Introduction
Modern molecular biological studies have confirmed
that hypertrophic cardiomyopathy (HCM) is an autosomal dominant
genetic disease caused by the genetic mutation of the myocardial
sarcomere gene (1–4), and it has an incidence rate of ~1/500
(5,6). HCM is a common cause of sudden
cardiac death in children and young individuals (7). The examination of the left
ventricular functions is important for patients with HCM,
particularly in the evaluation of treatment efficacy and long-term
follow-up. The conventional view considers that cardiac involvement
is common with HCM and represents as left ventricular diastolic
dysfunction (8–11). However, molecular studies have
identified that in HCM, the disorder of systolic function occurs
initially and the cardiac hypertrophy is a compensatory response
(12,13). Currently, sufficient evidence
indicates that despite the reductions of left ventricular systolic
functions in patients with HCM (14,15),
such as significant reductions in the left ventricular strain and
strain rate, the left ventricular ejection fraction (LVEF) of the
majority of patients remains normal or enhanced (16). Therefore, the use of LVEF to
evaluate the global left ventricular systolic functions is not able
to truly reflect the impaired condition of the left ventricular
systolic functions.
A number of novel ultrasound technologies, including
tissue Doppler imaging (TDI), TDI-derived strain imaging and
two-dimensional (2D) speckle tracking, are used to detect the
reductions in left ventricular longitudinal systolic functions in
patients with HCM. However, these techniques significantly depend
on image quality and are time-consuming. The tissue motion annular
displacement (TMAD) technique is a novel technology developed in
accordance with speckle-tracking technology. This technique is able
to assess the left ventricular longitudinal systolic functions by
tracking the positions of the mitral annulus in the left
ventricular systolic phase. TMAD quickly evaluates the left
ventricular longitudinal systolic functions and does not require
high image quality (17). In the
present study, TMAD technology was used to assess the left
ventricular global and segmental systolic functions in patients
with HCM to provide a novel method of evaluating the left
ventricular longitudinal systolic functions in these patients.
Subjects and methods
Subjects
A total of 65 patients with HCM were selected. The
inclusion criteria (18) were as
follows: 2D ultrasound showed asymmetric hypertrophy in the left
ventricular walls; septum/posterior wall ratio was ≥1.5; the
thickness of the interventricular septum was ≥15 mm; no significant
or mild thickening was found in segments of LV wall except
interventricular septum. The exclusion criteria were as follows:
Ventricular hypertrophy caused by simple apical HCM; hypertension;
coronary heart and valvular diseases; and LVEF <50%. According
to the resting pressure gradient of the left ventricular outflow
tract (PGlvot), the subjects were divided into the obstructive HCM
(HOCM) group [PGlvot ≥30 mmHg] and the non-obstructive HCM (NOHCM)
group (PGlvot <30 mmHg). The HOCM group comprised 16 cases (nine
males and seven females, with a mean age of 42.7±11.7 years) and
the NOHCM group comprised 49 cases (30 males and 19 females, with a
mean age of 43.0±17.4 years). The 48 participants in the healthy
control group were selected in the same period and according to the
following conditions: Gender- and age-matched (30 males and 18
females, with a mean age of 41.2±14.5 years); normal results in the
physical examinations, echocardiography, electrocardiography (ECG)
and biochemical tests; and did not have coronary heart disease,
hypertension, valvular disease, various arrhythmias and various
systemic diseases. This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of The Fourth Military Medical University (Xi’an, China). Written
informed consent was obtained from all participants. The general
information of the study subjects is shown in Table I.
 | Table IClinical characteristics of the three
groups. |
Table I
Clinical characteristics of the three
groups.
| Parameter | HOCM (n=16) | NOHCM (n=49) | Controls (n=48) | P-value |
|---|
| Male, n (%) | 9 (56.3) | 30 (61.2) | 30 (62.5) | 0.906 |
| Age (years) | 42.7±11.7 | 43.0±17.4 | 41.2±14.5 | 0.836 |
| Height (cm) | 165.3±7.0 | 167.4±8.1 | 167.7±6.0 | 0.601 |
| Weight (kg) | 64.6±10.8 | 67.3±12.8 | 63.9±9.6 | 0.314 |
| BSA
(m2) | 1.7±0.2 | 1.8±1.1 | 1.7±0.1 | 0.649 |
| HR (beats/min) | 71.0±12.2 | 74.0±11.9 | 71.8±9.8 | 0.535 |
| Systolic BP
(mmHg) | 113.6±10.4 | 110.2±10.5 | 109.5±10.7 | 0.451 |
| Diastolic BP
(mmHg) | 67.5±6.9 | 68.8±6.4 | 68.0±4.8 | 0.700 |
| Drug therapy, n
(%) |
| ACE inhibitor | 8 (50.0) | 17 (34.7) | 0 | - |
| ARB | 4 (25.0) | 21 (42.9) | 0 | - |
| β-blocker | 7 (43.8) | 16 (32.7) | 0 | - |
| Calcium-channel
blocker | 7 (43.8) | 18 (36.7) | 0 | - |
| Aspirin | 10 (62.5) | 31 (63.3) | 0 | - |
Standard ultrasound examination
The iE33 xMatrix UCG system (S5-1 transducer;
Philips Healthcare, Andover, MA, USA) with external QLAB
quantitative analysis software, version 8.1 (Philips Healthcare)
was used. The left ventricular end-diastolic diameter,
interventricular septum and posterior left ventricular wall
thickness were measured along the left ventricular long-axis view.
The septum/posterior wall ratio was calculated. The pulsed wave
(PW) sampling volumes were placed under the mitral valves of the
apical four-, two- and three-chamber views. The early and late
mitral inflow velocities (E and A, respectively) were measured, and
E/A was calculated. In the TDI mode, PW sampling volumes were
placed in the interventricular septum, lateral wall, anterior wall,
inferior wall, anterior septum, and the ring side of the
posterior-wall mitral valve. Early and late myocardial diastolic
velocities (Em and Am) were measured, and the mean values were
obtained for the calculation of Em/Am and E/Em. When the PW
sampling volume was placed on the aortic valve of the aortic
long-axis view, the PGlvot was measured. The measurements of the
aforementioned ultrasound data were based on the American Society
of Echocardiography guidelines and requirements (19).
Strain imaging data acquisition
All subjects were imaged in the left lateral
position, with synchronous ECG display. The 2D images of three
consecutive cardiac cycles of the apical four-, two- and
three-chamber views were collected. The depth and fan size were
adjusted to reach the maximum frame frequency (>50 frames/sec).
Data were stored in a removable hard disk for offline analysis.
QLAB software, version 8.1 was used to perform the following:
Importation of the 2D grey-scale images; selection of the cardiac
motion quantification (CMQ) module; sketching of the endocardium
and epicardium in the region of interest (ROI) interface; and
adjustment of the ROI width to match the myocardial thickness.
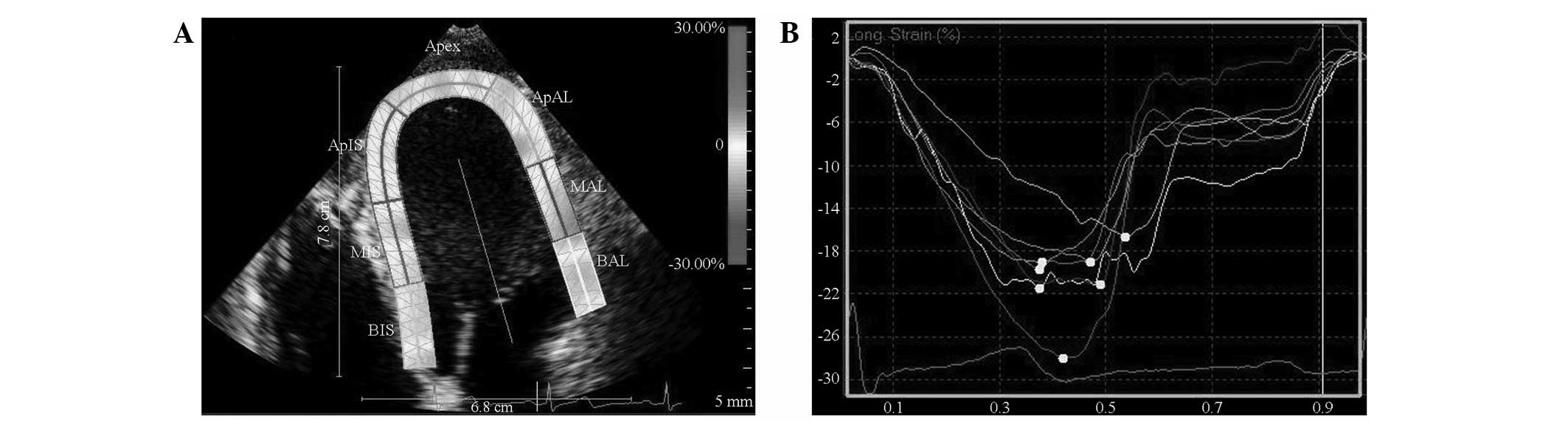
After completely outlining the endocardium and epicardium, the
software was run to automatically track the ROI cardiac echo spots
and provide all myocardial segmental longitudinal strain curves and
peaks (Fig. 1). The left
ventricular longitudinal segmental strain was read. The average
values of the basal, middle and apical segment of the left
ventricular wall represented its strain. The ventricular wall
segments selected for the analysis were the interventricular
septum, lateral wall, inferior wall, anterior wall, anterior septum
and posterior wall. The strains measured by the software included
the basal, middle and apical segments of the corresponding wall,
the mean value of which represented the longitudinally segmental
strain (LSseg) of the ventricular wall. When more than
two segments of the wall were not effectively tracked, the track
was considered ineffective.
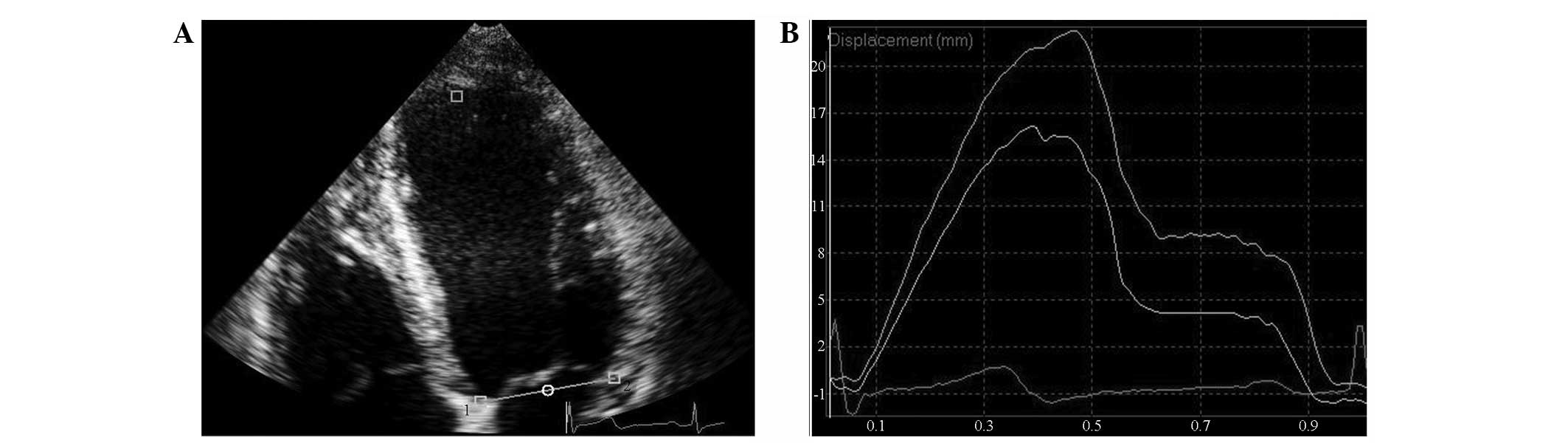
TMAD data acquisition
TMAD data were measured in the apical four-, two-
and three-chamber views. The 2D grey-scale images were imported
into QLAB software, version 8.1. Subsequently, the CMQ module was
selected to outline three points in the ROI. Two points were placed
in each of the interventricular septum and lateral wall side of the
mitral ring of the apical four-chamber heart, the anterior
interventricular septum, and the posterior wall side of the apical
three-chamber heart, and anterior wall side and inferior wall side
of the apical two-chamber heart. The third point was fixed on the
surface of the apical endocardium (Fig. 2). The software automatically
tracked the synchronous displacement curves of two sampling points
and the corresponding segmental mitral annular values of
displacement (MADseg). The average value of the six wall
segments was considered to represent the global displacement of the
mitral ring (MADglobal). The ratio of the midpoint MAD
of the apical four-, two- and three-chamber views to the left
ventricular long-axis was recorded as normalized midpoint
displacement of the mitral ring [TMAD midpt (%)].
Intra- and interobserver variability
Five healthy subjects and five patients with HCM
were randomly selected, in which the TMAD of a total of 60 segments
was measured. The MAD values of all segments were measured by
doctors A and B. The difference reflected the interobserver error.
Doctor B repeated the measurement of the same index of the above
points two weeks later. From this measurement, the difference was
used to reflect the intraobserver error. The degree of variation of
these differences was then calculated. The error percentage of each
measured value was used as the variability index, that is,
(x1−x2)/[(x1+x2)/2] × 100, where ×1 is the result of the first
measurement and ×2 is the result of the second measurement.
Statistical analysis
All data were analysed using SPSS statistical
analysis software, version 16.0 (SPSS, Inc., Chicago, IL, USA). The
normally distributed measurement data were expressed as mean ±
standard deviation. The quantitative parameters of the three groups
were compared using analysis of variance. The least significant
difference method was used for pairwise comparison. Pearson’s
correlation was used to analyse the intergroup relationship and the
χ2 test was used to compare the intergroup countable
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
General information
No statistical differences were identified in the
gender ratio, age, height, weight, body surface area, heart rate or
blood pressure among the three groups (P>0.05; Table I).
Conventional echocardiographic
parameters
The interventricular septal thickness (IVST), left
ventricular posterior wall thickness (LVPWT), septum/posterior wall
ratio and left atrial volume (LAV) of the HOCM and NOHCM groups
were increased compared with those of the healthy control group.
Additionally, the left ventricular end-diastolic volume (LVEDV),
early peak mitral inflow velocity (MV E), Em and peak myocardial
systolic velocity (Sm) of the HOCM and NOHCM groups decreased, and
the late peak mitral inflow velocity (MV A) and E/Em ratio of the
HOCM and NOHCM groups increased compared with those of the healthy
control group, whereas Am decreased. No significant differences
were identified in the ejection fraction (EF), left ventricular
end-systolic volume (LVESV) and left ventricular internal diameter
at end diastole (LVIDd) among the three groups (Table II).
 | Table IIEchocardiographic characteristics of
the three groups. |
Table II
Echocardiographic characteristics of
the three groups.
| Parameter | HOCM | NOHCM | Controls | P-value |
|---|
| IVST(mm) | 24.3±3.1a | 20.2±3.5a | 7.5±1.1 | <0.001 |
| LVPWT (mm) | 11.5±3.2a | 10.5±2.9a | 7.1±1.1 | <0.001 |
| Septum/posterior
wall ratio | 2.2±0.6a | 2.1±0.6a | 1.1±0.1 | <0.001 |
| LVIDd (mm) | 41.8±3.5 | 44.6±5.1 | 44.3±3.0 | 0.053 |
| LVEDV (ml) | 60.5±15.9a | 67.0±17.6a | 74.7±14.7 | 0.006 |
| LVESV (ml) | 23.3±7.0 | 27.0±9.2 | 27.6±5.3 | 0.134 |
| EF biplane (%) | 64.1±6.2 | 61.3±7.8 | 62.0±3.6 | 0.304 |
| LAV biplane
(ml) | 48.7±18.9a | 42.5±19.1a | 27.1±6.2 | <0.001 |
| PGlvot (mmHg) | 67.4±16.9a | 10.7±7.8a | 4.6±1.4 | <0.001 |
| MV E (cm/sec) | 71.1±20.4a | 71.0±22.0a | 84.6±17.9 | 0.003 |
| MV A (cm/sec) | 92.0±30.2a,b | 71.9±27.2a | 63.7±15.9 | <0.001 |
| MV E/A | 0.86±0.37a | 1.11±0.54a | 1.44±0.63 | 0.001 |
| Sm (cm/sec) | 5.9±2.1a,b | 6.7±1.8a | 8.3±1.6 | 0.032 |
| Em (cm/sec) | 3.5±1.0a | 4.7±2.2a | 9.3±2.2 | <0.001 |
| Am (cm/sec) | 6.6±2.2a | 6.5±1.8a | 7.5±1.5 | 0.022 |
| Em/Am | 0.55±0.11a,b | 0.76±0.39a | 1.3±0.48 | <0.001 |
| E/Em | 21.0±7.3a,b | 16.8±7.0a | 9.3±1.6 | <0.001 |
Comparison of the left ventricular
segmental strains and global strain (LSglobal)
The LSseg and LSglobal of the
HOCM and NOHCM groups significantly increased compared with the
healthy control group (P<0.01). No statistically significant
difference was identified in the left ventricular segmental strains
and LSglobal between the HOCM and NOHCM groups (Table III).
 | Table IIIComparison of the left ventricular
segmental and global strains of the three groups. |
Table III
Comparison of the left ventricular
segmental and global strains of the three groups.
| Parameter | HOCM | NOHCM | Controls | P-value |
|---|
|
SRIS | −14.6±4.4a | −15.7±4.8a | −23.1±2.4 | <0.001 |
|
SRAL | −14.9±4.1a | −16.2±5.5a | −23.1±3.0 | <0.001 |
|
SRAN | −13.7±4.0a | −14.6±4.4a | −22.6±2.5 | <0.001 |
|
SRIN | −17.0±4.8a | −17.3±6.1a | −23.8±3.5 | <0.001 |
|
SRAS | −14.0±4.1a | −13.0±6.1a | −20.5±3.1 | <0.001 |
|
SRIL | −17.0±5.3a | −16.4±6.8a | −19.3±6.1 | <0.001 |
|
LSglobal | −15.2±3.2a | −15.6±4.5a | −18.6±5.0 | <0.001 |
Comparison of segmental MADs and
MADglobal
The six MADs and MADglobal in the HOCM
and NOHCM groups were significantly reduced compared with those of
the healthy control group (P<0.01). No statistically significant
differences were identified in the MADglobal and
segmental MAD values of the HOCM and NOHCM groups (Table IV).
 | Table IVMADs of the three groups. |
Table IV
MADs of the three groups.
| Parameter | HOCM | NOHCM | Controls | P-value |
|---|
| MADIS
(mm) | 8.3±2.2a | 8.4±2.9a | 13.1±2.3 | <0.001 |
| MADAL
(mm) | 9.7±3.3a | 9.3±3.8a | 13.9±2.5 | <0.001 |
| MADAN
(mm) | 8.4±3.0a | 9.1±3.7a | 13.1±2.2 | <0.001 |
| MADIN
(mm) | 8.4±3.0a | 8.2±3.2a | 13.5±2.2 | <0.001 |
| MADAS
(mm) | 8.2±2.2a | 7.5±3.3a | 12.4±2.1 | <0.001 |
| MADIL
(mm) | 10.3±2.7a | 8.8±3.6a | 13.8±2.3 | <0.001 |
|
MADglobal (mm) | 8.9±1.9a | 8.6±2.8a | 13.3±1.8 | <0.001 |
| TMAD midpt (%) | 11.5±2.4a | 11.0±3.8a | 16.7±2.4 | <0.001 |
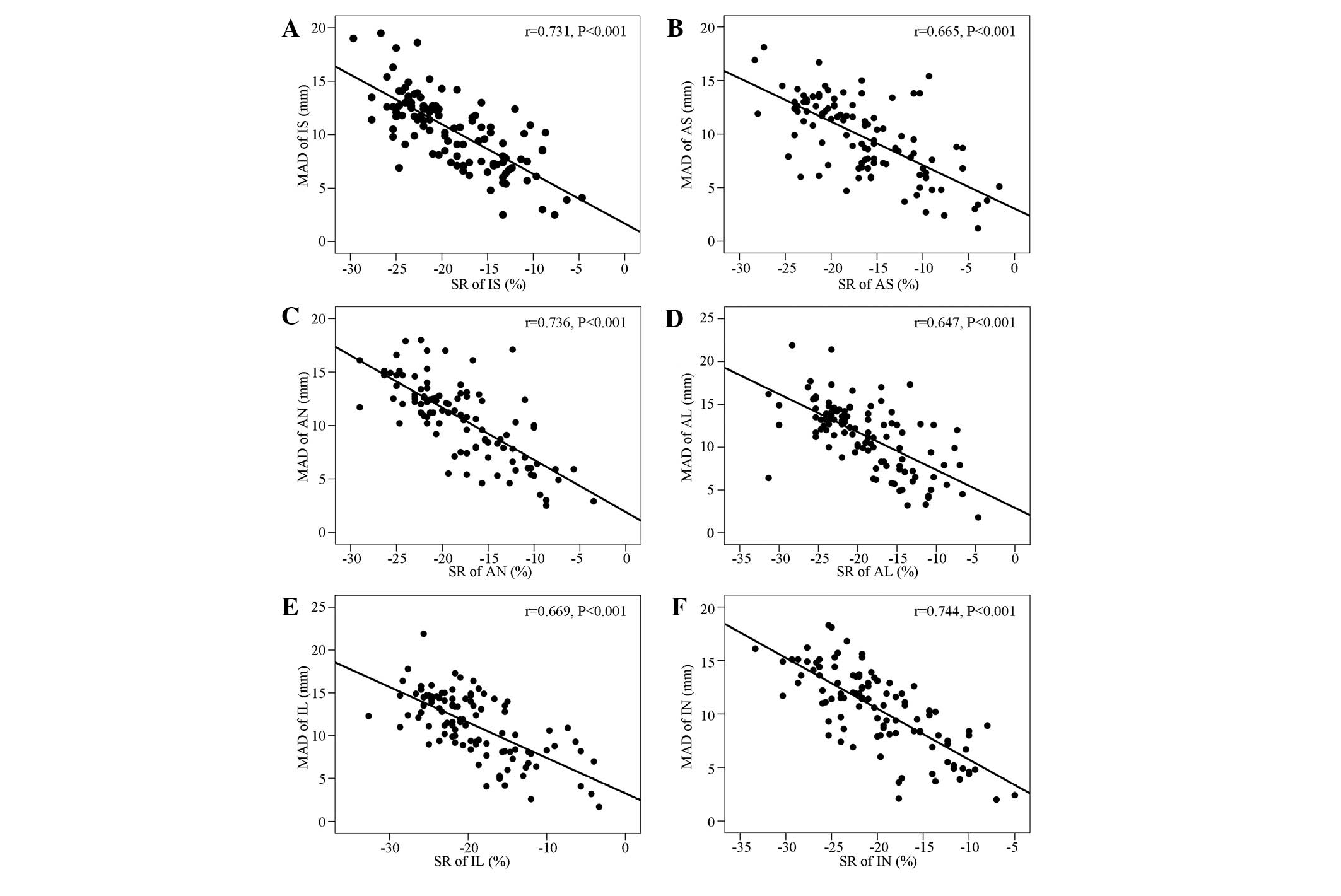
Correlation analysis of the displacements
of the six mitral ring sites and the left ventricular wall
strain
Significant negative correlations were identified
between the displacements of the six mitral ring sites and the left
ventricular wall strain (Fig.
3).
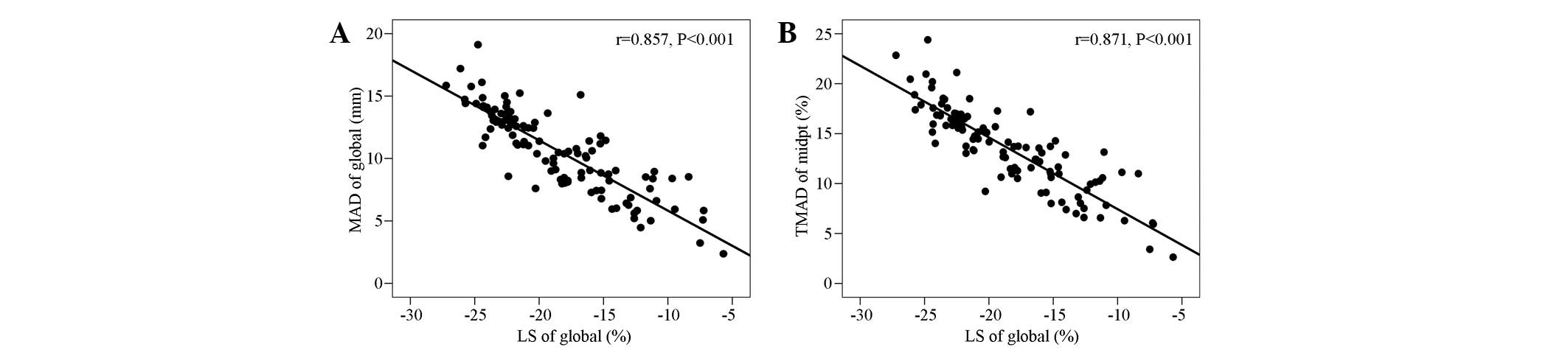
Correlation analysis of
MADglobal and TMAD midpt with the
LSglobal
The MADglobal was significantly
negatively correlated with the LSglobal (r=−0.857,
P<0.001). The TMAD midpt (%) was also significantly negatively
correlated with LSglobal (r=−0.871, P<0.001; Fig. 4).
Repeatability test
No significant differences were identified in the
TMAD and speckle-tracking imaging (STI) between the intra-
(5.24±2.60 vs. 5.83±2.51) and interobserver variabilities
(6.99±3.32 vs. 6.47±2.80) (P>0.05).
Discussion
TMAD technology was used in the present study to
evaluate the left ventricular longitudinal systolic functions of
patients with HCM. Through a comparison conducted using 2D-STI, the
LVEF in the HCM group was found to be non-significantly reduced
compared with that in healthy controls. However, the left
ventricular global and segmental longitudinal systolic functions of
the patients with HCM were observed to be significantly reduced. In
addition, the MADs were significantly negatively correlated with
the left ventricular segmental strains. Furthermore,
MADglobal and TMAD midpt values were significantly
negatively correlated with the LSglobal values.
Therefore, we considered that it is possible to use TMAD to quickly
and accurately assess the left ventricular longitudinal global and
segmental functions of patients with HCM. Additionally, MAD may be
more sensitive than LVEF in terms of identifying the left
ventricular global and segmental systolic dysfunction.
The main pathological changes associated with HCM
are myocardial hypertrophy, muscle cell disorder, contractile
protein dysfunction and interstitial fibrosis (20). Of these changes, the first to occur
is the left ventricular systolic dysfunction, whereas the
myocardial hypertrophy is the compensatory response (12,13).
More advanced technologies and further studies (14,15,21,22)
have confirmed that the left ventricular longitudinal systolic
functions in patients with HCM are reduced. Therefore, the
detection of left ventricular longitudinal systolic functions in
patients with HCM is important for the early detection of systolic
dysfunction, disease progression monitoring, therapeutic review and
prognosis evaluation. However, these novel techniques significantly
depend on image quality, and assessment using poor quality images
would be difficult to accomplish.
The mitral annulus is part of the fibrous skeleton
of the heart. This structure has a large number of muscle fibres
attached to the skeleton, which are arranged longitudinally
throughout the space between the apex and base (23). The contraction of longitudinal
muscle fibres shortens the ventricular cavity along the long-axis,
leading to the mitral annulus moving downward towards the apex and
the intraventricular blood gravity being relatively fixed in the
position of the apex (24).
Therefore, it is possible to use the apex as a reference point. It
may be possible to use the displacement of the mitral annulus as it
moves towards the apex to reflect left ventricular shortening. A
study (25) detected that the
shortening of the left ventricular long-axis contributed to almost
70% of the LVEF. Studies have demonstrated that the MAD may have
important clinical value in the application of the left ventricular
longitudinal functions to the evaluation of early diagnosis of
heart damage (26), heart disease
efficacy evaluation (27),
prediction of cardiovascular events (28) and prognosis of certain heart
diseases (29).
The present study observed that the MADs of the six
mitral annulus points in the HCM group were lower compared with
those of the healthy control group. This result suggests that the
reduction of the left ventricular segmental systolic functions in
the patients with HCM not only occurs in the hypertrophic
myocardial segments, but also in the non-hypertrophic myocardial
segments. The left ventricular longitudinal myocardial fibres are
mainly distributed under the endocardium, which may be more
susceptible to pathological factors. The MAD is the indirect
reflection of the contractile function of these longitudinal
myocardial fibres. Therefore, the evaluation of the MAD on the left
ventricular longitudinal systolic function has potential value in
the early diagnosis of the left ventricular damage associated with
HCM. Certain studies of patients with diastolic heart failure have
shown that isolated diastolic heart failure is rare. The majority
of cases are associated with left ventricular longitudinal systolic
dysfunction. Nishikage et al (26) observed that in patients with
hypertension with a normal LVEF, the left ventricular systolic
dysfunction occurred prior to the longitudinal diastolic
dysfunction. Thus, MAD is an early reflection of the left
ventricular systolic dysfunction. In addition, MAD is able to
sensitively reveal the left ventricular systolic dysfunction. As
indicated by the present study, the detection and evaluation of the
left ventricular early systolic dysfunction associated with HCM by
measuring the MAD is likely to provide a novel perspective.
The present study revealed that the LVEF did not
significantly differ between the HCM and control groups, regardless
of the type of HCM (cHCM or nHCM). However, the representative
index of left ventricular global systolic function, namely,
MADglobal, was reduced, which suggested that patients
with HCM suffered from left ventricular global longitudinal
systolic dysfunction. However, MADseg and
MADglobal were the actual displacements, by which the
mitral annulus moved directly to the apex. These displacements were
not considered in relation to the actual heart size. Therefore,
comparisons among different individuals were adversely affected.
Similarly to the LVEF, the ratio of TMAD midpt to the left
ventricular long axis was used. It was observed that the left
ventricular TMAD midpt of the patients with HCM was reduced. The
contraction of the heart and volume deformation includes the
shortening of the cardiac long and short axes. The TMAD midpt may
be a practical and feasible indicator for evaluating the
longitudinal shortening rate in patients with HCM, which is worthy
of further study.
The 2D-STI method is able to track 2D ultrasound
myocardial spot positions through a frame-by-frame process and
determine the myocardial strain through the spot position-change
rate to reflect the left ventricular contractile function (14); it overcomes angle dependence and
possesses a good correlation with cardiac magnetic resonance
(30,31). 2D-STI also accurately assesses the
left ventricular global and segmental systolic functions. In the
present study, the detection of left ventricular 2D strain in HCM
through 2D-STI and further comparison with the TMAD data revealed
that the 2D-STI detection of LSglobal and the left
ventricular segmental strains had a good correlation with the TMAD
detection of the MADglobal and MADseg.
Therefore, TMAD was able to accurately assess the left ventricular
longitudinal global and segmental systolic functions. TMAD is
advantageous due to its low-quality image requirements and rapid
measurement (17).
In conclusion, TMAD was able to accurately and
quickly evaluate the left ventricular longitudinal global and
segmental systolic functions with low-quality image requirements
and good reproducibility. In addition, TMAD may be used for the
evaluation of left ventricular longitudinal systolic functions in
patients with HCM. The proposed technique exhibits possible
potential prognostic value in the early diagnosis, disease
progression monitoring, efficacy assessment and prognosis
evaluation of HCM.
This study included a small number of cases.
Therefore, it was not possible to determine the MAD normal
reference values. Furthermore, the impact factors affecting MAD
remain unclear. MAD was simply the reflection of the ventricular
wall segmental overall function at the mitral annular attachment
point, and was not able to reveal the systolic functions of the
corresponding basal, middle and apical segments. Therefore, the
information regarding left ventricular motion in the patients with
HCM provided by the TMAD data was less than that provided by the
strain data. All limitations of this study necessitate further
study.
Acknowledgements
This article was supported by international S&T
Cooperation Program of China (2014DFA31980), International Science
& Technology Cooperation Program of Shanxi (2013KW33-03) and
Shanxi Science & Technology tackling key problem
(2012K15-01-03).
References
|
1
|
Marian AJ, Salek L and Lutucuta S:
Molecular genetics and pathogenesis of hypertrophic cardiomyopathy.
Minerva Med. 92:435–451. 2001.PubMed/NCBI
|
|
2
|
Niimura H, Patton KK, McKenna WJ, et al:
Sarcomere protein gene mutations in hypertrophic cardiomyopathy of
the elderly. Circulation. 105:446–451. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watkins H, Conner D, Thierfelder L, et al:
Mutations in the cardiac myosin binding protein-C gene on
chromosome 11 cause familial hypertrophic cardiomyopathy. Nat
Genet. 11:434–437. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waldmüller S, Sakthivel S, Saadi AV, et
al: Novel deletions in MYH7 and MYBPC3 identified in Indian
families with familial hypertrophic cardiomyopathy. J Mol Cell
Cardiol. 35:623–636. 2003.PubMed/NCBI
|
|
5
|
Fananapazir L and Epstein ND: Prevalence
of hypertrophic cardiomyopathy and limitations of screening
methods. Circulation. 92:700–704. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maron BJ, Gardin JM, Flack JM, Gidding SS,
Kurosaki TT and Bild DE: Prevalence of hypertrophic cardiomyopathy
in a general population of young adults. Echocardiographic analysis
of 4111 subjects in the CARDIA Study Coronary Artery Risk
Development in (Young) Adults. Circulation. 92:785–789. 1995.
View Article : Google Scholar
|
|
7
|
Maron BJ, Doerer JJ, Haas TS, Tierney DM
and Mueller FO: Sudden deaths in young competitive athletes:
analysis of 1866 deaths in the United States, 1980–2006.
Circulation. 119:1085–1092. 2009.
|
|
8
|
Maron BJ, Spirito P, Green KJ, Wesley YE,
Bonow RO and Arce J: Noninvasive assessment of left ventricular
diastolic function by pulsed Doppler echocardiography in patients
with hypertrophic cardiomyopathy. J Am Coll Cardiol. 10:733–742.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YT, Chang KC, Hu WS, Wang SJ and
Chiang BN: Left ventricular diastolic function in hypertrophic
cardiomyopathy: assessment by radionuclide angiography. Int J
Cardiol. 15:185–193. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nihoyannopoulos P, Karatasakis G,
Frenneaux M, McKenna WJ and Oakley CM: Diastolic function in
hypertrophic cardiomyopathy: relation to exercise capacity. J Am
Coll Cardiol. 19:536–540. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsumura Y, Elliott PM, Virdee MS,
Sorajja P, Doi Y and McKenna WJ: Left ventricular diastolic
function assessed using Doppler tissue imaging in patients with
hypertrophic cardiomyopathy: relation to symptoms and exercise
capacity. Heart. 87:247–251. 2002. View Article : Google Scholar
|
|
12
|
Rust EM, Albayya FP and Metzger JM:
Identification of a contractile deficit in adult cardiac myocytes
expressing hypertrophic cardiomyopathy-associated mutant troponin T
proteins. J Clin Invest. 103:1459–1467. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bottinelli R, Coviello DA, Redwood CS, et
al: A mutant tropomyosin that causes Hypenrophic cardiomyopathy is
expressed in vivo and associated with an increased calcium
sensitivity. Circ Res. 82:106–115. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serri K, Reant P, Lafitte M, et al: Global
and regional myocardial function quantification by two-dimensional
strain: application in hypertrophic cardiomyopathy. J Am Coll
Cardiol. 47:1175–1181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abozguia K, Nallur-Shivu G, Phan TT, et
al: Left ventricular strain and untwist in hypertrophic
cardiomyopathy: relation to exercise capacity. Am Heart J.
159:825–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wigle ED, Rakowski H, Kimball BP and
Williams WG: Hypertrophic cardiomyopathy. Clinical spectrum and
treatment. Circulation. 92:1680–1692. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buss SJ, Mereles D, Emami M, et al: Rapid
assessment of longitudinal systolic left ventricular function using
speckle tracking of the mitral annulus. Clin Res Cardiol.
101:273–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gersh BJ, Maron BJ, Bonow RO, et al: 2011
ACCF/AHA guideline for the diagnosis and treatment of hypertrophic
cardiomyopathy: a report of the American College of Cardiology
Foundation/American Heart Association task force on practice
guidelines. Developed in collaboration with the American
Association for Thoracic Surgery, American Society of
Echocardiography, American Society of Nuclear Cardiology, Heart
Failure Society of America, Heart Rhythm Society, Society for
Cardiovascular Angiography and Interventions, and Society of
Thoracic Surgeons. J Am Coll Cardiol. 58:e212–e260. 2011.
|
|
19
|
Lang RM, Bierig M, Devereux RB, et al;
Chamber Quantification Writing Group; American Society of
Echocardiography’s Guidelines and Standards Committee; European
Association of Echocardiography. Recommendations for chamber
quantification: a report from the American Society of
Echocardiography’s Guidelines and Standards Committee and the
Chamber Quantification Writing Group, developed in conjunction with
the European Association of Echocardiography, a branch of the
European Society of Cardiology. J Am Soc Echocardiogr.
18:1440–1463. 2005.PubMed/NCBI
|
|
20
|
Maron BJ: Hypertrophic cardiomyopathy: a
systematic review. JAMA. 287:1308–1320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weidemann F, Mertens L, Gewillig M and
Sutherland GR: Quantitation of localized abnormal deformation in
asymmetric nonobstructive hypertrophic cardiomyopathy: a velocity,
strain rate, and strain Doppler myocardial imaging study. Pediatr
Cardiol. 22:534–537. 2001. View Article : Google Scholar
|
|
22
|
Yang H, Sun JP, Lever HM, et al: Use of
strain imaging in detecting segmental dysfunction in patients with
hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 16:233–239.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greenbaum RA, Ho SY, Gibson DG, Becker AE
and Anderson RH: Left ventricular fibre architecture in man. Br
Heart J. 45:248–263. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez F, Tibayan FA, Glasson JR, et
al: Fixed-apex mitral annular descent correlates better with left
ventricular systolic function than does free-apex left ventricular
long-axis shortening. J Am Soc Echocardiogr. 17:101–107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carlsson M, Ugander M, Mosén H, Buhre T
and Arheden H: Atrioventricular plane displacement is the major
contributor to left ventricular pumping in healthy adults,
athletes, and patients with dilated cardiomyopathy. Am J Physiol
Heart Circ Physiol. 292:H1452–H1459. 2007. View Article : Google Scholar
|
|
26
|
Nishikage T, Nakai H, Lang RM and Takeuchi
M: Subclinical left ventricular longitudinal systolic dysfunction
in hypertention with no evidence of heart failure. Circ J.
72:189–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Q, Fung JW, Yip GW, et al:
Improvement of left ventricular myocardial short-axis, but not
long-axis function or torsion after cardiac resynchronisation
therapy: an assessment by two-dimensional speckle tracking. Heart.
94:1464–1471. 2008. View Article : Google Scholar
|
|
28
|
Ballo P, Barone D, Bocelli A, Motto A and
Mondillo S: Left ventricular longitudinal systolic dysfunction is
an independent marker of cardiovascular risk in patients with
hypertension. Am J Hypertens. 21:1047–1054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sveälv BG, Olofsson EL and Andersson B:
Ventricular long-axis function is of major importance for long-term
survival in patients with heart failure. Heart. 94:284–289.
2008.PubMed/NCBI
|
|
30
|
Amundsen BH, Helle-Valle T, Edvardsen T,
et al: Noninvasive myocardial strain measurement by speckle
tracking echocardiography: validation against sonomicrometry and
tagged magnetic resonance imaging. J Am Coll Cardiol. 47:789–793.
2006. View Article : Google Scholar
|
|
31
|
Korinek J, Wang J, Sengupta PP, et al:
Two-dimensional strain - a Doppler-independent ultrasound method
for quantitation of regional deformation: validation in vitro and
in vivo. J Am Soc Echocardiogr. 18:1247–1253. 2005. View Article : Google Scholar : PubMed/NCBI
|