|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members. Strategies for
subtypes - dealing with the diversity of breast cancer: highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adnane L, Trail PA, Taylor I and Wilhelm
SM: Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that
targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases
VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 407:597–612.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilhelm S, Carter C, Lynch M, et al:
Discovery and development of sorafenib: a multikinase inhibitor for
treating cancer. Nat Rev Drug Discov. 5:835–844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar
|
|
6
|
Yu C, Bruzek LM, Meng XW, et al: The role
of Mcl-1 downregulation in the proapoptotic activity of the
multikinase inhibitor BAY 43-9006. Oncogene. 24:6861–6869. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Escudier B, Eisen T, Stadler WM, et al;
TARGET Study Group. Sorafenib in advanced clear-cell renal-cell
carcinoma. New Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llovet JM, Ricci S, Mazzaferro V, et al;
SHARP Investigators Study Group. Sorafenib in advanced
hepatocellular carcinoma. New Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
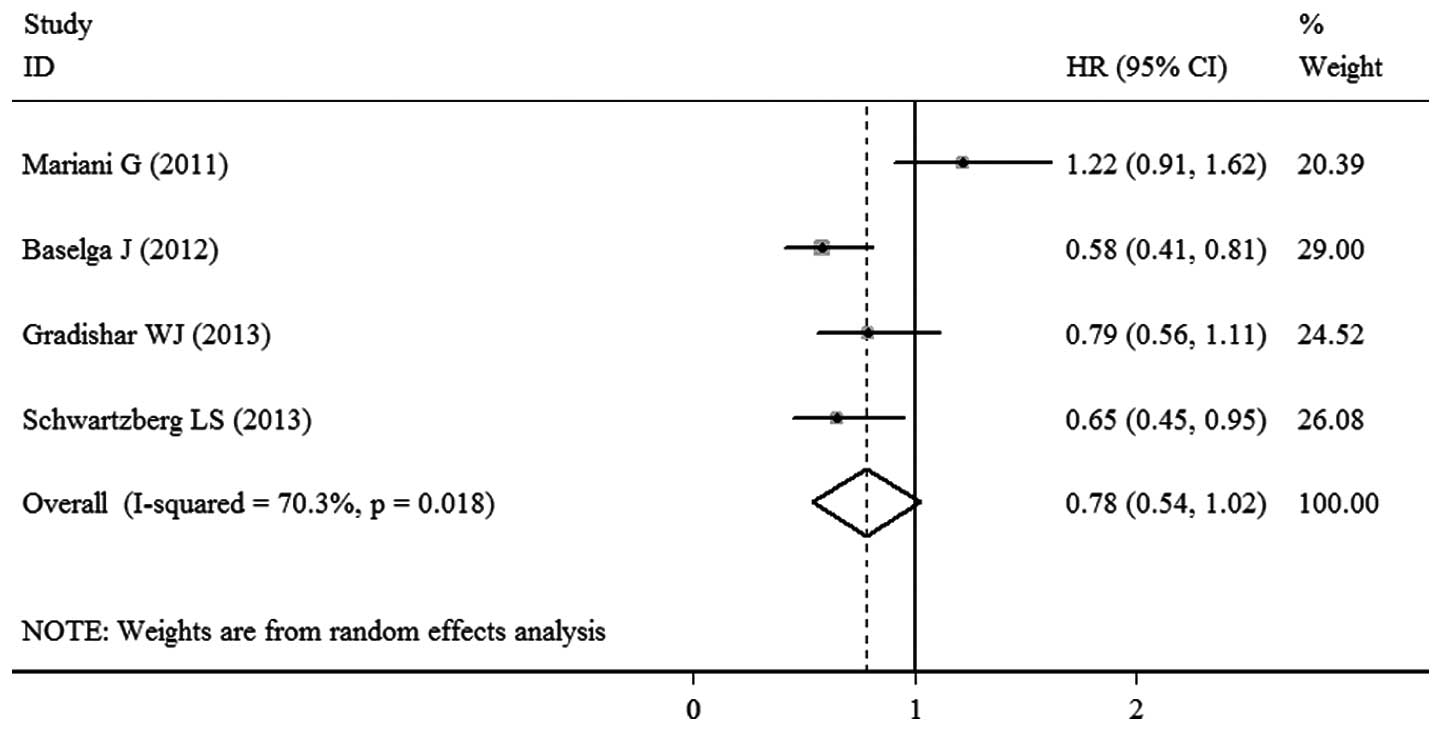
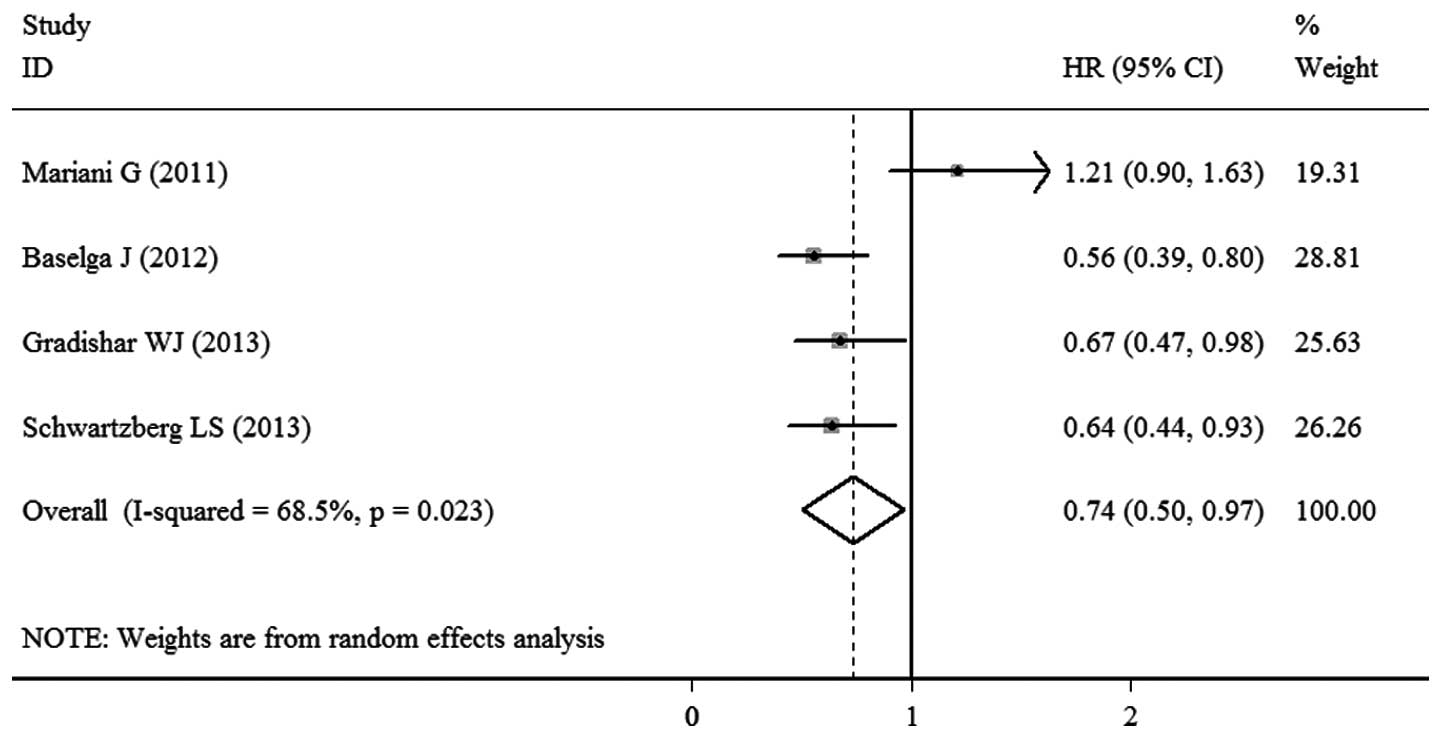
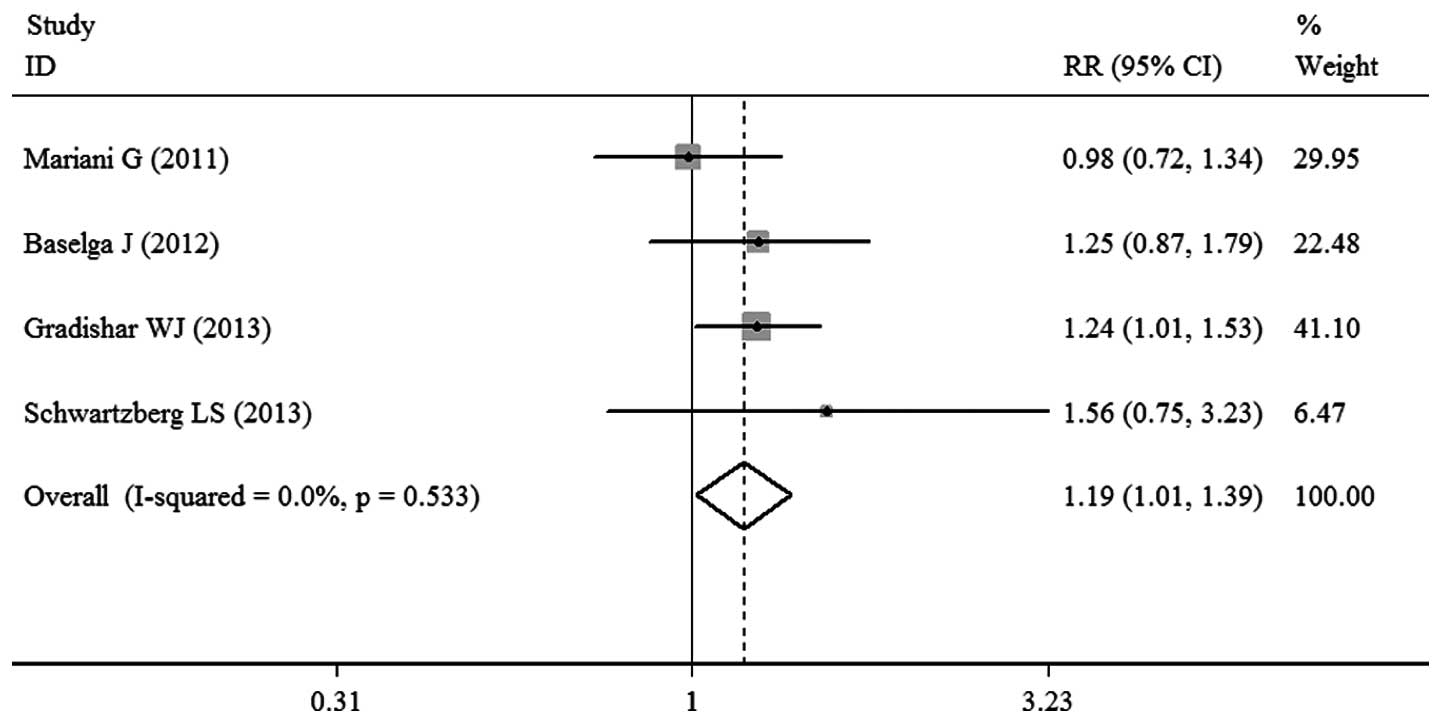
Mariani G, Burdaeva O, Roman L, et al: A
double-blind, randomized phase lib study evaluating the efficacy
and safety of sorafenib (SOR) compared to placebo (PL) when
administered in combination with docetaxel and/or letrozole in
patients with metastatic breast cancer (MBC): FM-B07-01 Trial. Eur
J Cancer. 47:102011. View Article : Google Scholar
|
|
11
|
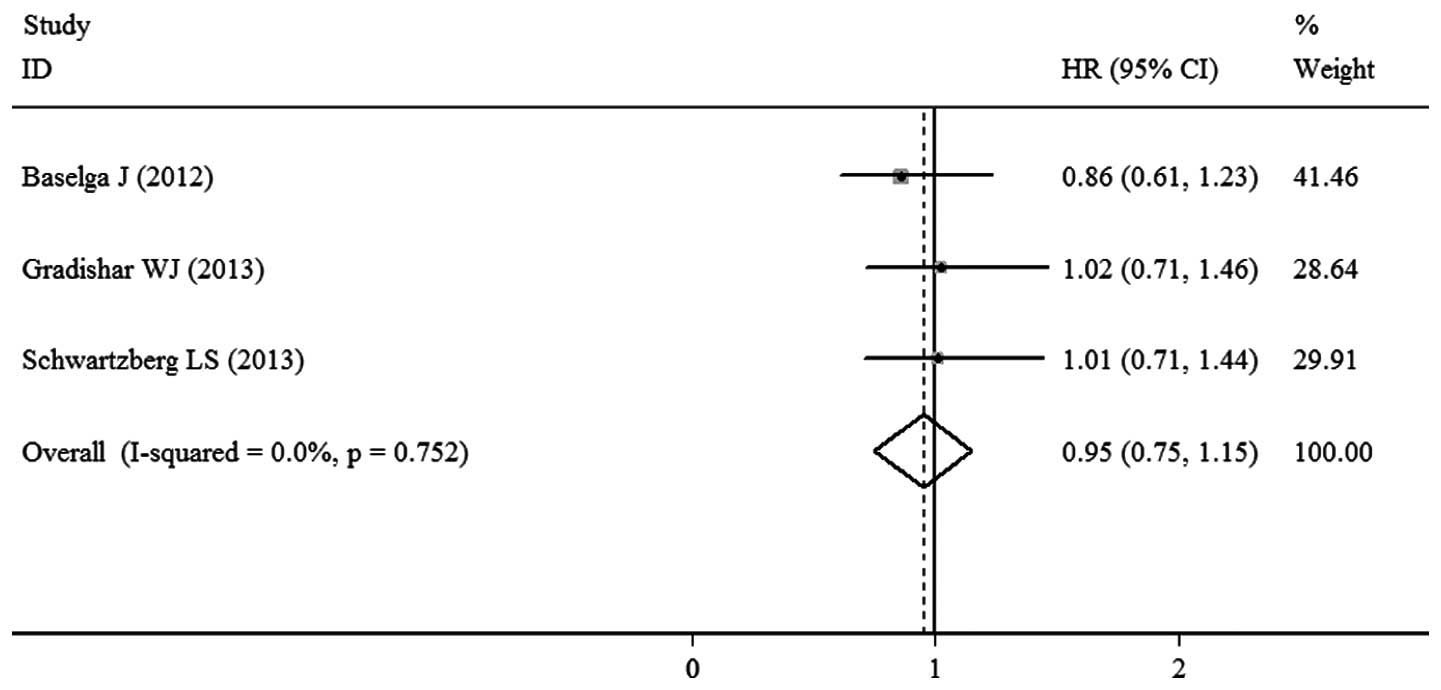
Baselga J, Segalla JG, Roché H, et al:
Sorafenib in combination with capecitabine: an oral regimen for
patients with HER2-negative locally advanced or metastatic breast
cancer. J Clin Oncol. 30:1484–1491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gradishar WJ, Kaklamani V, Sahoo TP, et
al: A double-blind, randomised, placebo-controlled, phase 2b study
evaluating sorafenib in combination with paclitaxel as a first-line
therapy in patients with HER2-negative advanced breast cancer. Eur
J Cancer. 49:312–322. 2013. View Article : Google Scholar
|
|
13
|
Schwartzberg LS, Tauer KW, Hermann RC, et
al: Sorafenib or placebo with either gemcitabine or capecitabine in
patients with HER-2-negative advanced breast cancer that progressed
during or after bevacizumab. Clin Cancer Res. 19:2745–2754. 2013.
View Article : Google Scholar
|
|
14
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
15
|
Moreno-Aspitia A, Morton RF, Hillman DW,
et al: Phase II trial of sorafenib in patients with metastatic
breast cancer previously exposed to anthracyclines or taxanes:
North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J
Clin Oncol. 27:11–15. 2009. View Article : Google Scholar
|
|
16
|
Bianchi G, Loibl S, Zamagni C, et al:
Phase II multicenter, uncontrolled trial of sorafenib in patients
with metastatic breast cancer. Anticancer Drugs. 20:616–624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isaacs C, Herbolsheimer P, Liu MC, et al:
Phase I/II study of sorafenib with anastrozole in patients with
hormone receptor positive aromatase inhibitor resistant metastatic
breast cancer. Breast Cancer Res Treat. 125:137–143. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spigel DR, Hainsworth JD, Burris HA 3rd,
et al: A pilot study of adjuvant doxorubicin and cyclophosphamide
followed by paclitaxel and sorafenib in women with node-positive or
high-risk early-stage breast cancer. Clin Adv Hematol Oncol.
9:280–286. 2011.PubMed/NCBI
|
|
19
|
Lyons JF, Wilhelm S, Hibner B and Bollag
G: Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer.
8:219–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanne JH: FDA cancels approval for
bevacizumab in advanced breast cancer. BMJ. 343:d76842011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller K, Wang M, Gralow J, et al:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. New Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miles DW, Chan A, Dirix LY, et al: Phase
III study of bevacizumab plus docetaxel compared with placebo plus
docetaxel for the first-line treatment of human epidermal growth
factor receptor 2-negative metastatic breast cancer. J Clin Oncol.
28:3239–3247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miles D, Harbeck N, Escudier B, et al:
Disease course patterns after discontinuation of bevacizumab:
pooled analysis of randomized phase III trials. J Clin Oncol.
29:83–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elting J, Bigwood D, Brown-Shimer S, et
al: Biomarkers associated with clinical outcomes in TARGETs, a
Phase III single-agent, placebo-controlled study of sorafenib in
advanced renal cell carcinoma. Proc Amer Assoc Cancer Res.
47:683–684. 2006.
|
|
26
|
Abou-Alfa GK, Schwartz L, Ricci S, et al:
Phase II study of sorafenib in patients with advanced
hepatocellular carcinoma. J Clin Oncol. 24:4293–4300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jubb AM, Oates AJ, Holden S and Koeppen H:
Predicting benefit from anti-angiogenic agents in malignancy. Nat
Rev Cancer. 6:626–635. 2006. View
Article : Google Scholar : PubMed/NCBI
|