Introduction
Despite advances in diagnosis and treatment over
several decades, breast cancer remains the highest cause of cancer
mortality in females (1).
Metastasis and recurrence often contribute to the poor clinical
outcomes of breast cancer patients, and metastatic disease remains
incurable. Combination chemotherapy regimens have demonstrated
clinical benefits compared with single-agent regimens, but have
also shown increased toxicity. Thus, the development of new drugs
is critical to further improve and advance the treatment of breast
cancer. For human epidermal growth factor receptor 2
(HER2)-positive advanced breast cancer (ABC), trastuzumab in
combination with chemotherapy has become the standard first-line
adjuvant treatment (2). However,
with regard to HER2-negative ABC, there remain no effective
targeted therapies. Therefore, the development and testing of novel
agents that target pathways considered to be involved in the
pathogenesis of HER2-negative ABC is required.
Sorafenib is an oral multikinase inhibitor that
exhibits antiangiogenic and antiproliferative activity. The targets
of sorafenib include the Ras/Raf/mitogen-activated protein kinase
(MAPK) signaling pathway, platelet-derived growth factor receptor-α
and β, vascular endothelial growth factor receptors (VEGFRs)-1, 2
and 3 and c-KIT and FLT3 kinases (3,4).
These kinases play key roles in tumor cell proliferation, apoptosis
and tumor angiogenesis (5).
Compared with antiangiogenic monoclonal antibodies, including
bevacizumab, a potential advantage of sorafenib is that it inhibits
other receptors and intracellular signals involved in
tumorigenesis, potentially offering multiple pathway inhibition.
Preclinical studies have demonstrated that sorafenib decreases
tumor cell proliferation in vitro, inhibits angiogenesis and
induces apoptosis (6,7). In addition, sorafenib has been
approved for the treatment of advanced renal cell (RCC) and
unresectable hepatocellular carcinomas (HCC) (8,9).
With regard to breast cancer, several randomized controlled
clinical trials (RCTs) have compared sorafenib with a placebo for
the treatment of ABC, with four studies reporting their results
(10–13). In order to improve the evaluation
of the potential role of sorafenib in combination with known
effective palliative treatments for HER2-negative ABC, a
meta-analysis was conducted to assess the efficacy and safety of
sorafenib treatment in patients with HER2-negative ABC.
Materials and methods
Literature search and selection
An extensive search of PubMed (www.ncbi.nlm.nih.gov/pubmed), EMBASE (www.elsevier.com/online-tools/embase),
the Cochrane Library Databases (www.thecochranelibrary.com), American Society of
Clinical Oncology (www.asco.org) and the European Society
for Medical Oncology (www.esmo.org) was conducted up to
September 2013, using the following search terms: ‘Advanced breast
cancer’ and ‘sorafenib’. In addition, associated keywords and their
synonyms were included in the search strategy and reference lists
were scanned for additional publications. RCTs that investigated
the efficacy of sorafenib in HER2-negative locally
recurrent/metastatic breast cancer were considered to be eligible.
Trials had to meet the following inclusion criteria. Firstly, all
the patients had a confirmed pathological diagnosis of
HER2-negative advanced/metastatic breast cancer and were randomly
assigned to treatment. Secondly, sorafenib or sorafenib-based
therapy was administered to the research group and compared with
placebo or placebo-based therapy that was administered to the
control group. Finally, the studies provided information on
survival rates, including progression-free survival (PFS), time to
progression (TTP), overall survival (OS) and data regarding the
overall response rate (ORR) and adverse events (AEs).
Data extraction and quality
assessment
Data extraction was performed independently by two
authors according to the aforementioned inclusion criteria. The
following information was collected: First author, year of
publication, methodological quality, number of patients, patient
characteristics, hazard ratios (HRs) and 95% confidence intervals
(CIs) for PFS, TTP and OS, data regarding AEs, details of subgroup
analysis and the number of patients acquired overall response (the
sum of complete and partial tumour responses to drugs) that was
assessed with Response Evaluation Criteria In Solid Tumors.
The quality of each retrieved study was
independently assessed by the two authors, in accordance with the
instrument reported by Jadad et al (14). Firstly, it was determined whether
the trial reported an appropriate randomization method (score 0–2).
Secondly, it was determined whether the trials reported an
appropriate blinding method (score 0–2) and finally, whether the
trials reported withdrawals and dropouts (score 0–1). The final
score ranged between 0 and 5 for each study with higher scores
indicating better methodology.
Statistical analysis
Statistical analyses were performed using Stata 12.0
statistical software (StataCorp LP, College Station, TX, USA) for
meta-analysis. Survival outcome data, including PFS, TTP and OS,
were polled using the time-to-event HRs and 95% CIs as the
operational measures, while relative risk (RR) for overall response
to treatment and odds ratios (OR) for various types of toxicity
were also calculated. Statistical heterogeneity among the trials
was analyzed using the χ2 test and the I2
measure of inconsistency; P<0.1 or I2>50%
indicated significant heterogeneity. Fixed-effects models were used
in all analyses unless heterogeneity existed (P<0.1 or
I2>50%). All statistical tests were two-sided and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Literature search
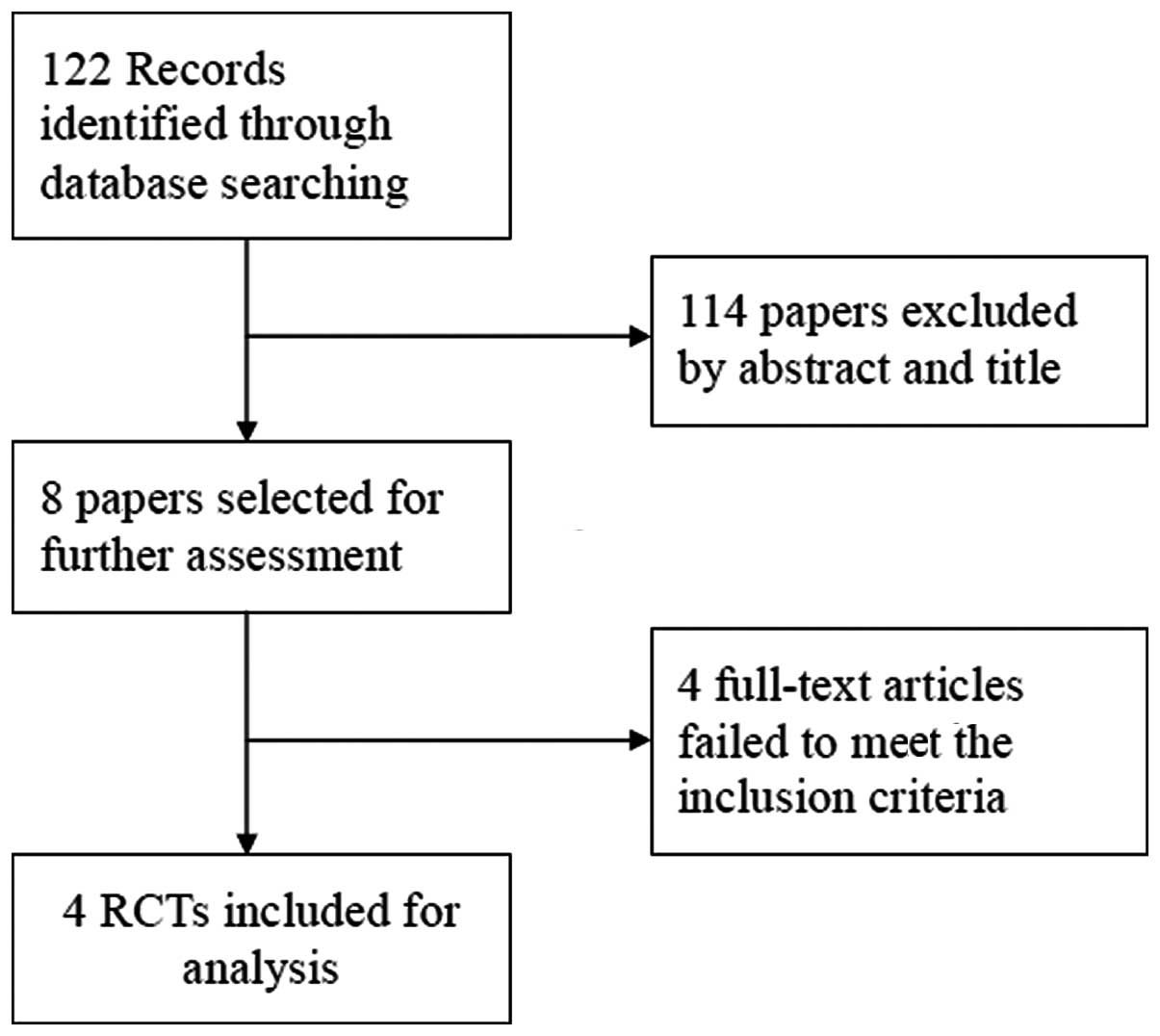
A total of 122 potentially relevant studies were
identified by the literature search. Following the review of each
publication, 114 studies were excluded as they were not relevant to
the aim of the present study, while eight relevant studies were
selected for detailed evaluation. Four studies were excluded prior
to analysis (15–18). Ultimately, four RCTs, including
four phase IIB studies, were selected for analysis, involving a
total of 844 patients (10–13).
The study search process is shown in Fig. 1.
Study characteristics
Characteristics of the four studies are shown in
Table I. The four studies were
phase IIB randomized, double-blind, placebo-controlled screening
trials that were known collectively as Trials to Investigate the
Effects of Sorafenib and were undertaken in patients with
HER2-negative ABC. All the studies reported PFS and TTP data
following treatment, as well as ORRs and AEs. Three studies
reported the OS rates. The total number of included patients was
844. Among them, 426 patients received sorafenib-based therapy and
418 patients received placebo-based therapy. All the patients
received treatment until disease progression, intolerable toxicity,
consent was withdrawn or treatment was discontinued for other
reasons.
 | Table IBaseline characteristics of the four
eligible RCTs used in the meta-analysis. |
Table I
Baseline characteristics of the four
eligible RCTs used in the meta-analysis.
| Parameter | FM-B07-01 | SOLTI-0701 | NU07B1 | AC01B07 |
|---|
| Author | Mariani et al
(10) | Baselga et al
(11) | Gradishar et
al (12) | Schwartzberg et
al (13) |
| Publish year | 2011 | 2012 | 2013 | 2013 |
| Population | Italy, Germany,
Poland, Russia | Spain, France,
Brazil | India, USA,
Brazil | USA |
| Phase | IIB | IIB | IIB | IIB |
| Sample size, n
(S/P) | 208 (111/107) | 229 (115/114) | 237 (119/118) | 160 (81/79) |
| Age, years, S/P | NC | 55.1/54.4 | 50.6/53.1 | 53.5/54.2 |
| Therapy line | First | First/second | First | First/second |
| Treatment | SOR + DOC and/or LET
vs. PLA + DOC and/or LET | SOR + CAP vs. PLA +
CAP | SOR + PAC vs. PLA +
PAC | SOR + GEM/CAP vs. PLA
+ GEM/CAP |
| Design | Parallel
randomized | Parallel
randomized | Parallel
randomized | Parallel
randomized |
| Blinding | Double-blind | Double-blind | Double-blind | Double-blind |
| Multicenter | Yes | Yes | Yes | Yes |
| Allocation
concealment | Yes | Yes | Yes | Yes |
| ITT analysis | Yes | Yes | Yes | Yes |
| Survival
analysis | PFS/TTP | PFS/TTP/OS | PFS/TTP/OS | PFS/TTP/OS |
| HR | Reported in text | Reported in text | Reported in text | Reported in text |
| Quality score | 5 | 5 | 5 | 5 |
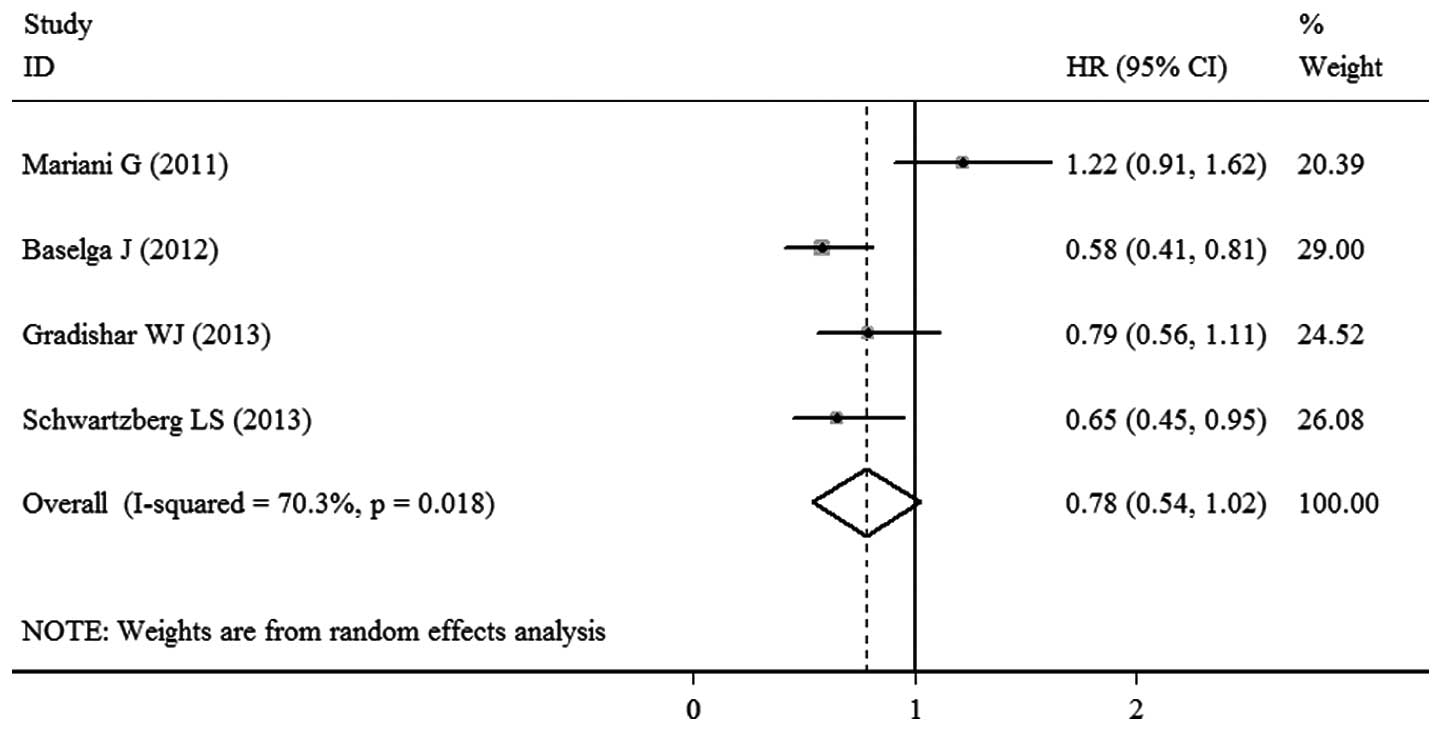
PFS
Data regarding PFS were available in the four
trials. Significant heterogeneity was observed among the studies
(P=0.018; I2=70.3%), thus, the pooled HR for PFS was
calculated using a random-effects model, with a result of 0.78 (95%
CI, 0.54–1.02; P<0.00001; Fig.
2). This result demonstrated that there was a statistically
significant difference between the sorafenib- and placebo-based
therapy groups with regard to PFS.
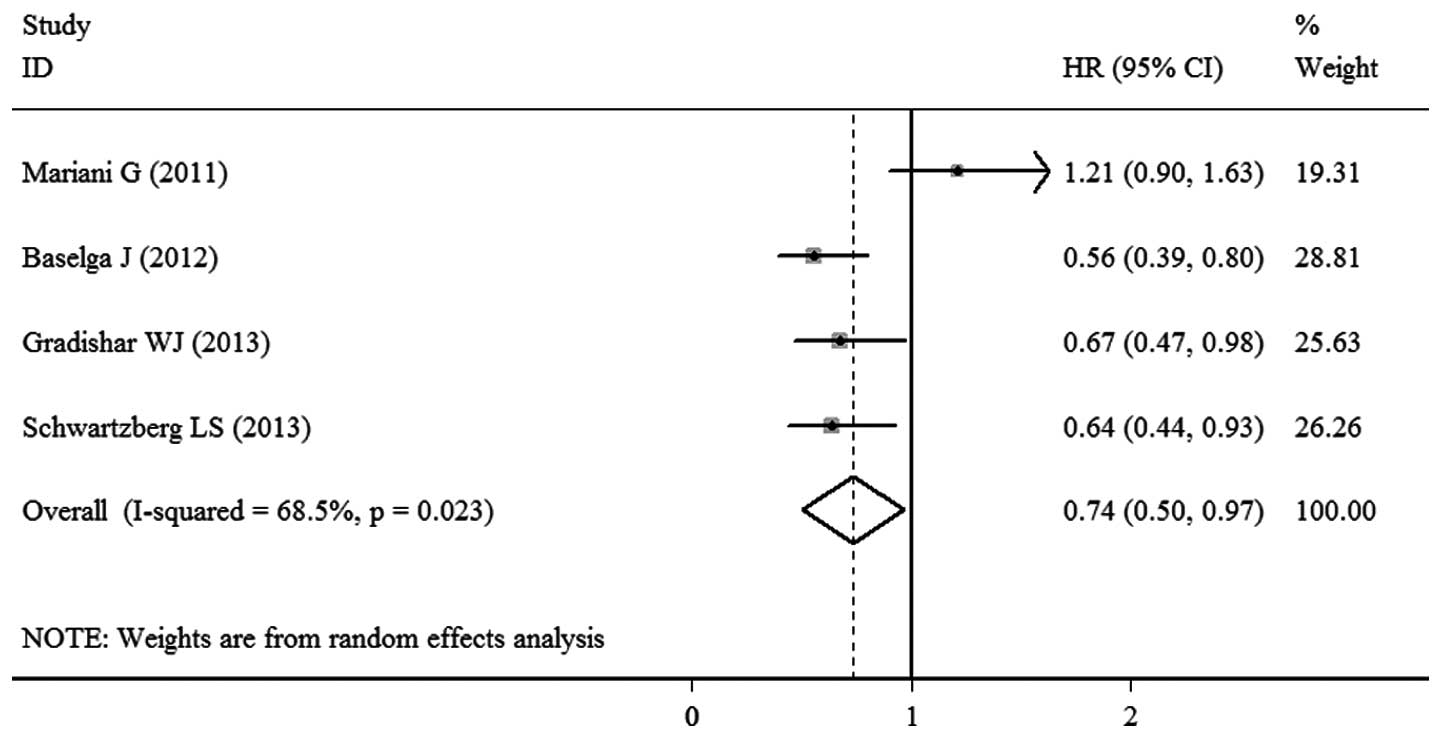
TTP
TTP data were available in the four trials. Due to
the significant heterogeneity among the studies (P=0.023;
I2=68.5%), a random-effects model was used. A
statistically significant difference was observed between the
sorafenib- and placebo-based therapy groups, as the pooled HR of
the four RCTs was 0.74 (95% CI, 0.50–0.97; P<0.00001; Fig. 3).
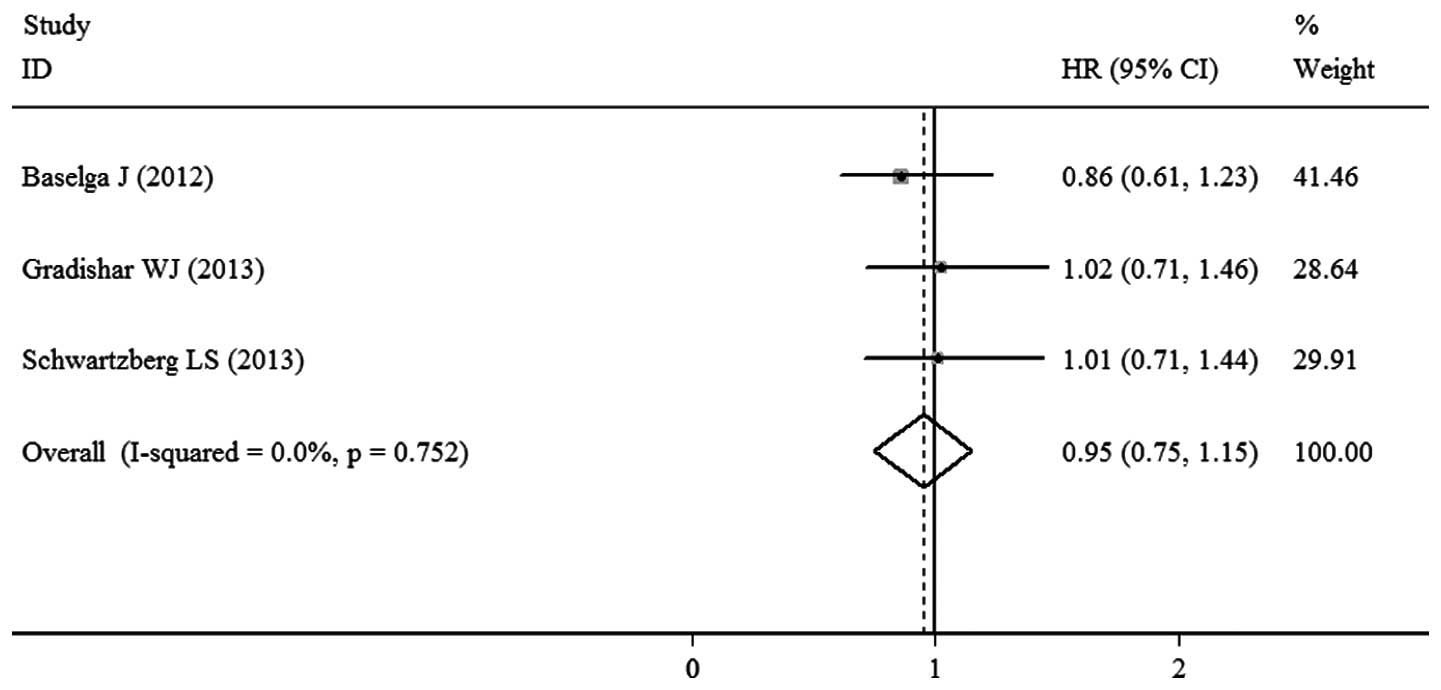
OS
OS was reported in three studies and the pooled HR
of these RCTs was 0.95 (95% CI, 0.75–1.15; P<0.00001; Fig. 4). This result indicated that there
was no significant difference between the sorafenib- and
placebo-based therapy groups with regard to OS. No significant
heterogeneity (P=0.752; I2=0.0%)was observed and the
pooled HR for OS was calculated using the fixed-effects model.
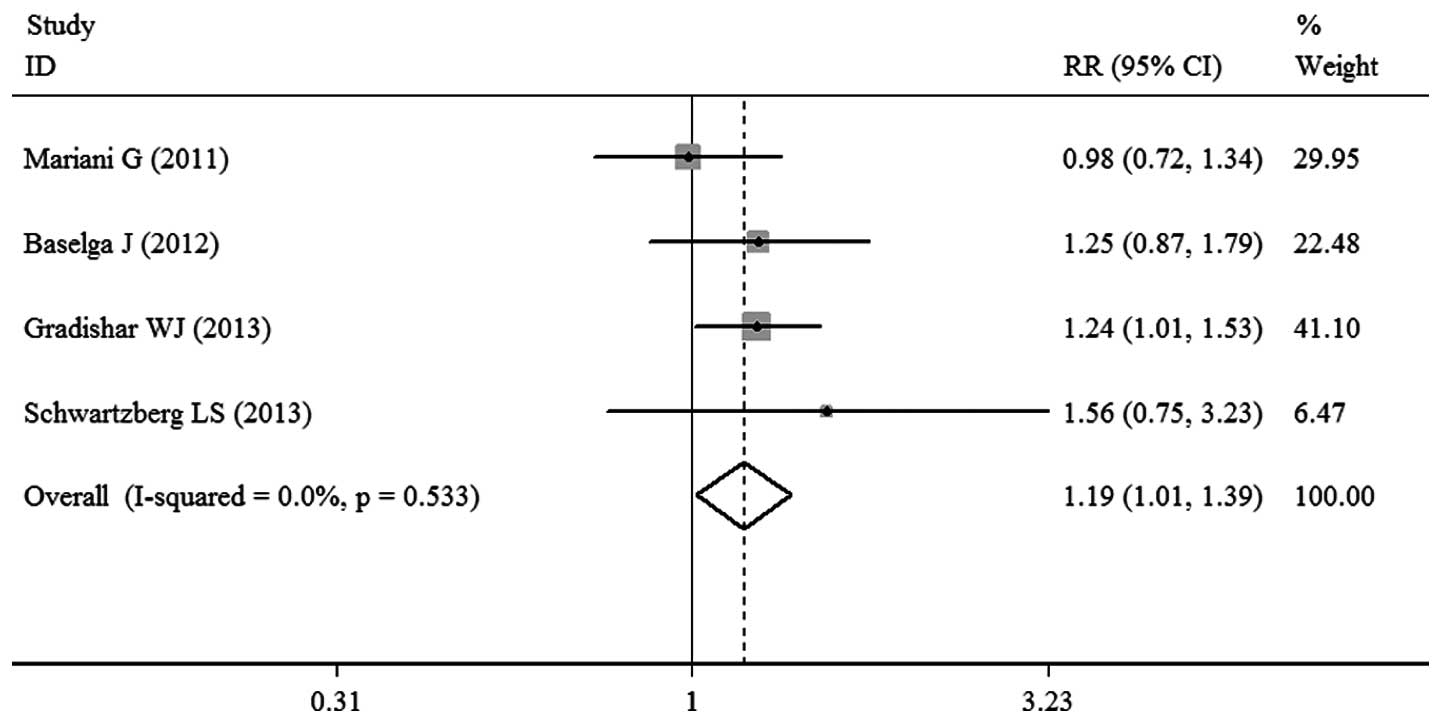
ORR
ORR was reported in the four trials. There was no
significant heterogeneity observed among the studies (P=0.533;
I2=0.0%), thus, a fixed-effects model for meta-analysis
was used. The pooled RR value was 1.19 (95% CI, 1.01–1.39; P=0.033;
Fig. 5), demonstrating that
sorafenib-based therapy did not significantly improve the ORR.
AEs
All the trials provided information on multiple
toxicities/AEs following treatment. However, only the incidence of
major grade 3/4 AEs was assessed in the present study. The pooled
result revealed that the risks of hand-foot skin syndrome, anemia,
fatigue, rash and stomatitis were increased in the patients who
received sorafenib-based therapy. However, there was no significant
difference in other grade 3/4 AEs (Table II).
 | Table IIMeta-analysis results for the major
grade 3/4 AEs. |
Table II
Meta-analysis results for the major
grade 3/4 AEs.
| | SOR group | PLA group | | |
|---|
| |
|
| | |
|---|
| AE | Evaluable trials,
n | N1 | A1, n (%) | N2 | A2, n (%) | OR (95% CI) | P-value |
|---|
| Hypertension | 4 | 417 | 7 (1.7) | 414 | 7 (1.7) | 0.99
(0.34–2.84) | 0.098 |
| Diarrhea | 4 | 417 | 17 (4.1) | 414 | 9 (2.2) | 1.92
(0.85–4.37) | 0.119 |
| Hand-foot
syndrome | 4 | 417 | 127 (30.1) | 414 | 24 (5.8) | 7.83
(4.87–12.59) | 0.000 |
| Rash | 4 | 417 | 24 (5.8) | 414 | 2 (0.5) | 7.73
(2.50–23.95) | 0.000 |
| Stomatitis | 3 | 306 | 15 (4.9) | 307 | 0 (0.0) | 11.60
(2.18–61.64) | 0.004 |
| Asthenia | 3 | 338 | 14 (4.1) | 337 | 7 (2.1) | 1.98
(0.81–4.84) | 0.135 |
| Fatigue | 3 | 306 | 24 (7.8) | 307 | 12 (3.9) | 2.18
(1.04–4.57) | 0.039 |
| Mucositis | 3 | 306 | 6 (2.0) | 307 | 5 (1.6) | 1.19
(0.38–3.74) | 0.763 |
|
Thrombocytopenia | 3 | 306 | 13 (4.2) | 307 | 6 (2.0) | 1.81
(0.19–17.19) | 0.607 |
| Neutropenia | 4 | 417 | 54 (13.0) | 414 | 44 (10.6) | 1.26
(0.81–1.96) | 0.302 |
Discussion
Sorafenib is an oral multikinase inhibitor that
inhibits the Ras/Raf/MAPK signaling pathway, as well as
platelet-derived growth factor receptors-α and β, VEGFRs-1, 2 and 3
and c-KIT and FLT3 kinases, to prevent tumor growth by
antiangiogenic, antiproliferative and/or proapoptotic effects. The
first phase I clinical trial evaluating oral sorafenib in patients
with advanced solid tumors was initiated in July 2000 (19) and since then, sorafenib has been
used to treat advanced RCC and HCC. With regard to breast cancer,
two single-arm phase II studies demonstrated that sorafenib as a
single agent, although well-tolerated, did not exhibit activity
when measured by tumor shrinkage in patients with metastatic breast
cancer (15–16). Thus, the role of sorafenib in the
treatment of metastatic breast cancer has focused on combinations
with standard therapies. For HER2-negative ABC patients, the lack
of a specific target therapy has limited the benefits of treatment.
Therefore, a new targeted agent is required that represents a novel
approach to anticancer therapy, while also providing a higher
therapeutic index. Several RCTs have investigated the efficacy of
sorafenib in combination with systemic therapies for the treatment
of HER2-negative ABC (by fluorescence in situ hybridization
or immunohistochemistry), however, the results have been
inconsistent. The SOLTI-0701 (11)
and AC01B07 (13) trials
demonstrated that anticancer activity was achieved when sorafenib
was added to capecitabine and gemcitabine/capecitabine,
respectively. By contrast, the NU07B1 (12) and FM-B07-01 (10) trials did not demonstrate a clinical
benefit when sorafenib was added to paclitaxel and
docetaxel/letrozole, respectively. Therefore, we hypothesized that
it was necessary to integrate the data from these RCTs and evaluate
the evidence with the aim of offering a more comprehensive insight
into sorafenib-based therapy in patients with HER2-negative ABC.
Thus, physicians and patients may make better-informed decisions
regarding the most appropriate adjuvant therapy.
The results of the present meta-analysis
demonstrated that sorafenib-based therapies provide a clinically
modest, but statistically significant, PFS benefit (HR, 0.78; 95%
CI, 0.54–1.02) in HER2-negative ABC patients. In addition,
sorafenib therapy prolonged the TTP (HR, 0.74; 95% CI, 0.50–0.97),
but failed to improved OS (HR, 0.95; 95% CI, 0.75–1.15) and ORR
(RR, 1.19; 95% CI, 1.01–1.39). The majority of AEs associated with
the addition of sorafenib were mild to moderate in severity.
However, certain grade 3/4 AEs occurred more frequently in
sorafenib-treated patients as compared with placebo-treated
patients, including hand-foot skin syndrome, anemia, fatigue, rash
and stomatitis.
Therefore, the development program for sorafenib in
HER2-negative ABC has demonstrated encouraging activity when used
in combination with selected chemotherapies, although the clinical
benefit was relatively small.
In each of the RCTs, the primary endpoint was PFS
and the starting sorafenib dose was 400 mg administered twice
daily. However, the chemotherapies selected varied. The NU07B1
study (12) evaluated sorafenib in
combination with paclitaxel as a first-line therapy. The study
found that sorafenib-based chemotherapy improved disease control,
but did not significantly improve the PFS, with a median PFS time
of 6.9 months in sorafenib-treated patients and 5.6 months in
placebo-treated patients (HR, 0.788; 95% CI, 0.558–1.112;
P=0.1715). No significant difference was observed between the
treatment groups with regard to OS, however, the addition of
sorafenib was associated with statistically significant
improvements in TTP and ORR. The SOLTI-0701 study (11) combined sorafenib with first- or
second-line capecitabine. The addition of sorafenib to capecitabine
resulted in a significant improvement in PFS when compared with
placebo-based treatment (median PFS, 6.4 vs. 4.1 months; HR, 0.58;
95% CI, 0.41–0.81; P=0.001). Sorafenib was favored across the
subgroups, including first- and second-line treatments, but there
was no significant improvement for OS and ORR. In the AC01B07 trial
(13), sorafenib was combined with
gemcitabine or capecitabine in patients with HER2-negative ABC
whose cancer had progressed during or following bevacizumab
treatment. A clinically small, but statistically significant, PFS
benefit was observed in the sorafenib patients (median PFS, 3.4 vs.
2.7 months; HR, 0.65; 95% CI, 0.45–0.95; P=0.02). Statistically
significant improvements were was also observed in TTP, but there
were no improvements to OS and ORR. These results support a
potential role for sorafenib, when combined with gemcitabine or
capecitabine, in the treatment of breast cancer that has progressed
during or following the application of bevacizumab. The FM-B07-01
trial (10) investigated the
potential benefit of sorafenib as an addition to the standard
therapeutic approach in patients with HER-2 negative ABC, based on
hormone receptor and visceral disease status. Patients with
triple-negative breast cancer received docetaxel for a maximum of
six cycles, patients with positive hormone receptor and visceral
disease received docetaxel followed by letrozole and patients with
positive hormone receptor and non-visceral disease received
letrozole. However, there were no improvements to PFS, TTP or ORR
associated with the addition of sorafenib to these regimens; OS
data are pending.
Overall, the development program for sorafenib in
HER2-negative ABC has demonstrated encouraging activity when used
in combination with select chemotherapies. The current
meta-analysis provides two types of clinically relevant evidence
that further supports the development of sorafenib for the
treatment of HER2-negative ABC. Firstly, the present study
demonstrated activity for sorafenib when added to selected
chemotherapeutic agents in various clinical scenarios, despite the
PFS benefit being limited. In addition, as indicated by one of the
RCTs, sorafenib may be able to overcome resistance in patients
previously treated with bevacizumab by targeting multiple
angiogenic pathways. Considering that the conditional approval of
bevacizumab for breast cancer was revoked by the US Food and Drug
Administration in 2011 (20),
sorafenib may be developed as a novel approach to anticancer
therapy in metastatic breast cancer. Secondly, although the
incidence of specific AEs was increased in sorafenib patients,
combination therapy was tolerable. Despite the high frequency of
treatment discontinuations due to AEs, the sorafenib regimen
exhibited a clinically manageable toxicity profile and no new or
unexpected side effects were observed with this combination.
In view of the RCTs analyzed in the present study,
there are specific questions that require further discussion and
consideration. Firstly, the improvements in PFS and TTP with the
addition of sorafenib did not translate into prolonged OS. This
observation also occurred when bevacizumab was administered with
standard chemotherapy for the treatment of metastatic breast cancer
(21,22). One possibility is that the
differences in postprogression treatments between the groups may
have affected the OS outcome. In addition, it is possible that
treatment with sorafenib adversely impacted the postprogression
survival rate, by affecting tumor growth or toxicities. However,
there was no evidence of postprogression mortalities associated
with drug toxicity (23).
Furthermore, dose interruptions and reductions were more common in
the combination regimen, which may have affected the OS outcome.
Other possible explanations include statistical chance or potential
imbalances in baseline prognostic factors. Secondly, the variety of
selected chemotherapeutic agents and the unselected patient
population may have resulted in various outcomes. Subgroup analyses
in each of the RCTs based on stratification factors and other
baseline characteristics, including age, hormone receptor status
and the presence of visceral disease, did not identify any patient
subpopulations with statistically significant improvements.
However, patients who had received prior adjuvant chemotherapy
generally exhibited a greater PFS or TTP with the combination
treatment. Validated biomarkers are likely to improve the
understanding behind the variability in responses to antiangiogenic
therapies across patient populations. Thus, the identification and
validation of appropriate biomarkers for improved patient selection
are required. The tumor growth-inhibitory effects of sorafenib may
be attributed to the inhibition of tumor angiogenesis (24) and molecular markers involved in
angiogenesis may be candidates. Preliminary biomarker evaluations
have indicated that baseline soluble VEGFR and phosphorylated
extracellular signal-regulated kinase levels are indicative of the
sorafenib response in RCC (25)
and HCC (26), respectively.
However, at present, there are no proven biomarkers for selecting
patients with ABC that are likely to benefit from antiangiogenic
therapy (27). Thus, further study
should be considered.
To the best of our knowledge, the present study is
the first meta-analysis of RCTs comparing sorafenib- and
placebo-based therapies for the treatment of HER2-negative ABC. As
a meta-analysis, there are limitations that should be discussed.
Firstly, although an individual patient data meta-analysis provides
a more robust estimate of an association, obtaining individual
patient data for the study was almost impossible. Therefore, a
meta-analysis based on aggregated data from published literature
was conducted. Secondly, the literature search was performed in
limited databases. The total number of included studies and sample
size were relatively small, which may have affected the validity of
the meta-analysis to a certain extent. Possible publication bias is
also a potential limitation of the present study, although this was
not detected statistically. However, the four trials included were
double-blind, randomized, placebo-controlled and performed on an
intention-to-treat analysis. Therefore, the data extraction from
these trials is reliable. In addition, a random-effects model was
used to analyze PFS and TTP due to the significant heterogeneity
observed among the studies. Since the random-effects model reduced
the effect of large samples with better quality, this model was not
as stable as the fixed-effects model.
In conclusion, the meta-analysis of the present
study demonstrated that the addition of sorafenib to selected first
or second-line chemotherapies provides statistically significant
improvements in PFS and TTP for the treatment of HER2-negative ABC.
However, the OS or ORR failed to improve significantly. The
incidence of grade 3/4 AEs was generally higher in patients
administered a combination of sorafenib and chemotherapy.
Therefore, the sorafenib-based therapy regimen evaluated in the
present study is not currently recommended for routine clinical
practice in the treatment of HER-2 negative ABC, until further
investigation and larger prospective clinical trials provide more
data. Future issues for the development of sorafenib also include
the identification and validation of appropriate biomarkers for
improved patient selection.
Acknowledgements
The authors thank the patients and clinical
investigators who were involved in the studies selected for the
meta-analysis.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members. Strategies for
subtypes - dealing with the diversity of breast cancer: highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adnane L, Trail PA, Taylor I and Wilhelm
SM: Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that
targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases
VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 407:597–612.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilhelm S, Carter C, Lynch M, et al:
Discovery and development of sorafenib: a multikinase inhibitor for
treating cancer. Nat Rev Drug Discov. 5:835–844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar
|
|
6
|
Yu C, Bruzek LM, Meng XW, et al: The role
of Mcl-1 downregulation in the proapoptotic activity of the
multikinase inhibitor BAY 43-9006. Oncogene. 24:6861–6869. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Escudier B, Eisen T, Stadler WM, et al;
TARGET Study Group. Sorafenib in advanced clear-cell renal-cell
carcinoma. New Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llovet JM, Ricci S, Mazzaferro V, et al;
SHARP Investigators Study Group. Sorafenib in advanced
hepatocellular carcinoma. New Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mariani G, Burdaeva O, Roman L, et al: A
double-blind, randomized phase lib study evaluating the efficacy
and safety of sorafenib (SOR) compared to placebo (PL) when
administered in combination with docetaxel and/or letrozole in
patients with metastatic breast cancer (MBC): FM-B07-01 Trial. Eur
J Cancer. 47:102011. View Article : Google Scholar
|
|
11
|
Baselga J, Segalla JG, Roché H, et al:
Sorafenib in combination with capecitabine: an oral regimen for
patients with HER2-negative locally advanced or metastatic breast
cancer. J Clin Oncol. 30:1484–1491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gradishar WJ, Kaklamani V, Sahoo TP, et
al: A double-blind, randomised, placebo-controlled, phase 2b study
evaluating sorafenib in combination with paclitaxel as a first-line
therapy in patients with HER2-negative advanced breast cancer. Eur
J Cancer. 49:312–322. 2013. View Article : Google Scholar
|
|
13
|
Schwartzberg LS, Tauer KW, Hermann RC, et
al: Sorafenib or placebo with either gemcitabine or capecitabine in
patients with HER-2-negative advanced breast cancer that progressed
during or after bevacizumab. Clin Cancer Res. 19:2745–2754. 2013.
View Article : Google Scholar
|
|
14
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
15
|
Moreno-Aspitia A, Morton RF, Hillman DW,
et al: Phase II trial of sorafenib in patients with metastatic
breast cancer previously exposed to anthracyclines or taxanes:
North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J
Clin Oncol. 27:11–15. 2009. View Article : Google Scholar
|
|
16
|
Bianchi G, Loibl S, Zamagni C, et al:
Phase II multicenter, uncontrolled trial of sorafenib in patients
with metastatic breast cancer. Anticancer Drugs. 20:616–624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isaacs C, Herbolsheimer P, Liu MC, et al:
Phase I/II study of sorafenib with anastrozole in patients with
hormone receptor positive aromatase inhibitor resistant metastatic
breast cancer. Breast Cancer Res Treat. 125:137–143. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spigel DR, Hainsworth JD, Burris HA 3rd,
et al: A pilot study of adjuvant doxorubicin and cyclophosphamide
followed by paclitaxel and sorafenib in women with node-positive or
high-risk early-stage breast cancer. Clin Adv Hematol Oncol.
9:280–286. 2011.PubMed/NCBI
|
|
19
|
Lyons JF, Wilhelm S, Hibner B and Bollag
G: Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer.
8:219–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanne JH: FDA cancels approval for
bevacizumab in advanced breast cancer. BMJ. 343:d76842011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller K, Wang M, Gralow J, et al:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. New Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miles DW, Chan A, Dirix LY, et al: Phase
III study of bevacizumab plus docetaxel compared with placebo plus
docetaxel for the first-line treatment of human epidermal growth
factor receptor 2-negative metastatic breast cancer. J Clin Oncol.
28:3239–3247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miles D, Harbeck N, Escudier B, et al:
Disease course patterns after discontinuation of bevacizumab:
pooled analysis of randomized phase III trials. J Clin Oncol.
29:83–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elting J, Bigwood D, Brown-Shimer S, et
al: Biomarkers associated with clinical outcomes in TARGETs, a
Phase III single-agent, placebo-controlled study of sorafenib in
advanced renal cell carcinoma. Proc Amer Assoc Cancer Res.
47:683–684. 2006.
|
|
26
|
Abou-Alfa GK, Schwartz L, Ricci S, et al:
Phase II study of sorafenib in patients with advanced
hepatocellular carcinoma. J Clin Oncol. 24:4293–4300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jubb AM, Oates AJ, Holden S and Koeppen H:
Predicting benefit from anti-angiogenic agents in malignancy. Nat
Rev Cancer. 6:626–635. 2006. View
Article : Google Scholar : PubMed/NCBI
|