regulated
kinases-mitogen-activated protein kinase and STAT3 signaling
pathways (14,25). It has been hypothesized that
associations exist among miRNA, the immune system and viral
replication. Sen et al revealed that STAT3 promoted VZV
replication (23). However, it
remains unknown as to whether miRNAs regulate the mechanism of VZV
replication.
In the present study, the expression of miR-21 was
observed to be upregulated significantly in MeWo cells following
infection with VZV. Upregulation of miR-21 also occurred in HELF
cells following VZV infection; however, the increase in miR21
levels in HELF cells was not found to be significant. To confirm
the effect of miR-21 on VZV replication, MeWo and HELF cell lines
were transfected with miR-21-mimic. The transfection was shown to
stimulate VZV replication, which indicates that miR-21 plays an
important role as a promoter in VZV replication.
STAT3 responds to a variety of signals, including
growth factors, cytokines and oncogenes. STAT3 is modulated by the
miR-17-92 cluster, which affects tumorigenesis (26,27).
Furthermore, the miR-21/STAT3 interaction has been studied
extensively. Expression of miR-21 and upstream STAT3 occurs
simultaneously in myeloma cells and the knockdown of STAT3
prohibits the upregulation of miR-21 (28). However, conflicting results have
been produced with regard to the miR-21/STAT3 interaction in human
glioma cells. In one study, it was found that increased STAT3
expression resulted in the downregulation of miR-21. This was
confirmed by a further study that evaluated changes in the
expression of miR-21 with the overexpression or knockdown of STAT3
(29). The conflicting results
indicate that the miR-21/STAT3 interaction may be largely
determined by the microenvironment.
In the present study, miR-21 was overexpressed in
MeWo and HELF cell lines by transfection with miR-21-mimic. The
upregulation of STAT3 occurred concurrently with miR-21
overexpression in these cell lines. This was further supported by
evaluating miR-21 expression in cells with silenced STAT3 genes, as
shown in Fig. 4B. The observations
demonstrated that the miR-21/STAT3 interaction was reinforced
mutually in MeWo and HELF cell lines. In addition, previous
observations indicated that the upregulation of miR-21 had positive
effects on VZV replication. Subsequently, the association between
the STAT3/miR-21 interaction and its effects on viral replication
and evasion of the host immune system required investigation.
Specifically, the roles that miR-21/STAT3 interactions play in VZV
replication were considered. When miR-21 was upregulated, the viral
titer when STAT3 was knocked down was found to be significantly
lower compared with the viral titer for non-silenced STAT3. This
result indicated that the miR-21/STAT3 interaction plays a positive
role in VZV replication. Additionally, when STAT3 was knocked down,
a comparison of the viral titers in cells transfected with
miR-21-mimic with those in cells transfected with miR-control
indicated that the mechanism by which miR-21 promotes VZV
replication is strictly regulated by STAT3, .
Several viruses have been shown to directly or
indirectly decrease the expression of factors associated with the
host innate immune system, including proteins, genes and cell
signaling pathways, in order to enhance their own replication
(30). VZV replicates not only by
preventing IFN induction via inhibition of the IFN regulatory
factor 3 mediated IFN-β pathway, but also by inhibiting certain
signaling pathways, including NF-κB and STAT1 pathways (31–33).
Conversely, host cellular factors may also be used to viral
advantage. The activation of STAT3 and upregulation of survivin is
a necessary mechanism of VZV pathogenesis and is important for the
pathogenesis of lytic and tumorigenic herpesviruses. The present
study of VZV replication further supports the hypothesis that
viruses use cellular factors to their advantage.
In conclusion, cellular miR-21 was found to be a
promoting factor of VZV replication. The study also demonstrated
that STAT3 was involved in the mechanism by which miR-21 modulates
VZV replication. In addition, the enforced expression of miR-21 was
shown to promote the replication of VZV by activating STAT3 in
vitro. This study increased the understanding of the mechanism
of VZV replication, a critical part of the VZV pathogenesis.
Acknowledgements
The study was supported by a grant from the Southern
Medical University and General Hospital of Guangzhou Military
Command of PLA.
References
|
1
|
Gilden D, Mahalingam R, Nagel MA,
Pugazhenthi S and Cohrs RJ: Review: The neurobiology of varicella
zoster virus infection. Neuropathol Appl Neurobiol. 37:441–463.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoleri O, Olmez N, Oztura I, Sengül I,
Günaydin R and Memiş A: Segmental zoster paresis of the upper
extremity: a case report. Arch Phys Med Rehabil. 86:1492–1494.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oliver SL, Brady JJ, Sommer MH, et al: An
immunoreceptor tyrosine-based inhibition motif in varicella-zoster
virus glycoprotein B regulates cell fusion and skin pathogenesis.
Proc Natl Acad Sci USA. 110:1911–1916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arvin AM, Moffat JF, Sommer M, et al:
Varicella-zoster virus T cell tropism and the pathogenesis of skin
infection. Curr Top Microbiol Immunol. 342:189–209. 2010.PubMed/NCBI
|
|
5
|
Zerboni L, Ku CC, Jones CD, Zehnder JL and
Arvin AM: Varicella-zoster virus infection of human dorsal root
ganglia in vivo. Proc Natl Acad Sci USA. 102:6490–6495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berkhout B and Jeang KT: RISCy business:
MicroRNAs, pathogenesis, and viruses. J Biol Chem. 282:26641–26645.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grassmann R and Jeang KT: The roles of
microRNAs in mammalian virus infection. Biochim Biophys Acta.
1779:706–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartels CL and Tsongalis GJ: MicroRNAs:
novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang J, Wang F, Argyris E, et al:
Cellular microRNAs contribute to HIV-1 latency in resting primary
CD4+ T lymphocytes. Nat Med. 13:1241–1247. 2007.PubMed/NCBI
|
|
10
|
Gao L, Guo XK, Wang L, et al: MicroRNA 181
suppresses porcine reproductive and respiratory syndrome virus
(PRRSV) infection by targeting PRRSV receptor CD163. J Virol.
87:8808–8812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung YJ, Kim JW, Park SJ, et al:
c-Myc-mediated overexpression of miR-17-92 suppresses replication
of hepatitis B virus in human hepatoma cells. J Med Virol.
85:969–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin J, Tang S, Xia L, et al: MicroRNA-501
promotes HBV replication by targeting HBXIP. Biochem Biophys Res
Commun. 430:1228–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye X, Hemida MG, Qiu Y, Hanson PJ, Zhang
HM and Yang D: MiR-126 promotes coxsackievirus replication by
mediating cross-talk of ERK1/2 and Wnt/β-catenin signal pathways.
Cell Mol Life Sci. 70:4631–4644. 2013.PubMed/NCBI
|
|
14
|
Kumarswamy R, Volkmann I and Thum T:
Regulation and function of miRNA-21 in health and disease. RNA
Biol. 8:706–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Chen J, Wang H, et al: HCV-induced
miR-21 contributes to evasion of host immune system by targeting
MyD88 and IRAK1. PLoS Pathog. 9:e10032482013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keck K, Volper EM, Spengler RM, et al:
Rational design leads to more potent RNA interference against
hepatitis B virus: factors affecting silencing efficiency. Mol
Ther. 17:538–547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosato P, Anastasiadou E, Garg N, et al:
Differential regulation of miR-21 and miR-146a by Epstein-Barr
virus-encoded EBNA2. Leukemia. 26:2343–2352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Fits L, van Kester MS, Qin Y, et
al: MicroRNA-21 expression in CD4+ T cells is regulated by STAT3
and is pathologically involved in Sézary syndrome. J Invest
Dermatol. 131:762–768. 2011.
|
|
19
|
Jarnicki A, Putoczki T and Ernst M: Stat3:
linking inflammation to epithelial cancer - more than a ‘gut’
feeling? Cell Div. 5:142010.PubMed/NCBI
|
|
20
|
Al Zaid Siddiquee K and Turkson J: STAT3
as a target for inducing apoptosis in solid and hematological
tumors. Cell Res. 18:254–267. 2008.PubMed/NCBI
|
|
21
|
Wang Z, Luo F, Li L, et al: STAT3
activation induced by Epstein-Barr virus latent membrane protein1
causes vascular endothelial growth factor expression and cellular
invasiveness via JAK3 and ERK signaling. Eur J Cancer.
46:2996–3006. 2010. View Article : Google Scholar
|
|
22
|
Punjabi AS, Carroll PA, Chen L and
Lagunoff M: Persistent activation of STAT3 by latent Kaposi’s
sarcoma-associated herpesvirus infection of endothelial cells. J
Virol. 81:2449–2458. 2007.PubMed/NCBI
|
|
23
|
Sen N, Che X, Rajamani J, et al: Signal
transducer and activator of transcription 3 (STAT3) and survivin
induction by varicella-zoster virus promote replication and skin
pathogenesis. Proc Natl Acad Sci USA. 109:600–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holtick U, Vockerodt M, Pinkert D, et al:
STAT3 is essential for Hodgkin lymphoma cell proliferation and is a
target of tyrphostin AG17 which confers sensitization for
apoptosis. Leukemia. 19:936–944. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kohanbash G and Okada H: MicroRNAs and
STAT interplay. Semin Cancer Biol. 22:70–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ventura A, Young AG, Winslow MM, et al:
Targeted deletion reveals essential and overlapping functions of
the miR-17 through 92 family of miRNA clusters. Cell. 132:875–886.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carraro G, El-Hashash A, Guidolin D, et
al: miR-17 family of microRNAs controls FGF10-mediated embryonic
lung epithelial branching morphogenesis through MAPK14 and STAT3
regulation of E-Cadherin distribution. Dev Biol. 333:238–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Löffler D, Brocke-Heidrich K, Pfeifer G,
et al: Interleukin-6 dependent survival of multiple myeloma cells
involves the Stat3-mediated induction of microRNA-21 through a
highly conserved enhancer. Blood. 110:1330–1333. 2007.PubMed/NCBI
|
|
29
|
Ohno M, Natsume A, Kondo Y, et al: The
modulation of microRNAs by type I IFN through the activation of
signal transducers and activators of transcription 3 in human
glioma. Mol Cancer Res. 7:2022–2030. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cullen BR: MicroRNAs as mediators of viral
evasion of the immune system. Nat Immunol. 14:205–210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sen N, Sommer M, Che X, White K, Ruyechan
WT and Arvin AM: Varicella-zoster virus immediate-early protein 62
blocks interferon regulatory factor 3 (IRF3) phosphorylation at key
serine residues: a novel mechanism of IRF3 inhibition among
herpesviruses. J Virol. 84:9240–9253. 2010. View Article : Google Scholar
|
|
32
|
Zhu H, Zheng C, Xing J, et al:
Varicella-zoster virus immediate-early protein ORF61 abrogates the
IRF3-mediated innate immune response through degradation of
activated IRF3. J Virol. 85:11079–11089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sloan E, Henriquez R, Kinchington PR,
Slobedman B and Abendroth A: Varicella-zoster virus inhibition of
the NF-κB pathway during infection of human dendritic cells: role
for open reading frame 61 as a modulator of NF-κB activity. J
Virol. 86:1193–1202. 2012.
|