Introduction
The pathophysiology of traumatic spinal cord injury
(SCI) is thought to divide into two stages (1). As the primary insult, the direct
mechanical damage cannot be therapeutically influenced. However,
the secondary damage, including electrolyte abnormalities, free
radical formation, edema, vascular ischemia, posttraumatic
inflammatory reaction, apoptosis and other processes, may be
targeted with various therapeutic interventions. It has been shown
that inflammatory processes play an important role in post-SCI
secondary injury (2–4). Therefore, it is important to develop
a therapy that reduces the evolution of the secondary damage in
SCI.
The spinal cord is a glucocorticoid-responsive
tissue and it contains substantial amounts of receptors for
adrenocortical steroids (5,6). It
has been demonstrated that glucocorticoid drugs enhance functional
recovery and induce regenerative responses following SCI in humans
and experimental animals (7,8).
Methylprednisolone (MP) is a synthetic glucocorticoid and the only
therapeutic agent approved by the Food and Drug Administration for
reducing the extent of the post-traumatic inflammatory reaction
following acute SCI (9,10). Although the application of MP after
SCI is associated with a wide array of anti-inflammatory effects,
including anti-lipid peroxidation (11,12)
and attenuation of the formation of deleterious prostanoids
(prostaglandin F2α and thromboxane A2) (13), the long-term administration of this
therapeutic steroid results in a variety of side-effects, such as
downregulation of the expression of several inflammatory genes and
an inhibitory effect on the proliferation of endogenous neural
progenitor cells following SCI (14).
Aminoguanidine (AG), a small water-soluble compound,
has been widely used for the prevention of the chronic tissue
complications of diabetes mellitus in humans (15). Previous studies have shown that AG
reduces the extent of brain edema in animal models of surgical
brain injury (16), stroke
(17) and post-traumatic brain
injury (18). Notably, Pearse
et al demonstrated that AG improved the motor functions of
injured spinal cords in rats and may have a potential role in the
treatment of acute SCI (19).
However, high doses of AG lead to nonspecific and potentially toxic
effects (20), which limits its
usefulness clinically. Treating a single target with a low dose of
therapeutic agent is unlikely to achieve complete inhibition of the
inflammation due to the complexity and redundancy of the
inflammatory response associated with SCI. It has been shown that
the strategy of targeting multiple proinflammatory pathways may be
more effective than targeting a single effector molecule (21,22).
To the best of our knowledge, the therapeutic effects of the
simultaneous administration of AG and MP have not previously been
evaluated. Thus, to determine whether MP and AG act
synergistically, SCI was induced in rats and the effects of MP and
AG were determined in the present study.
Materials and methods
Experimental animal
Sixty Wistar adult female rats (200–240 g) were
obtained from Jilin University (Changchun, China). All animals were
enclosed in ventilated, humidity- (50–60%) and
temperature-controlled (22±1°C) rooms with a 12/12-h light/dark
cycle for approximately two weeks. The animals were housed on
sawdust and received food pellets and water ad libitum. All
animal procedures were performed in accordance with the Guide for
the Care and Use of Laboratory Animals of the National Institutes
of Health (1996) and were approved by the Jilin University
Committee on Animal Research.
Surgical procedure of SCI
The rats were anesthetized with a cocktail of 40
mg/kg ketamine, 4 mg/kg xylazine and 0.9 mg/kg acepromazine
administered by intraperitoneal (IP) injection. A dorsal incision
was made to expose the T10 vertebra and a laminectomy was
performed, leaving the spinal segment exposed. Following exposure
of the T10 segment by laminectomy, the animals received a moderate
contusion using a New York University impactor (W.M. Keck Center
for Collaborative Neuroscience, Rutgers the State University of New
Jersey, Piscataway, NJ, USA) that provides a contusion of 12.5 g.cm
as previously described (23).
Following the surgery, 10 ml 0.9% sodium chloride and 30 mg/kg
sulfadiazine and trimethoprim were injected subcutaneously. Access
to food was facilitated by placing softened food pellets directly
in the bottom of each cage. The state of hydration and
gastrointestinal function were monitored daily. The rats were
weighed daily for the first seven days postsurgery and then weighed
weekly. Post-surgical care included the manual expression of
bladders twice a day until bladder function returned, as well as
injections of sulfadiazine and trimethoprim twice a day for up to
one week.
Experimental groups
The rats were randomly allocated into the following
groups: Group 1: Sham surgery group, the animals were subjected to
identical surgical procedures without impaction; group 2: Control
group, the rats received an IP injection of the carrier solution (5
ml/kg of 5% dimethylsulfoxide in 0.9% normal saline) following SCI;
group 3: MP group, MP (0.75 mg/kg, IP) was administered at 1 and 4
h after SCI according to the methods of Messina et al
(22); group 4: AG group, AG (75
mg/kg, IP) was administered at 1 and 4 h after SCI; and group 5: AG
and MP group, AG (75 mg/kg, IP) and MP (0.75 mg/kg, IP) were
administered at 1 and 4 h after SCI. One rat of each group was used
in each of the following experiments.
Determination of spinal cord water
content
The spinal cords collected 24 h after treatment.
Spinal cord edema was evaluated by determining the water content of
the spinal cord as previously described with minor revision
(21). In brief, the injured
spinal cords for all groups were dried for 48 h at 80°C for
determination of the dry weight. The values for the water content
in the spinal cord tissues were obtained based on the following
calculation: Hemispheric water content (%) = (wet weight - dry
weight)/wet weight × 100.
Myeloperoxidase (MPO) activity
The levels of MPO activity, an indicator of
polymorphonuclear leukocyte accumulation, were determined in the
spinal cord tissues according to the methods of a previous study
(24) at 24 h after SCI. MPO
activity was defined as the quantity of enzyme required to degrade
1 μmol of peroxide per min at 37°C and was expressed in U/g of wet
tissue.
Behavioral assessments
Behavioral assessments were determined using the
Basso, Beattie, and Bresnahan (BBB) score and grid-walking test.
Gross BBB locomotor recovery following contusive SCI was scored in
an open field according to the locomotor rating scale of 0
(complete paralysis) to 21 (normal locomotion) (25). BBB testing was performed at 24 h
prior to SCI, 24 h and 3 days post-injury, and once weekly
thereafter up to eight weeks post-injury. Each rat was observed for
4 min by three blinded investigators. To assess the locomotion in
all groups, the ability of rats to walk on an irregularly
horizontal wire grid was determined as described by a previous
study (26). The rats were allowed
to walk on the grid weekly and tested at eight weeks after the
contusive SCI. Each rat was allowed to walk around freely for 4
min. If a hind paw protruded entirely through the grid, with all
toes and the heel extended below the wire surface, it was counted
as a misstep. Furthermore, the total number of steps taken with the
hindlimb of the same side was also counted. The results are shown
as a percentage of missteps.
Measurement of tumor necrosis factor-α
(TNF-α) and interleukin-1β (IL-1β) levels following SCI
To evaluate the TNF-α and IL-1β tissue levels,
sections of the spinal cord tissues, collected at 24 h after SCI,
were homogenized as previously described (21) in phosphate-buffered saline
containing 2 mmol/l phenylmethylsulfonyl fluoride (Sigma Chemical
Co., Milan, Italy). The assay was performed using a commercial
colorimetric kit (rat TNF-α commercial colorimetric kit and rat
IL-1β commercial colorimetric kit; Calbiochem-Novabiochem
Corporation, San Diego, CA, USA) according to the manufacturer’s
instructions. All detections were performed in duplicate serial
dilutions.
Western blot analysis
Western blot analysis was performed to investigate
the expression levels of the Bcl-2-associated X (Bax) and B-cell
lymphoma 2 (Bcl-2) proteins in an extract from the injured spinal
cord at 24 h after SCI. Following sacrifice under deep anesthesia
by transcardial saline infusion, the experimental rat spinal cord
tissue (1.5-mm long, centered at the injury site) was quickly
removed and homogenized by sonication in radioimmunoprecipitation
assay lysis buffer. The samples were centrifuged at 12,000 × g for
1 h. The protein concentration of the soluble materials was
determined by the Coomassie Brilliant Blue G-250 dye-binding method
(Thermo Fisher, Rockford, IL, USA). The protein lysates (15 μg per
lane for each sample) were fractioned by 10% SDS-PAGE, followed by
transfer to nitrocellulose membranes (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). The membranes were blocked in blocking
buffer (5% nonfat dairy milk dissolved in Tris-buffered saline with
Tween 20 and PBS with Tween 20) overnight at 4°C. The blots were
then incubated with anti-Bax and anti-Bcl-2 rabbit polyclonal
antibodies (dilution 1:500; Santa Cruz Biotechnology, Inc.) for 2
h. The Bax and Bcl-2 protein bands on these immunoblots were
visualized using enhanced chemiluminescence (ECL) western blotting
kit (Santa Cruz Biotechnology, Inc.). The Bax and Bcl-2 protein
bands and GAPDH bands were scanned using the ChemiImager 5500
system with the corresponding software, version 2.03 (Informer
Technologies, Inc., Dallas, TX, USA), and the integrated density
values were calculated using FluorChem software, version 2.0
(Informer Technologies, Inc.) and normalized with those of
GAPDH.
Statistics analysis
The statistical package SPSS software, version 19.0
(SPSS, Inc., Chicago, IL, USA) was used for all analyses. One-way
analysis of variance followed by Bonferroni’s post hoc test were
utilized to determine the significant differences among multiple
groups. All values are expressed as the mean ± standard deviation.
In general, P<0.05 was considered to indicate a statistically
significant difference.
Results
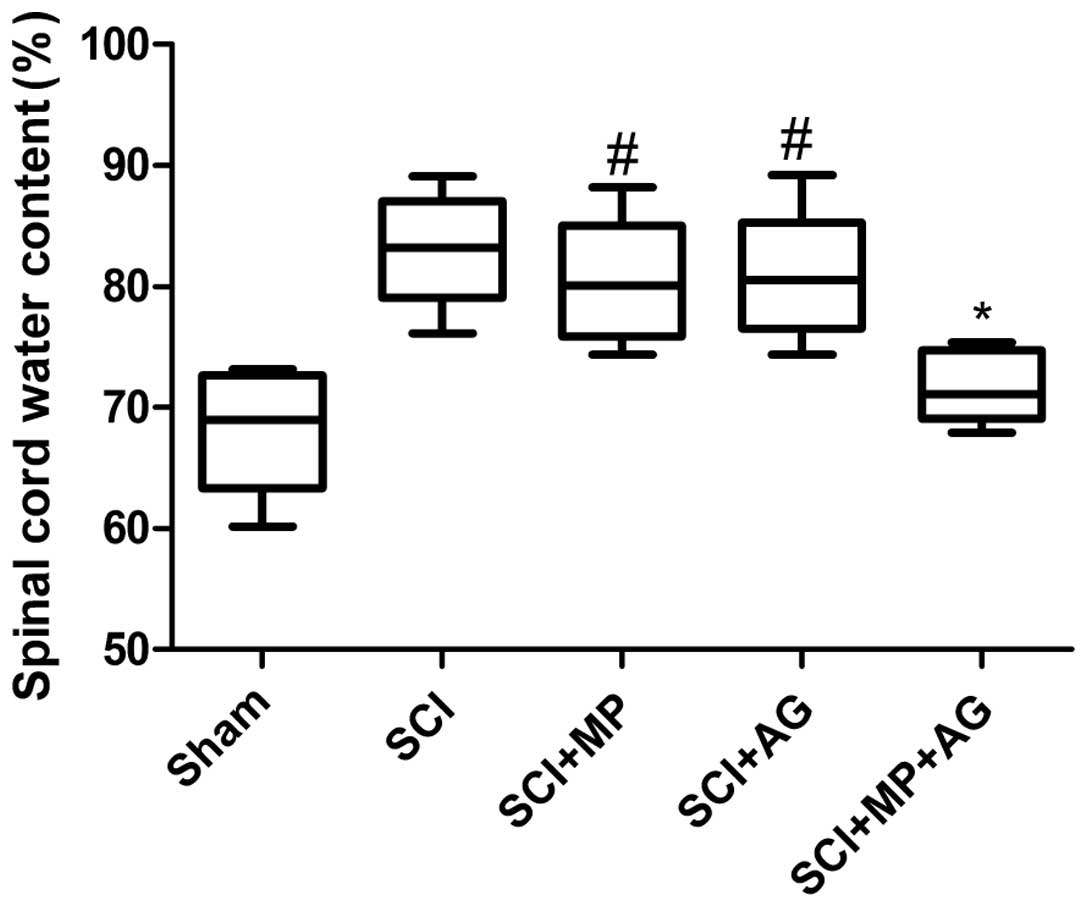
Effect of the combination therapy on
spinal cord water content
In the present study, the effect of combination
therapy with AG (75 mg/kg) and MP (0.75 mg/kg) on the spinal cord
water content at 24 h after SCI was investigated. As shown in
Fig. 1, the combination therapy
had significant anti-edematous activity compared with that observed
in the control group (SCI group), whereas in the single treatment
groups (the MP and AG groups) the levels of cerebral edema did not
significantly change compared with those of the control group at 24
h after SCI. However, the levels of cerebral edema in the single
treatment groups were significantly increased compared with those
of the sham group. These data showed that the combination therapy
with AG and MP significantly ameliorated the increased water
content of the injured spinal cords.
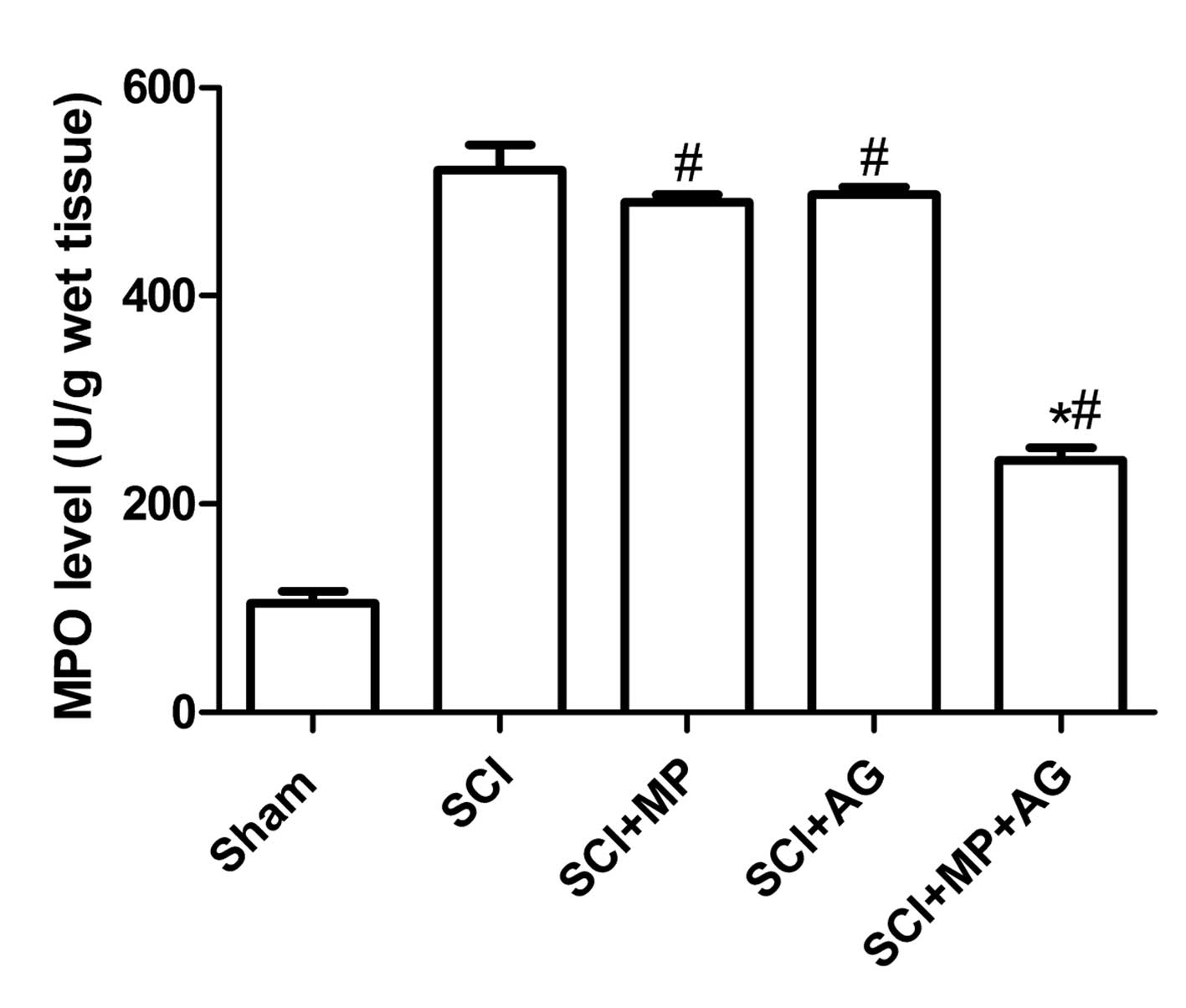
Effect of the combination therapy on
neutrophil infiltration
The effect of combination therapy with AG (75 mg/kg)
and MP (0.75 mg/kg) on neutrophil infiltration was investigated by
measuring the tissue levels of MPO activity. MPO activity was
significantly elevated in the spinal cord at 24 h after injury in
the rats subjected to SCI when compared with those of the rats in
the sham surgery group (Fig. 2).
The levels of MPO activity were significantly reduced by the
combination therapy with AG (75 mg/kg) and MP (0.75 mg/kg) compared
with those of the rats in the control group (Fig. 2). However, administering either of
the compounds as a single treatment did not reduce the levels of
neutrophil infiltration in the injured spinal cord compared with
those of the rats in the control group (Fig. 2).
Combination treatment with MP and AG
results in functional recovery following SCI
To determine whether the AG and MP combination
treatment-mediated tissue protection and repair also had an effect
on functional recovery, the BBB locomotor test was performed at 1
day, 3 days and weekly up to eight weeks after SCI (Fig. 3A). At 1 day after SCI, BBB score of
all rats was regarded as 0. In the following days, the locomotor
performance substantially improved and reached a relative plateau
at the third week. A minor but not statistically significant
increase of the BBB scores was observed in the AG and MP groups
from the fifth week after SCI. The scores in the AG and MP
combination treatment group were consistently higher than those in
the other groups and the differences between BBB scores of the AG
and MP combination group and those of the other three groups were
statistically significant starting from the third week and
continuing until the eight week. The rats subjected to sham surgery
all achieved maximal scores in the BBB test (data not shown).
Results from the grid walking test also showed that the percentage
of missteps of hind paws was markedly reduced in the
combination-treated rats compared with that in the other groups
(Fig. 3B).
Effect of the combination therapy on the
expression levels of TNF-α and IL-1β following SCI
To test whether the combination therapy with MP and
AG modulated the inflammatory process through regulation of the
secretion of proinflammatory cytokines, TNF-α and IL-1β levels in
the spinal cord tissues were analyzed. Substantial increases in the
levels of TNF-α and IL-1β production were identified in the spinal
cord tissue samples collected from SCI rats at 24 h after SCI
(Fig. 4). The combination therapy
significantly reduced the spinal cord levels of TNF-α and IL-1β
compared with those of the control group (Fig. 4). Furthermore, the MP treatment
group exhibited markedly reduced spinal cord levels of TNF-α and
IL-1β compared with those of the control group (SCI group), whereas
the AG treatment group did not show clearly reduced TNF-α and IL-1β
levels compared with those of the control group (SCI group).
Western blot analysis of Bax and
Bcl-2
At 24 h after SCI, the appearance of Bax in the
spinal cord homogenates was investigated by western blot analysis.
The Bax expression levels were appreciably increased in the spinal
cords from the rats subjected to SCI, compared with those in the
spinal cord from the sham rats (Fig.
5). However, the combination therapy with MP and AG
significantly reduced Bax expression levels compared with those in
the control group (Fig. 5).
Furthermore, whole extracts from the spinal cord of each rat were
also analyzed to detect Bcl-2 expression levels. Combination
treatment of the rats with MP and AG significantly increased the
SCI induced inhibition of Bcl-2 expression (Fig. 5). These results indicate that
combination therapy with MP and AG inhibits apoptosis following
SCI.
Discussion
The present study provides convincing evidence that
the combination of MP with AG significantly reduced the levels of
spinal cord edema and improved the damaged motor function caused by
SCI in rats, whereas a single treatment did not significantly
improve them.
Although the two compounds have each been
extensively studied, to our knowledge, this study is the first to
show an enhanced neurological outcome from combining a clinically
applied therapy, MP, with AG following SCI. The enhanced viability
and regenerative capacity of neurons and functional recovery
supported by MP and AG combination treatment has practical and
conceptual implications due to the proinflammatory effect on
neurons in vivo following experimental SCI (19,27,28).
Traumatic SCI results in severe inflammation, the
release of free oxygen radicals, a reduction of neural regeneration
and glial scar formation, all of which are detrimental to neural
function recovery (29). It is
unrealistic to expect to achieve disease remission by blocking a
single early mediator in the inflammatory cascade as a large number
of inflammatory mediators are involved in the secondary injury
processes following SCI. Therefore, the observed combination
therapy is of potential therapeutic interest and suggests that
strategies targeting multiple proinflammatory pathways may be more
effective than those targeting a single effector molecule. Yin
et al (30) showed that
rolipram and MP combination treatment promoted significant
neuroprotection in rats through reduced motor neuron death, a
minimized lesion cavity and increased regeneration of lesioned
corticospinal tract axons beyond the lesion site following SCI.
Genovese et al (31)
demonstrated that treatment of mice with a combination of
etanercept and dexamethasone (DEX) significantly reduced the
SCI-induced spinal cord changes and also improved the motor
function compared with the effects of etanercept or DEX treatment
used alone. Xu et al (21)
showed that combination therapy with AG and DEX significantly
exerted an important beneficial anti-inflammatory effect by
blocking the possible progression of SCI in rats. These studies
demonstrated that combination therapy significantly ameliorates
functional recovery following SCI compared with the effect of
single drug treatment. The present study showed that the treatment
of SCI rats with MP and AG, when administered as a combination
therapy but not as a single treatment, significantly reduced the
levels of neutrophil infiltration (MPO activity), cytokine
expression (TNF-α and IL-1β) and apoptosis (Bax and Bcl-2
expression) compared with those of untreated rats, and it
demonstrated that the combination therapy significantly improved
the recovery of limb function. These results further confirmed that
strategies targeting multiple proinflammatory pathways may be more
effective than those using a single drug molecule.
Spinal cord trauma initiates a sequence of events
that lead to secondary neuronal cell damage. An inflammatory
response develops within hours after injury and is characterized by
the infiltration of neutrophils and the activation of microglia
(32). Reactive microglia have
been considered to be at the center of the injury cascade (33). Through releasing molecules,
including TNF-α, IL-1β, reactive free radicals and nitric oxide,
microglia encourage early post-injury necrotic cell death, remote
cell apoptosis, tissue edema and axonal degeneration (34,35).
Thus, methods of modulating microglia activation via the inhibition
of cell cytokines to improve recovery following SCI are sought. The
present study clearly demonstrates significant increases in the
levels of TNF-α and IL-1β in rats with SCI. Combination therapy
with MP and AG significantly reduced the levels of cytokine
expression (TNF-α and IL-1β), which indicated that combination
therapy with MP and AG may be effective treatment method for
SCI.
In conclusion, the data imply that strategies
targeting multiple proinflammatory pathways may be more effective
than those targeting a single effector molecule. The combination
therapy with AG and MP was shown to significantly exert an
important, beneficial anti-inflammatory effect by blocking the
possible progression of SCI; however, the detailed molecular
mechanism by which the combination therapy with AG and MP treats
SCI is unclear and requires investigation in further studies.
Acknowledgements
This study was supported by Science and Technology
Research and Innovation Team Fund of Jilin province
(JL2011088).
References
|
1
|
Ambrozaitis KV, Kontautas E, Spakauskas B
and Vaitkaitis D: Pathophysiology of acute spinal cord injury.
Medicina (Kaunas). 42:255–261. 2006.(In Lithuanian).
|
|
2
|
Qiao F, Atkinson C, Kindy MS, Shunmugavel
A, Morgan BP, Song H and Tomlinson S: The alternative and terminal
pathways of complement mediate post-traumatic spinal cord
inflammation and injury. Am J Pathol. 177:3061–3070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beattie MS: Inflammation and apoptosis:
linked therapeutic targets in spinal cord injury. Trends Mol Med.
10:580–583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beck KD, Nguyen HX, Galvan MD, Salazar DL,
Woodruff TM and Anderson AJ: Quantitative analysis of cellular
inflammation after traumatic spinal cord injury: evidence for a
multiphasic inflammatory response in the acute to chronic
environment. Brain. 133:433–447. 2010. View Article : Google Scholar
|
|
5
|
Marlier LN, Csikós T, Rebaudengo N,
Borboni P, Patacchioli FR, Angelucci L, Privat A and Lauro R:
Distribution of glucocorticoid receptor mRNA in the rat spinal
cord. Neuroreport. 6:2245–2249. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang S, Lim G, Zeng Q, Sung B, Yang L and
Mao J: Central glucocorticoid receptors modulate the expression and
function of spinal NMDA receptors after peripheral nerve injury. J
Neurosci. 25:488–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonzalez R, Glaser J, Liu MT, Lane TE and
Keirstead HS: Reducing inflammation decreases secondary
degeneration and functional deficit after spinal cord injury. Exp
Neurol. 184:456–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hurlbert RJ: Strategies of medical
intervention in the management of acute spinal cord injury. Spine
(Phila Pa 1976). 31(11 Suppl): S16–S21. S362006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bracken MB: Methylprednisolone in the
management of acute spinal cord injuries. Med J Aust.
153:3681990.PubMed/NCBI
|
|
10
|
Bracken MB, Shepard MJ, Collins WF,
Holford TR, et al: A randomized, controlled trial of
methylprednisolone or naloxone in the treatment of acute spinal
cord injury. Results of the Second National Acute Spinal Cord
Injury Study. N Engl J Med. 322:1405–1411. 1990. View Article : Google Scholar
|
|
11
|
Eck JC, Nachtigall D, Humphreys SC and
Hodges SD: Questionnaire survey of spine surgeons on the use of
methylprednisolone for acute spinal cord injury. Spine (Phila Pa
1976). 31:E250–E253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hall ED and Braughler JM: Glucocorticoid
mechanisms in acute spinal cord injury: a review and therapeutic
rationale. Surg Neurol. 18:320–327. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bracken MB: Pharmacological treatment of
acute spinal cord injury: current status and future projects. J
Emerg Med. 11(Suppl 1): 43–48. 1993.PubMed/NCBI
|
|
14
|
Obermair FJ, Schröter A and Thallmair M:
Endogenous neural progenitor cells as therapeutic target after
spinal cord injury. Physiology (Bethesda). 23:296–304. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdel-Rahman E and Bolto WK: Pimagedine: a
novel therapy for diabetic nephropathy. Expert Opin Investig Drugs.
11:565–574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan D, Gu YT, Lv H, Tang T, Xu ZH, Shi XY,
Xue HL and Wang YJ: Role of aminoguanidine in brain protection in
surgical brain injury in rat. Neurosci Lett. 448:204–207. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugimoto K and Iadecola C: Effects of
aminoguanidine on cerebral ischemia in mice: comparison between
mice with and without inducible nitric oxide synthase gene.
Neurosci Lett. 331:25–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Louin G, Marchand-Verrecchia C, Palmier B,
Plotkine M and Jafarian-Tehrani M: Selective inhibition of
inducible nitric oxide synthase reduces neurological deficit but
not cerebral edema following traumatic brain injury.
Neuropharmacology. 50:182–190. 2006. View Article : Google Scholar
|
|
19
|
Pearse DD, Chatzipanteli K, Marcillo AE,
Bunge MB and Dietrich WD: Comparison of iNOS inhibition by
antisense and pharmacological inhibitors after spinal cord injury.
J Neuropathol Exp Neurol. 62:1096–1107. 2003.PubMed/NCBI
|
|
20
|
Campbell IL: Exacerbation of lymphocytic
choriomeningitis in mice treated with the inducible nitric oxide
synthase inhibitor aminoguanidine. J Neuroimmunol. 71:31–36. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu WB, Lv G, Wang YF, Lu XH, Huang T, Zhu
Y and Jia LS: Combination of dexamethasone and aminoguanidine
reduces secondary damage in compression spinal cord injury. Cell
Mol Neurobiol. 29:683–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Messina S, Bitto A, Aguennouz M, Mazzeo A,
Migliorato A, Polito F, Irrera N, Altavilla D, Vita GL, Russo M,
Naro A, De Pasquale MG, Rizzuto E, Musarò A, Squadrito F and Vita
G: Flavocoxid counteracts muscle necrosis and improves functional
properties in mdx mice: a comparison study with methylprednisolone.
Exp Neurol. 220:349–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Irizarry-Ramírez M, Willson CA,
Cruz-Orengo L, Figueroa J, et al: Upregulation of EphA3 receptor
after spinal cord injury. J Neurotrauma. 22:929–935.
2005.PubMed/NCBI
|
|
24
|
Mullane K: Neutrophil-platelet
interactions and post-ischemic myocardial injury. Prog Clin Biol
Res. 301:39–51. 1989.PubMed/NCBI
|
|
25
|
Basso DM, Beattie MS, Bresnahan JC,
Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ,
Nockels R, Perot PL, Salzman SK and Young W: MASCIS evaluation of
open field locomotor scores: effects of experience and teamwork on
reliability. Multicenter Animal Spinal Cord Injury Study. J
Neurotrauma. 13:343–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu P, Huang L, Zou J, Yu Z, Wang Y, Wang
X, Xu L, Liu X, Xu XM and Lu PH: Immunization with recombinant
Nogo-66 receptor (NgR) promotes axonal regeneration and recovery of
function after spinal cord injury in rats. Neurobiol Dis.
32:535–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takano T, Kang J, Jaiswal JK, Simon SM,
Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR and Nedergaard M:
Receptor-mediated glutamate release from volume sensitive channels
in astrocytes. Proc Natl Acad Sci USA. 102:16466–16471.
2005.PubMed/NCBI
|
|
28
|
Vesce S, Rossi D, Brambilla L and Volterra
A: Glutamate release from astrocytes in physiological conditions
and in neurodegenerative disorders characterized by
neuroinflammation. Int Rev Neurobiol. 82:57–71. 2007. View Article : Google Scholar
|
|
29
|
Loane DJ and Byrnes KR: Role of microglia
in neurotrauma. Neurotherapeutics. 7:366–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin Y, Sun W, Li Z, Zhang B, Cui H, Deng
L, Xie P, Xiang J and Zou J: Effects of combining
methylprednisolone with rolipram on functional recovery in adult
rats following spinal cord injury. Neurochem Int. 62:903–912. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Genovese T, Mazzon E, Crisafulli C,
Esposito E, Di Paola R, Muià C, Di Bella P, Meli R, Bramanti P and
Cuzzocrea S: Combination of dexamethasone and etanercept reduces
secondary damage in experimental spinal cord trauma. Neuroscience.
150:168–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McTigue DM, Tani M, Krivacic K, et al:
Selective chemokine mRNA accumulation in the rat spinal cord after
contusion injury. J Neurosci Res. 53:368–376. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beattie MS: Inflammation and apoptosis:
linked therapeutic targets in spinal cord injury. Trends Mol Med.
10:580–583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao W, Xie W, Le W, Beers DR, He Y,
Henkel JS, Simpson EP, Yen AA, Xiao Q and Appel SH: Activated
microglia initiate motor neuron injury by a nitric oxide and
glutamate-mediated mechanism. J Neuropathol Exp Neurol. 63:964–977.
2004.PubMed/NCBI
|
|
35
|
Morino T, Ogata T, Horiuchi H, Takeba J,
Okumura H, Miyazaki T and Yamamoto H: Delayed neuronal damage
related to microglia proliferation after mild spinal cord
compression injury. Neurosci Res. 46:309–318. 2003. View Article : Google Scholar : PubMed/NCBI
|