Introduction
A Krukenberg tumor is metastatic ovarian
mucin-filled signet-ring cell carcinoma (1), that exhibits fast progression and has
a poor outcome. Krukenberg tumors account for 1–2% of all ovarian
tumors (2) and the 5-year survival
rate for a patient with a Krukenberg tumor ranges between 12 and
23.4% (3). The morbidity of
pregnant patients with a Krukenberg tumor is even rarer and the
survival rate is usually poor (2–5). The
present study reported on a patient with gestational hypertension
in addition to a progressive Krukenberg tumor. A unilateral
encapsulated pedunculated solid and cystic mass with multiple
nodules in the solid portion was identified by ultrasound and
magnetic resonance imaging (MRI). However, pathological examination
confirmed that bilateral adnexa were also involved. The tumor had
three features in ultrasonography: increased quickly, had multiple
nodular components in the solid portion and had a main vessel with
small branches penetrating from the tumor pedicle into the solid
portion.
Case report
Patient history
A 31-year-old female, gravida 1, para 0, was
referred to Tianjin Medical University General Hospital (Tianjin,
China) at 32 weeks gestation due to nausea and vomiting, with a
blood pressure of 155/101 mmHg. The patient had experienced
epigastric discomfort for two weeks prior to admission, however,
did not see a doctor until the vomiting had persisted for three
days. The patient had gained 7 kg in the last month and had
previously had regular menses (4–5 days each time with a menstrual
cycle of 37 days, dysmenorrhea was negative). The last menstrual
period had been July 2, 2012, and the estimated date of confinement
was April 9, 2013. A uric human chorionic gonadotropin (HCG) test
was positive following 40 days of amenorrhea. Fetal movements were
felt at four months gestation and a four dimensional ultrasound
examination was normal at 28 weeks gestation. The patient had
previously experienced two episodes of stomach bleeding (one year
and two years ago) due to unknown reasons, but the gastroscopy
examinations had appeared to be normal. The patient’s mother had a
history of hypertension. Serological tests revealed an increased
number of white blood cells and mildly abnormal hepatic and renal
function, additionally the D-dimer levels were abnormally high
(Table I). A urine test revealed
that protein quantitation was 241.6 mg/dl, and positive for
urobilirubin (++) and ketone (+). Written informed patient consent
was obtained from the patient.
 | Table IPositive serological test. |
Table I
Positive serological test.
| Inspection item | Test result | Normal range |
|---|
| White blood cell,
×109 | 19.36 | 4–10 |
| Neutrophils, % | 87.7 | 50–70 |
| Hemoglobin, g/l | 103 | 110–150 |
| Serum total protein,
g/l | 54 | 63–82 |
| Serum albumin,
g/l | 29 | 35–50 |
| Globulin, g/l | 25 | 26–37 |
| Lactate
dehydrogenase, U/l | 456 | 94–250 |
| Serum urea,
mmol/l | 8.0 | 2.5–7.1 |
| Uric acid,
mmol/l | 60 | 140–414 |
| Calcium, mmol/l | 1.8 | 2.1–2.55 |
| D-Dimer, ng/l | 2107 | <500 |
| Fibrinogen, g/l | 1.39 | 1.8–4 |
Diagnosis
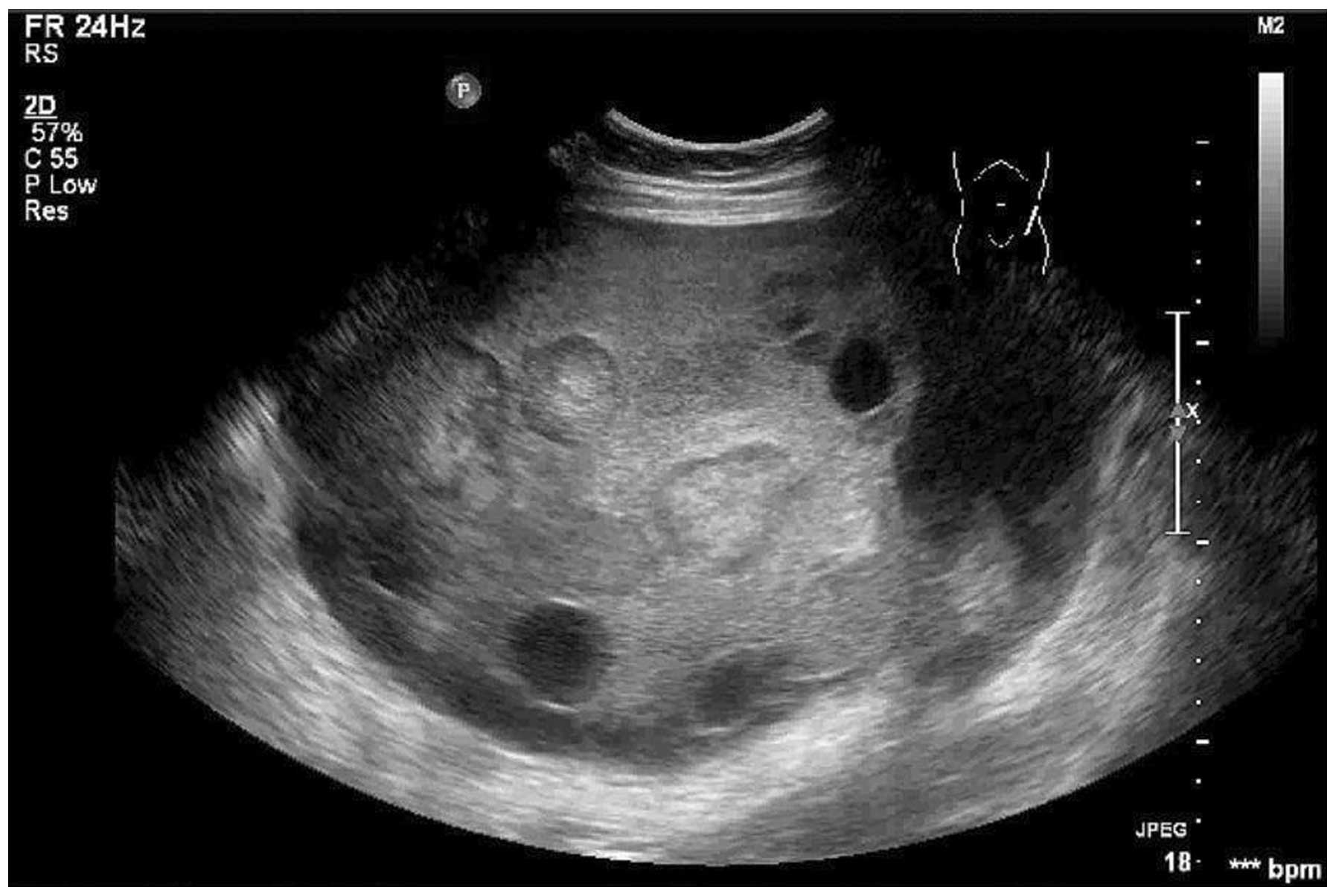
Transabdominal color Doppler examination revealed an
encapsulated pedunculated solid and cystic mass (20×18×13 cm) with
irregular but clear margins on the front left of the uterus
(Fig. 1). A main vessel was
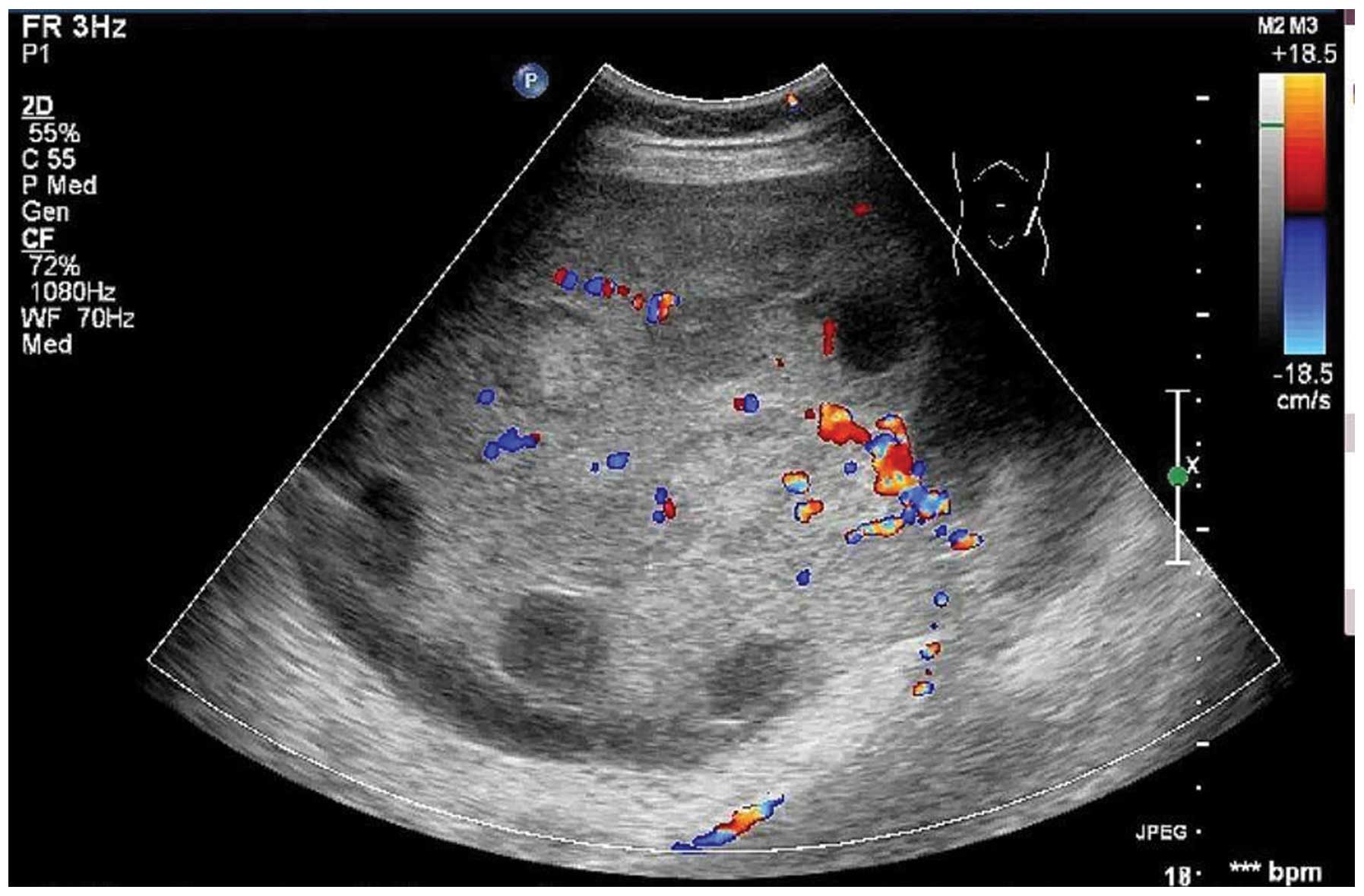
observed, with a pulsatility index of 0.76, a resistive index of
0.52 and a time-averaged maximum velocity of 27.63 (Fig. 2). There was a large quantity of
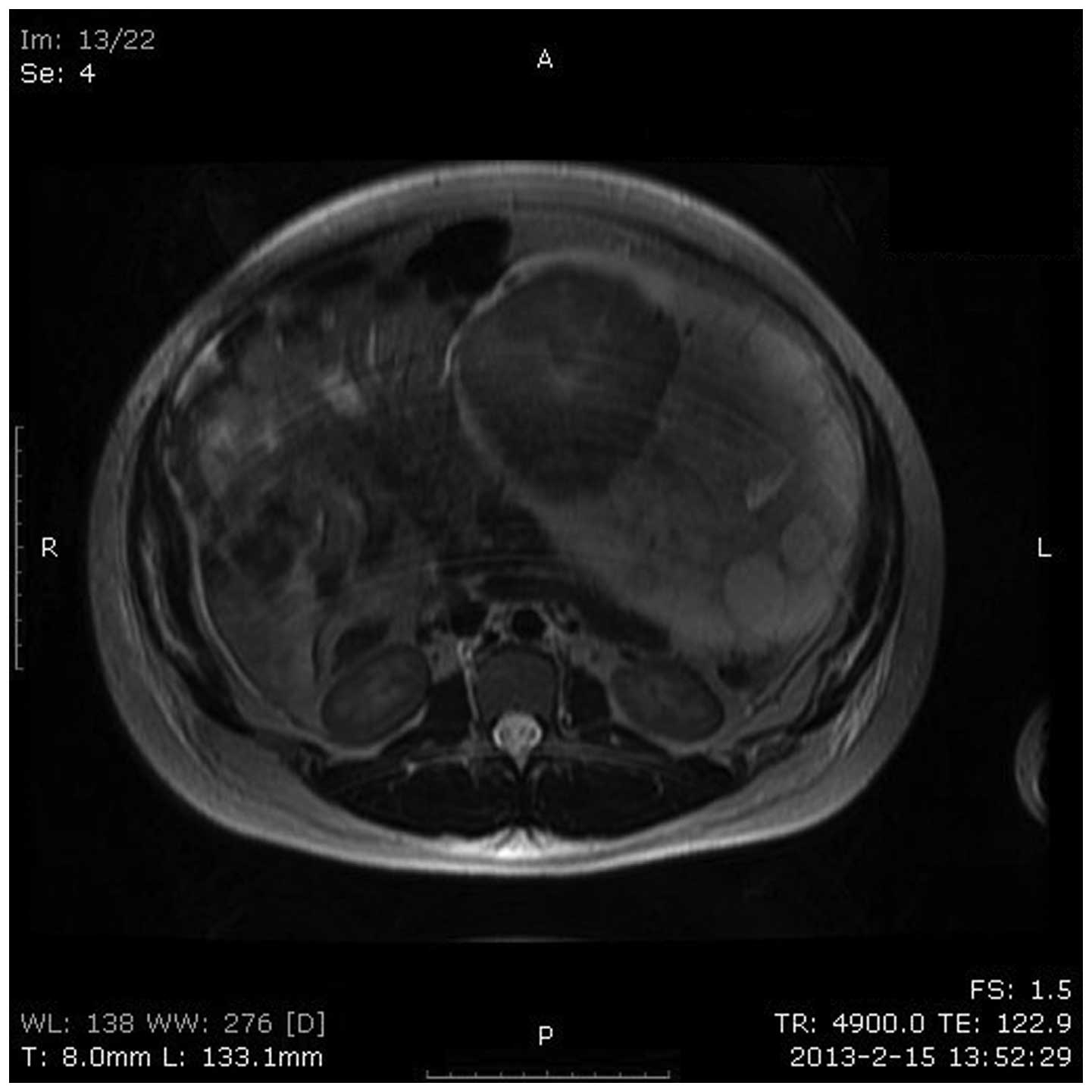
fluid surrounding the mass. From MRI, the patient was diagnosed
epithelial cancer and the imaging features were consistent with the
ultrasound in terms of location, size, external configuration and
internal structure (Fig. 3). Tumor
markers revealed α-fetoprotein, carcinoembryonic antigen (CEA),
cancer antigen 19-9, cancer antigen 125 and HCG levels to be
abnormally high (Table II).
Gastroscopy revealed a long ulcer lesion, 2.4 cm in length, in the
posterior wall of the middle of the gastric body. The diagnosis of
the biopsy was of a gastric ulcer.
 | Table IITumor marker. |
Table II
Tumor marker.
| Inspection item | Test result | Normal range |
|---|
| α-fetoprotein,
ng/ml | 542.2 | <8.1 |
| CEA, ng/ml | 20.64 | <5 |
| Cancer antigen 19-9,
U/ml | 204.01 | <37 |
| Cancer antigen 125,
U/ml | >600 | <30.2 |
| HCG, mIU/ml | >10,000 | <10 |
Treatment
At week 38, the patient underwent an abdominal
exploration and a healthy male infant weighing 1,300 g was
delivered by cesarean section, with Apgar scores of 6 and 8 at 1
and 5 min, respectively. The left uterine appendages were removed,
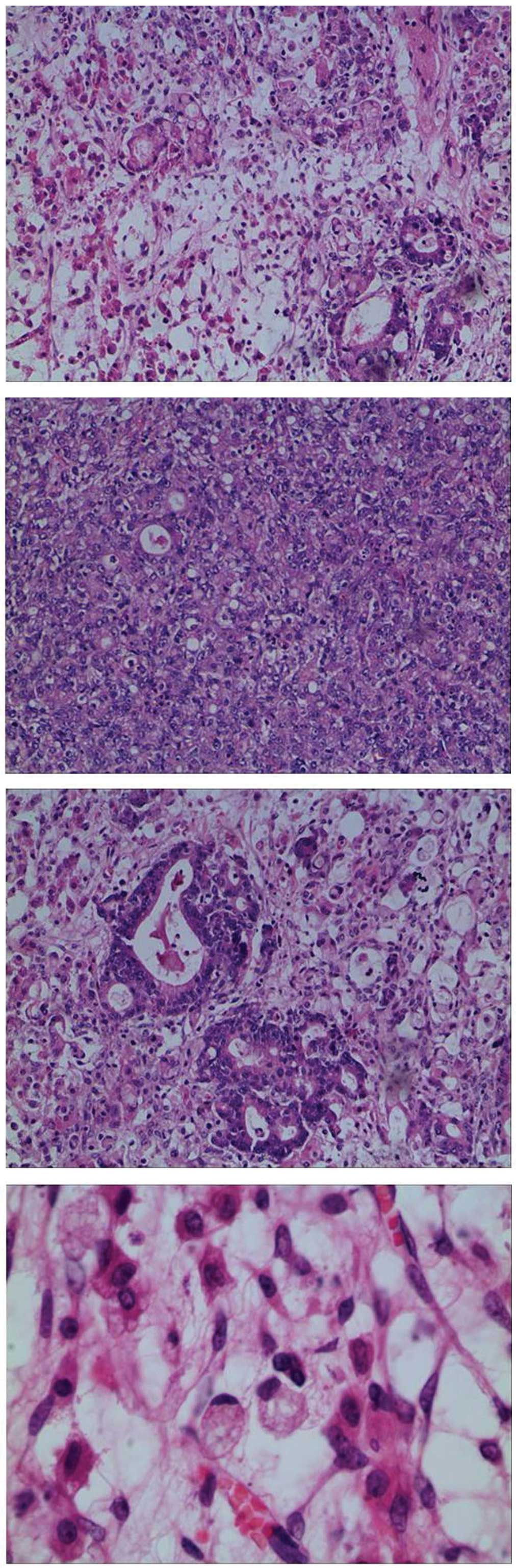
and subsequent pathological examination of the frozen section of
the left ovary revealed metastatic poorly-differentiated
adenocarcinoma (Fig. 4).
Subsequently, a right adnexectomy was performed. Pathological
examination revealed minimal invasion of the carcinoma tissue in
the right ovary, although the tissue appeared normal from the
abdominal exploration and imaging observations. Macropathological
analysis of the left ovary revealed multiple nodules (diameters,
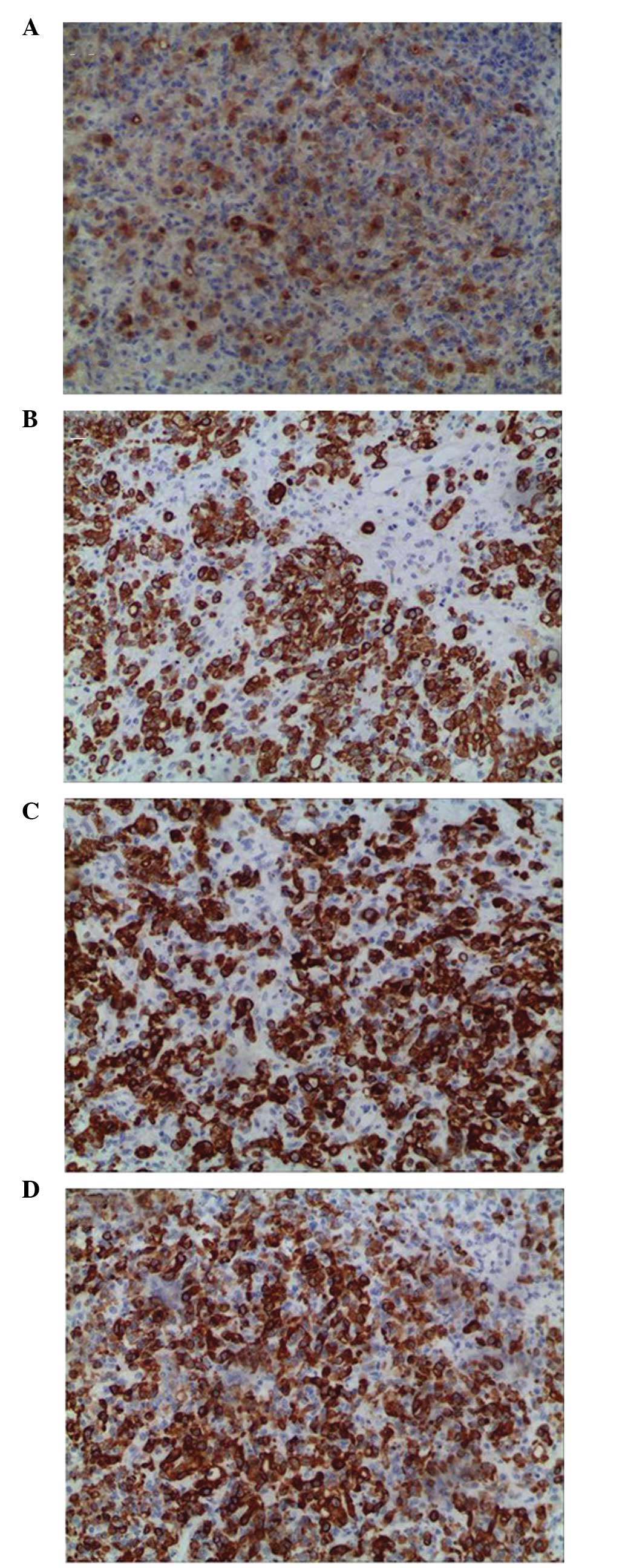
0.2–5 cm) in the mass. Immunohistochemistry results indicated that
CEA, cytokeratin (CK), CK7 and CK20 were positive (Fig. 5), while ascites were negative.
Follow-up
Five days after surgery, the patient underwent a
positron emission tomography-computed tomography scan. High levels
of radioactive uptake were detected in the lesser curvature wall of
the stomach, which indicated gastric carcinoma. The patient
underwent a total gastrectomy and regional lymph node dissection
combined with intraperitoneal chemotherapy. Postoperative pathology
revealed poorly-differentiated adenocarcinoma with signet-ring cell
carcinoma of the stomach, which infiltrated the serous membrane. In
addition, a cancerous embolus was observed in the blood
vessels.
Discussion
Krukenberg tumors are a type of metastatic ovarian
tumor. The primary tumor usually originates from the
gastrointestinal tract, primarily from the stomach or the colon and
rectum, although occasionally the tumors originate from the breast,
uterus, biliary tract, pancreas and kidney (1). The bloodstream, lymphatic system and
local implantation are common methods of Krukenberg tumor
metastasis (6). The tumor is
predominantly solid and often inflicts bilateral ovaries, with a
clear border and irregular shape, which occasionally exhibits a
single or multiple cystic structure. According to MRI (7) and pathology (1,6)
examinations, more than half of solid tumors exhibit a random
distribution of multiple nodular components. This is uncommon in
primary ovarian tumors and is useful in discriminating Krukenberg
tumors from primary ovarian tumors. However, sonographic
observations from existing case reports of Krukenberg tumors
commonly describe the tumors as roughly uniform solid masses with a
strong echo, but lacking internal nodular structure (3,8).
Sonographic observations in the present study revealed an
encapsulated pedunculated solid and cystic mass, with multiple
hyperechoic nodules in the solid portion, which was in accordance
with the MRI and pathological results. Furthermore, a main vessel
with small branches was observed to penetrate from the tumor
pedicle into the solid portion, with high speed and low resistance.
Compared with other imaging examinations, color Doppler sonography
is safer, inexpensive and more convenient for diagnosing these
diseases, particularly in pregnant patients (9). Thus, the sonographic observations of
Krukenberg tumors are important.
The level of ovarian hormones during pregnancy and a
rich blood flow contribute to tumor metastases. The most common
clinical manifestations of Krukenberg tumors during pregnancy are
nausea and vomiting, which are similar to symptoms often
experienced during pregnancy, thus, may be neglected. As the
gestational period increases and the circumference of the waist
increases, the pelvic neoplasm and ascites are difficult to locate
(4). Therefore diagnosis is often
delayed, as demonstrated by the present case. Disease progression
of Krukenberg tumors is often fast, thus, numerous patients present
with metastatic carcinoma earlier than the primary tumors. If a
patient has a history of primary gastrointestinal tumors or
gastrointestinal symptoms, including stomach bleeding, and suffer
from nausea and vomiting in the middle or later stages of
pregnancy, a Krukenberg tumor may be considered as a differential
diagnosis. With regard to the patient in the present case, the four
dimensional ultrasound examination was normal one month previously,
but a 20×18×13 cm mass was located 4 weeks later. The quick
progression may correlate with severe preeclampsia, which is
predominantly caused by placental hypoxia ischemia (10). Placental hypoxia may release a
variety of soluble factors, leading to a high dynamic blood
condition and accelerated renal blood flow, potentially promoting
tumor progression.
Examining the images in the current case only
located the ovarian neoplasm on the left side, but pathological
diagnosis indicated that the carcinoma had also invaded the right
ovary. This is due to tumor metastasis, particularly from the
stomach, which is usually via a blood or lymphatic channel and
leads to bilateral adnexa involvement. However, the degree of
involvement varies. Certain tumors may be located by image
examinations, while others cannot. Therefore, the diagnosis of a
Krukenberg tumor should depend on pathological confirmation. Once
unilateral lesions are located by image examination, it is
important to be aware that contralateral metastasis may also exist.
Thus, more care should be taken during abdominal exploration, as
resecting all metastases is important to improve the patient
prognosis.
In conclusion, Krukenberg tumors are difficult to
diagnose, progress fast and have a poor outcome, thus, improving
the understanding of Krukenberg tumors is of particular importance.
When a solid and cystic mass is identified in a pregnant woman by
ultrasound, with multiple hyperechoic nodules and a main vessel
with small branches in the solid portion, that may be a Krukenberg
tumor instead of a primary tumor, further examinations should be
performed to substantiate the diagnosis and identify the source,
this may help doctors to chose the best remedy and gain more
survival time for the patient.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30973040) and the
Technology Foundation of Tianjin Public Health Bureau of China (no.
2012KZ104).
References
|
1
|
Kiyokawa T, Young RH and Scully RE:
Krukenberg tumors of the ovary: a clinicopathologic analysis of 120
cases with emphasis on their variable pathologic manifestations. Am
J Surg Pathol. 30:277–299. 2006.
|
|
2
|
Duenas-Garcia OF, Diaz-Sotomayor M and
Chanana C: Bilateral ovarian krukenberg tumor in a full-term
pregnancy. ISRN Obstet Gynecol. 2011:6203802011.PubMed/NCBI
|
|
3
|
Testa AC, Licameli A, Di Legge A, et al:
Color Doppler sonographic features of a Krukenberg tumor in
pregnancy. J Ultrasound Med. 28:695–698. 2009.PubMed/NCBI
|
|
4
|
Ozdegirmenci O, Kayikcioglu F, Haberal A
and Ozfuttu A: Krukenberg tumor mimicking pregnancy luteoma.
Gynecol Endocrinol. 23:482–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stojnic J, Stefanovic A, Jeremic K, Kadija
S, Jeftovic M and Jeremic J: Krukenberg tumor of gastric origin in
pregnancy with dismal outcome. Eur J Gynaecol Oncol. 32:356–358.
2011.PubMed/NCBI
|
|
6
|
Qiu L, Yang T, Shan XH, Hu MB and Li Y:
Metastatic factors for Krukenberg tumor: a clinical study on 102
cases. Med Oncol. 28:1514–1519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ha HK, Baek SY, Kim SH, Kim HH, Chung EC
and Yeon KM: Krukenberg’s tumor of the ovary: MR imaging features.
AJR Am J Roentgenol. 164:1435–1439. 1995.
|
|
8
|
Chou MM, Ho ES, Lin NF and Lee YH: Color
Doppler sonographic appearance of a Krukenberg tumor in pregnancy.
Ultrasound Obstet Gynecol. 11:459–460. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Togashi K: Ovarian cancer: the clinical
role of US, CT, and MRI. Eur Radiol. 13:L87–L104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eiland E, Nzerue C and Faulkner M:
Preeclampsia 2012. J Pregnancy. 2012:5865782012. View Article : Google Scholar
|