Introduction
Acute respiratory distress syndrome (ARDS), a
clinically important complication of severe acute lung injuries
(ALIs) in humans, is a significant cause of morbidity and mortality
in critically ill patients (1).
Infectious etiologies, including sepsis and pneumonia, are the most
common causes of ALI (1,2). Histologically, ALI in humans is
characterised by a severe acute inflammatory response in the lungs
and neutrophilic alveolitis (1,3). The
physiological hallmark of ARDS is the disruption of the
alveolar-capillary membrane barrier, leading to the development of
noncardiogenic pulmonary oedema, in which a proteinaceous exudate
floods the alveolar spaces, impairs gas exchange and precipitates
respiratory failure (1,4,5).
Despite numerous studies investigating the early diagnostic and
pathogenetic factors of ALI, the current management of ALI is
predominantly supportive since specific therapies have not yet been
identified (6,7). Thus, novel strategies are required
for achieving effective treatment of ALI in clinical practice.
Rhodiola rosea (R. rosea) has been
widely used in traditional Chinese medicine for centuries (8). A previous study confirmed that the
plant exhibits various pharmacological effects, including improving
exercise endurance and fatigue, preventing high altitude sickness,
promoting blood circulation, eliminating toxins from the body and
treating various endemic diseases (9). R. rosea also has been
demonstrated to have antimicrobial and anticancer effects (9). Salidroside
(p-hydroxyphenethyl-β-D-glucoside;
C14H20O7) is a phenylpropanoid
glycoside that can be extracted from R. rosea and is
regarded as the most important bioactive component in the species
(10). Salidroside has been
reported to have various pharmacological effects, including
anti-aging, anticancer, hepatoprotective, antiviral and
antioxidative. In addition, salidroside has been shown to suppress
the release of prostaglandin E2 in vitro (11,12).
A previous study identified that R. rosea suppresses the
inflammatory response (13);
however, the specific mechanism remains unknown.
Peroxisome proliferator-activated receptors (PPARs)
are transcription factors belonging to the nuclear receptor
superfamily. The three known PPAR subtypes, α, γ and δ, have
various tissue distributions and are associated with selective
ligands. PPAR-α is predominantly expressed in tissues that
demonstrate high catabolism for fatty acids, including the liver,
heart, kidney and muscle tissues. PPAR-γ is expressed to the
greatest extent in white adipose tissue, where it plays a major
regulatory role in adipocyte differentiation and lipid metabolism.
PPAR-δ is ubiquitously expressed, thus, to date, no specific role
has been demonstrated for this isoform. Contradictory results have
been generated concerning the role of PPAR-γ in inflammatory models
in vivo. The activation of PPAR-γ has been shown to inhibit
inflammation in a model of inflammatory bowel disease (14) and a mouse model of atherosclerosis
(15).
Therefore, in the present study, the potential role
of salidroside for the treatment of ALI was evaluated using a rat
model of CLP-induced ALI.
Materials and methods
Rats
Adult male Sprague-Dawley rats, weighing 250±25 g
(supplied by The Field Surgery Institute, Kunming Medical
University, Kunming, China), were housed in 12-h light-dark
conditions with free access to water and standard laboratory chow.
The animal procedures were performed in strict accordance with
National Institutes of Health guidelines, which were approved by
the Ethics Committee of Kunming Medical University.
Reagents
A TRIzol kit was obtained from Gibco-BRL (Carlsbad,
CA, USA) and a reverse transcription kit was purchased from Takara
Biotechnology Co. Ltd. (Dalian, China). A polymerase chain reaction
(PCR) amplification reagent kit and the DNA ladder marker were
obtained from Sangon Biological Engineering Co. Ltd. (Shanghai,
China). β-actin was obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA) and tumour necrosis factor-α (TNF-α),
interleukin (IL)-6, -1β and -10 enzyme-linked immunosorbent assay
(ELISA) kits were obtained from Pierce Biotechnology Inc.
(Rockford, IL, USA). Salidroside (99% purity) was acquired from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China).
Model and groups
Using a random number table, 80 rats were randomly
divided into four groups: Normal control, sham-operation (sham),
sepsis model (model) and salidroside treatment (treatment) groups
(n=20 per group). The sepsis model was induced by cecal ligation
and puncture (CLP). Briefly, the animals were deprived of food, but
water was permitted for 6 h prior to surgery. Under light ether
anaesthesia, a laparotomy was performed through a midline abdominal
incision. The cecum was punctured twice at different sites with an
18-gauge needle and gently compressed until faeces were extruded.
The bowel was then returned to the abdomen and the abdominal
incision was closed in two layers. Animals in the model and
treatment groups were treated with 5 ml normal saline per 100 g
body weight subcutaneously at the completion of surgery to replace
the extracellular fluid sequestered during peritonitis (16). Animals in the sham group underwent
sham surgery, in which the cecum was neither ligated nor punctured.
Starting at 8 h prior to surgery, animals in the treatment group
were injected with 800 mg/kg salidroside every 8 h, while the
animals in the normal control, sham and model groups were
administered the same volume of normal saline. Subsequently, the
animals in each group were anaesthetised with ether 24 h
post-surgery, and the right internal carotid artery was isolated.
Blood was extracted (5 ml), centrifuged to collect the supernatant,
dispensed into two sterile tubes, sealed with sealing glue and
placed in freezer at −20°C for examination. All the animals were
sacrificed 24 h following surgery via anesthesia and tissue samples
were collected for further tests.
Extraction of RNA
For the isolation of lung tissue RNA, the rats were
humanely sacrificed and under aseptic conditions, the lung tissue
was removed and immediately frozen in liquid nitrogen. Prior to RNA
extraction, the lung samples were homogenised in TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) using a Mixer Mill 301. The total
RNA was extracted according to the manufacturer’s instructions. The
RNA samples were electrophoresed in agarose gel and visualised with
ethidium bromide for quality control.
cDNA synthesis and quantitative PCR
(qPCR)
RNA (3 μg) underwent reverse transcription with
reverse transcriptase for 1 h at 37°C to synthesise cDNA.
Quantitative changes in the mRNA expression levels were assessed
with qPCR (CFX; Bio-Rad, Hercules, CA, USA) using SYBR Green
detection, consisting of the SYBR Green PCR Master mix (Aria-tous,
Iran). The PCR master mix consisted of 0.5 units Taq
polymerase, 2-μl samples of each primer and 3-μl samples of each
cDNA sample in a final volume of 20 μl. The amplifications were
repeated three times. The oligonucleotide primer sequences are
presented in Table I. β-actin was
used as an endogenous control and each sample was normalised on the
basis of its β-actin content. The relative quantification of the
mRNA expression levels of the target genes was calculated using the
2−ΔΔCt method (17).
 | Table IPrimer sequences of the genes used to
validate the microarray analysis by qPCR. |
Table I
Primer sequences of the genes used to
validate the microarray analysis by qPCR.
| Gene | Primer
sequence | Length (bp) |
|---|
| PPAR-γ | F:
5′-ACAAGGACTACCCTTTACTGAAATTACC-3′
R: 5′-GTCTTCATAGTGTGGAGCAGAAATGCTG-3′ | 178 |
| NF-κβ | F:
5′-GCACGGATGACAGAGGCGTGTATAAGG-3′
R: 5′-GGCGGATGATCTCCTTCTCTCTGTCTG-3′ | 420 |
| Iκβ | F:
5′-TGCTGAGGCACTTCTGAG-3′
R: 5′-CTGTATCCGGGTGCTTGG-3′ | 421 |
| β-actin | F:
5′-GATTACTGCTCTGGCTCCTGC-3′
R: 5′-GACTCATCGTACTCCTGCTTGC-3′ | 190 |
Western blot analysis
Homogenised lung tissue was preserved in a
protease-inhibitor solution (Complete Mini; Roche). Western blot
analyses were performed for PPAR-γ, inhibitor-κβ (Iκβ) and NF-κβ
p65 using 20 μg protein. The PPAR-γ specimens were separated by
electrophoresis in a 10% Bis-Tris gel, while the NF-κβ p65 and Iκβ
specimens were separated by electrophoresis in a 3–8% Tris-acetate
gel. Following electrophoresis, the proteins were transferred to a
nitrocellulose membrane. Primary antibodies against β-actin,
PPAR-γ, phospho-PPAR-γ (Cell Signaling Technology, Inc., Danvers,
MA, USA), NF-κβ p65 and Iκβ (R&D Systems, Minneapolis, MN, USA)
were applied overnight at 4°C in Tris-buffered saline, which was
followed by the addition of anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (Millipore, Billerica,
MA, USA) for 1 h at room temperature (RT). Chemiluminescence
detection was performed with the Western Lightning detection
reagent (Perkin-Elmer, Waltham, MA, USA).
Immunohistochemistry
Immunostaining was performed on the lung sections
following antigen retrieval using Retrievagen A (Zymed, South San
Francisco, CA, USA) at 100°C for 20 min and the quenching of
endogenous peroxidases with 3% H2O2. The
sections were blocked with 2% bovine serum albumin in
phosphate-buffered saline, which was followed by staining with
primary anti-PPAR-γ, anti-NF-κβ p65 and Iκβ (BD Pharmingen, San
Jose, CA, USA) antibodies at RT for 1 h. The sections were washed
and secondary antibodies (R&D Systems) were applied, after
which the tissues were developed using Vectastain ABC (Vector
Laboratories, Burlingame, CA, USA) and 3,3′-diaminobenzidine
(Vector Laboratories). Using Image-Pro Plus image analysis software
(Media Cybernetics, Inc., Rockville, BD, USA), the PPAR-γ, Iκβ and
NF-κβ p65 positive expression levels in the lung tissue were
calculated and expressed in positive units.
Cytokine content measurement in the
serum
Serum levels of TNF-α, IL-1β, IL-6 and IL-10 in the
rats were measured using ELISA kits, according to the
manufacturer’s instructions (R&D Systems).
Determination of the total number of
cells and neutrophils
According to a previously described procedure
(18), the analysis of
bronchoalveolar lavage fluid (BALF) was performed by instilling
0.9% NaCl containing 0.6 mmol/l ethylenediaminetetraacetic acid in
two separate 0.5 ml aliquots. The fluid was recovered by gentle
suction and placed on ice for immediate processing. An aliquot of
the BALF was processed immediately for total and differential cell
counts. The remainder of the BALF was centrifuged and the
supernatant was removed aseptically and stored in individual
aliquots at −70°C. The total cell counts in the BALF were
determined using a haemocytometer and the number of neutrophils was
calculated as the percentage of neutrophils multiplied by the total
number of cells in the BALF sample. All analyses were performed in
a blind manner.
Elastase activity
Elastase activity was measured using an EnzChek
Elastase Assay kit (E-12056; Molecular Probes Europe, Leiden, The
Netherlands). Absorbance was measured at 515 nm with a microplate
reader (Infinite 200; Tecan, Männedorf, Switzerland).
Albumin concentration in the BALF
Albumin content in the BALF supernatants was
assessed using an ELISA kit for albumin (E91028Mu; Uscn Life
Science, Inc., Wuhan, China). Absorbance was measured at 450/540 nm
using a microplate reader.
Measurement of the pulmonary oedema
The right lungs of the rats were removed and the wet
weights were obtained. The lungs were weighed again following
drying for three days at 55°C. The wet to dry (W/D) ratio was
calculated as follows: W/D ratio = (wet weight - dry weight)/dry
weight (19). The lung water
content was calculated as the wet weight minus the dry weight and
the wet weight ratio of the lung tissue multiplied by 100.
Histopathological examination
Lung tissue was fixed in 10% formalin for 24 h,
which was followed by dehydration. The lung tissue was embedded in
paraffin wax, sectioned into 5-μm-thick slices and stained with
haematoxylin and eosin. Microphotography of the lung sections was
captured with a light microscope (Olympus, Tokyo, Japan). The
severity of the ALI was scored in a blind manner, as previously
described (6), according to the
categorical degree scoring (zero, minimal or no damage; four,
severe damage) of alveolar congestion, haemorrhaging, cell
infiltration into the airspace or vessel wall and thickness of the
alveolar wall. The mean score of five random areas per section per
animal was used for data analysis.
Statistical analysis
Quantitative data are presented as the mean ±
standard error of the mean (SEM) of at least three independent
experiments. The histological injury scoring data were analysed by
analysis of variance (ANOVA) followed by the Kruskal-Wallis
nonparametric test for comparison, which was then presented as a
box-and-whisker plot. The remaining data were analysed by ANOVA and
then with the Newman-Keuls test for comparison. For comparisons
among the groups, the unpaired Student’s t-test was used (GraphPad
Prism, GraphPad Software Inc., San Diego, CA, USA), in which
P<0.05 was considered to indicate a statistically significant
difference.
Results
Salidroside upregulates the expression
levels of PPAR-γ and Iκβ and blocks NF-κβ p65 expression in lung
tissue
To assess the potential role of salidroside in
CLP-induced ALI, the mRNA and protein expression levels of PPAR-γ,
NF-κβ p65 and Iκβ in the lung tissue of the lipopolysaccharide
(LPS)-induced ALI rats were determined using qPCR and western blot
analysis at 24 h after the CLP challenge. As shown in Figs. 1–4, the expression levels of PPAR-γ and Iκβ
were markedly reduced and the NF-κβ p65 expression levels were
markedly enhanced in the model group compared with those in the
normal control and sham groups. However, following the
administration of salidroside, the expression levels of PPAR-γ and
Iκβ were markedly upregulated and NF-κβ p65 expression levels were
significantly decreased. In combination, these observations
indicated that salidroside may be involved in the increased
expression levels of PPAR-γ and Iκβ and the reduced NF-κβ p65
expression levels in CLP-induced ALI. A negative correlation was
shown to exist between PPAR-γ and NF-κβ p65 mRNA expression levels,
as well as between PPAR-γ and NF-κβ p65 protein (r=−0.452,
P<0.05; r=0.613, P<0.05).
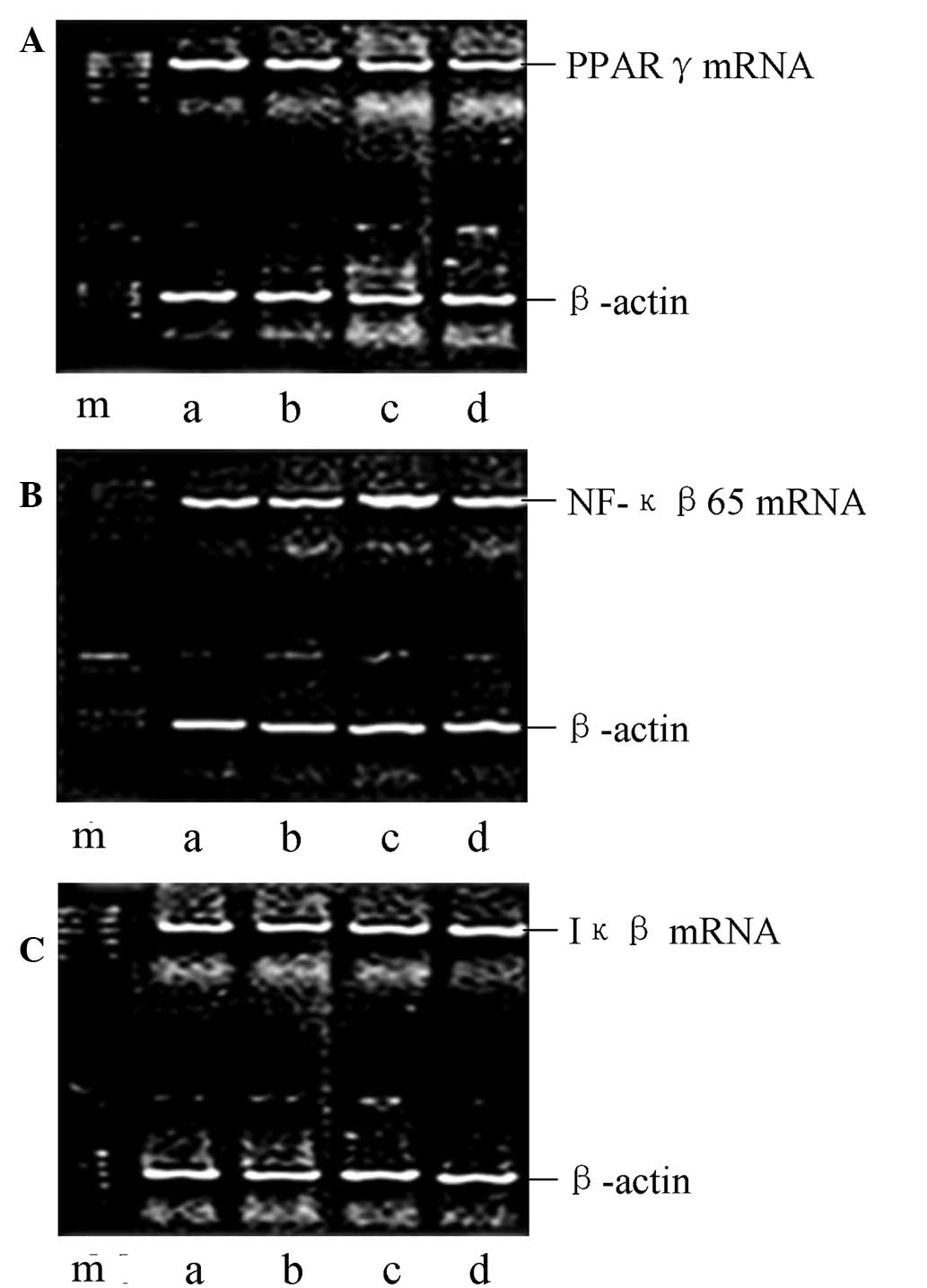 | Figure 1Effect of salidroside on the mRNA
expression levels of PPAR-γ, Iκβ and NF-κβ p65 in the lung tissue
of CLP-ALI rats, as determined by qPCR. Representative gels show
the mRNA expression levels of (A) PPAR-γ, (B) NF-κβ p65 and (C) Iκβ
in the four groups of rats: m, marker; a, normal control; b, sham
surgery; c, model; and d, treatment groups. PPAR-γ, peroxisome
proliferator-activated receptor γ; NF-κβ, nuclear factor-κβ; CLP,
cecal ligation and puncture; ALI, acute lung injury; qPCR,
quantitative polymerase chain reaction; Iκβ, inhibitor-Iκβ. |
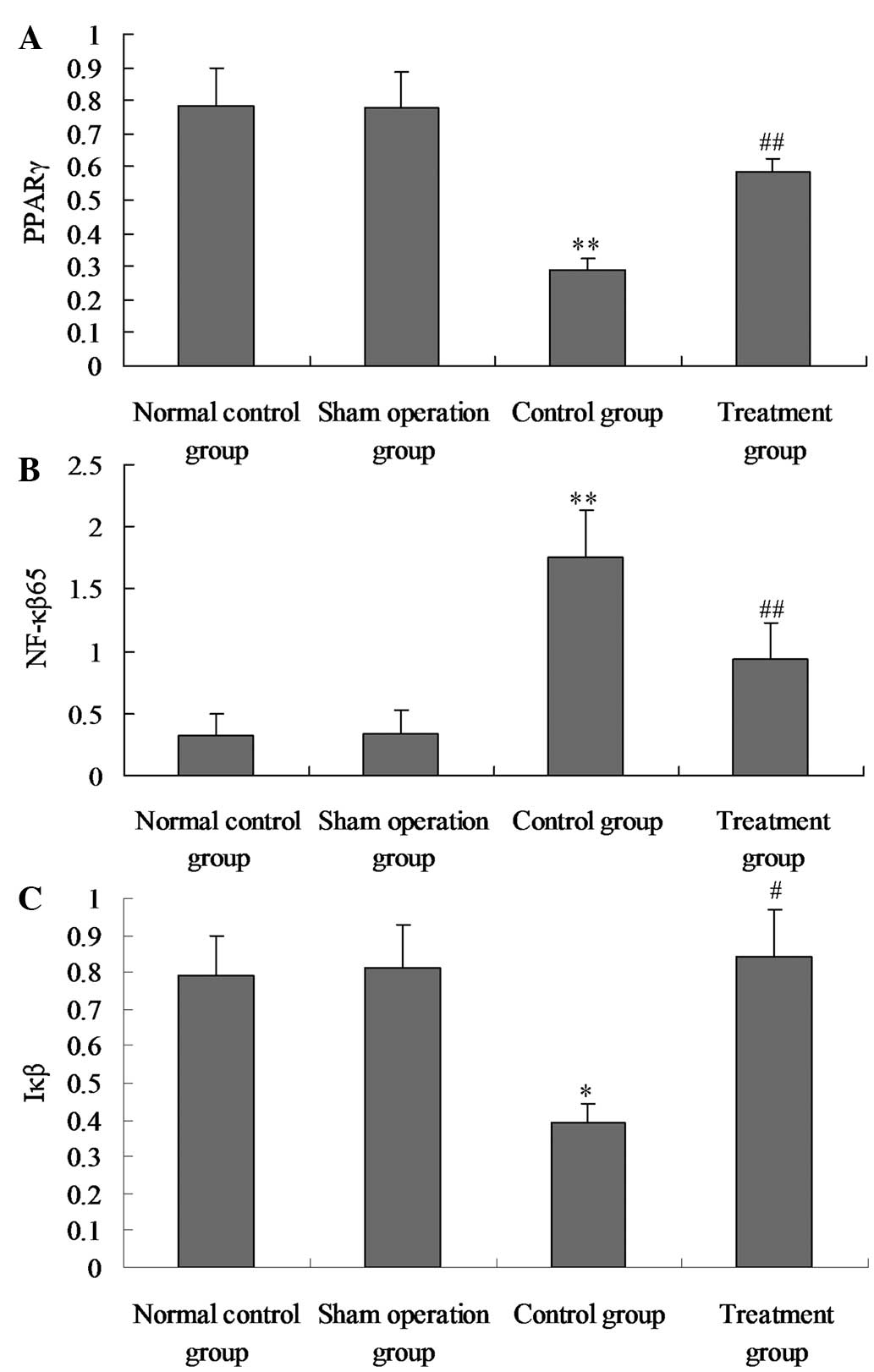
Salidroside increases PPAR-γ and Iκβ
activation in lung tissue, and inhibits NF-κβ p65 activation in
lung tissue
To study the effect of salidroside on the positive
expression of PPAR-γ, NF-κβ p65 and Iκβ in CLP-induced ALI,
immunohistochemical staining was performed on the lung sections. As
shown in Figs. 5 and 6, in the model group, the activation of
PPAR-γ and Iκβ was significantly suppressed and the activation of
NF-κβ p65 was significantly increased at 24 h after the CLP
challenge, as compared with the sham group. However, in the
CLP-induced rats that received salidroside, the positive expression
levels of PPAR-γ and Iκβ were significantly increased and the NF-κβ
p65 positive expression levels were markedly inhibited compared
with the model group. Therefore, salidroside may suppress the
positive expression of NF-κβ p65 and promote the positive
expression of PPAR-γ and Iκβ.
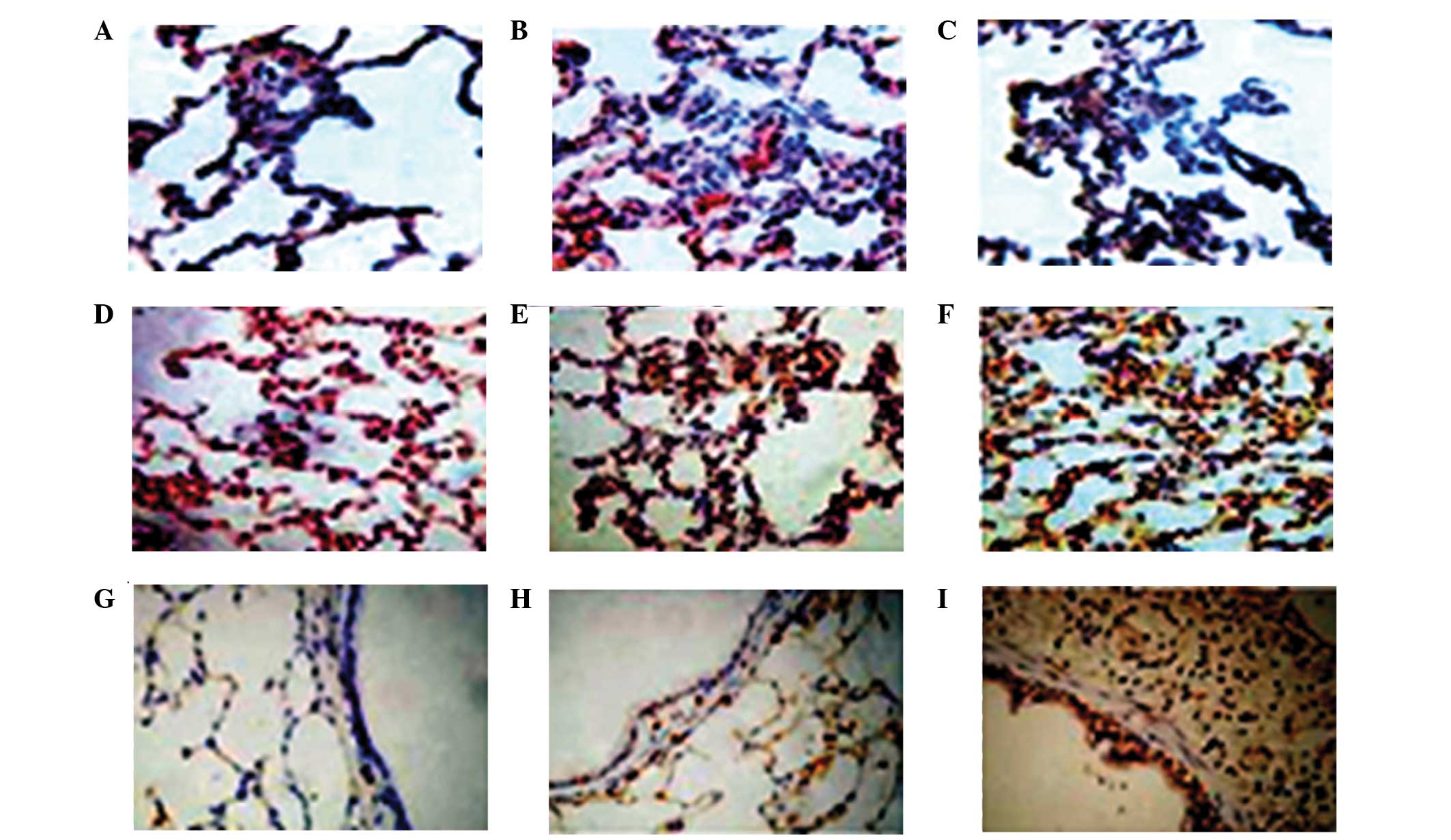 | Figure 5Effect of the administration of
salidroside on the PPAR-γ, NF-κβ p65 and Iκβ positive expression
levels in the lung tissue of CLP-ALI rats. The groups of rats were
challenged with CLP and treated with salidroside 24 h later.
Immunostaining was performed on the lung sections following antigen
retrieval using Retrievagen A. Representative immunostaining images
show the positive expression levels of PPAR-γ, NF-κβ and Iκβ in
three groups of rats (immunofluorescence staining; magnification,
×200). Positive expression levels of (A–C) NF-κβ p65 (A, sham
surgery group; B, model group; C, treatment group); (D–F) PPAR-γ
(D, sham surgery group; E, model group; F, treatment group); (G–I)
Iκβ (G, sham surgery group; H, model group; I, treatment group).
PPAR-γ, peroxisome proliferator-activated receptor γ; NF-κβ,
nuclear factor-κβ; CLP, cecal ligation and puncture; ALI, acute
lung injury; Iκβ, inhibitor-Iκβ. |
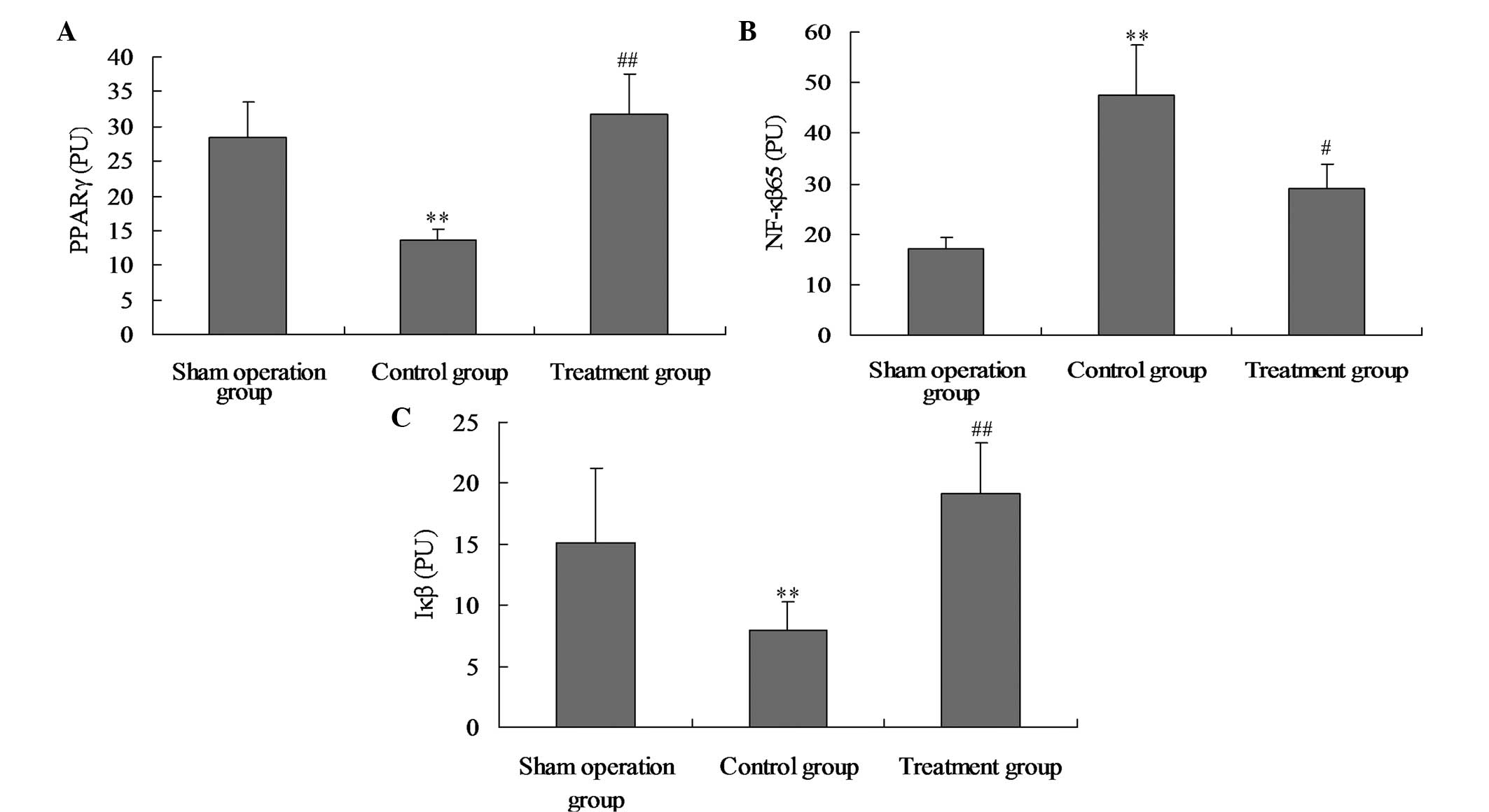 | Figure 6Effect of salidroside on (A) PPAR-γ,
(B) NF-κβ p65 and (C) Iκβ positive expression levels in the lung
tissue of CLP-ALI rats. The groups of rats were challenged with CLP
and treated with salidroside 24 h later. Immunostaining was
performed on the lung sections following antigen retrieval using
Retrievagen A. Using Image-Pro Plus image analysis software, the
PPAR-γ, Iκβ and NF-κβ p65 positive expression levels in lung tissue
were calculated. Data are presented as the mean ± standard
deviation of one experiment consisting of three replicates. The
experiments were performed in triplicate. *P<0.05 and
**P<0.01, vs. normal control and sham surgery groups.
#P<0.05 and ##P<0.01, vs. control
group. PPAR-γ, peroxisome proliferator-activated receptor γ; NF-κβ,
nuclear factor-κβ; CLP, cecal ligation and puncture; ALI, acute
lung injury; Iκβ, inhibitor-Iκβ. |
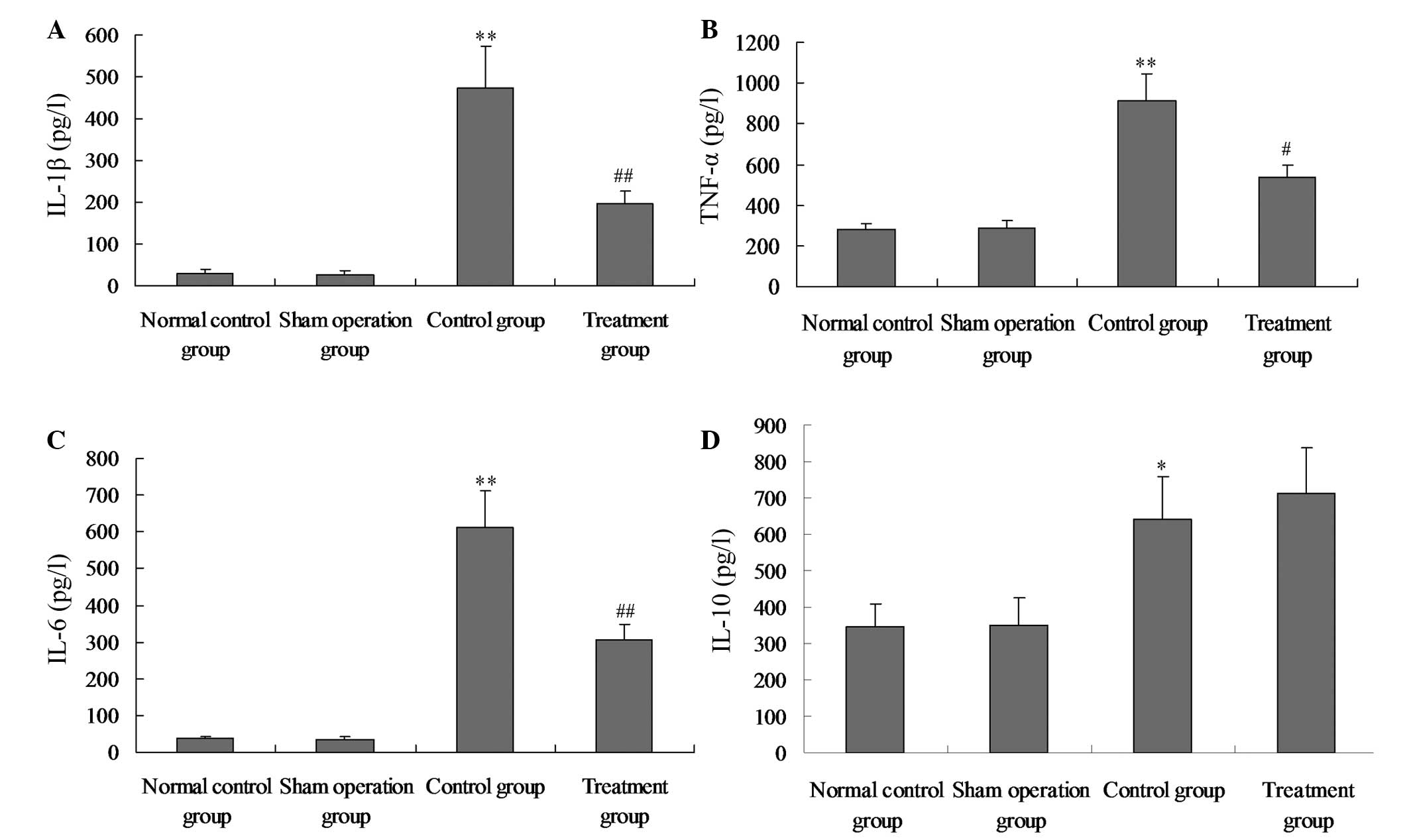
Salidroside reduces the production of
proinflammatory cytokines
To further assess the anti-inflammatory effect of
salidroside, the levels of proinflammatory and anti-inflammatory
cytokines in the plasma were detected. The level of proinflammatory
cytokines, including TNF-α, IL-1β and IL-6, were all significantly
elevated in the plasma in response to the LPS challenge (Fig. 7A–C; P<0.05). By contrast, the
administration of salidroside effectively reduced the levels of
proinflammatory cytokines (P<0.05) as shown in Fig. 7A–C. Consistent with these
observations, the administration of salidroside after a 24 h
interval further increased the level of anti-inflammatory cytokines
to relatively high levels in the plasma (Fig. 7D).
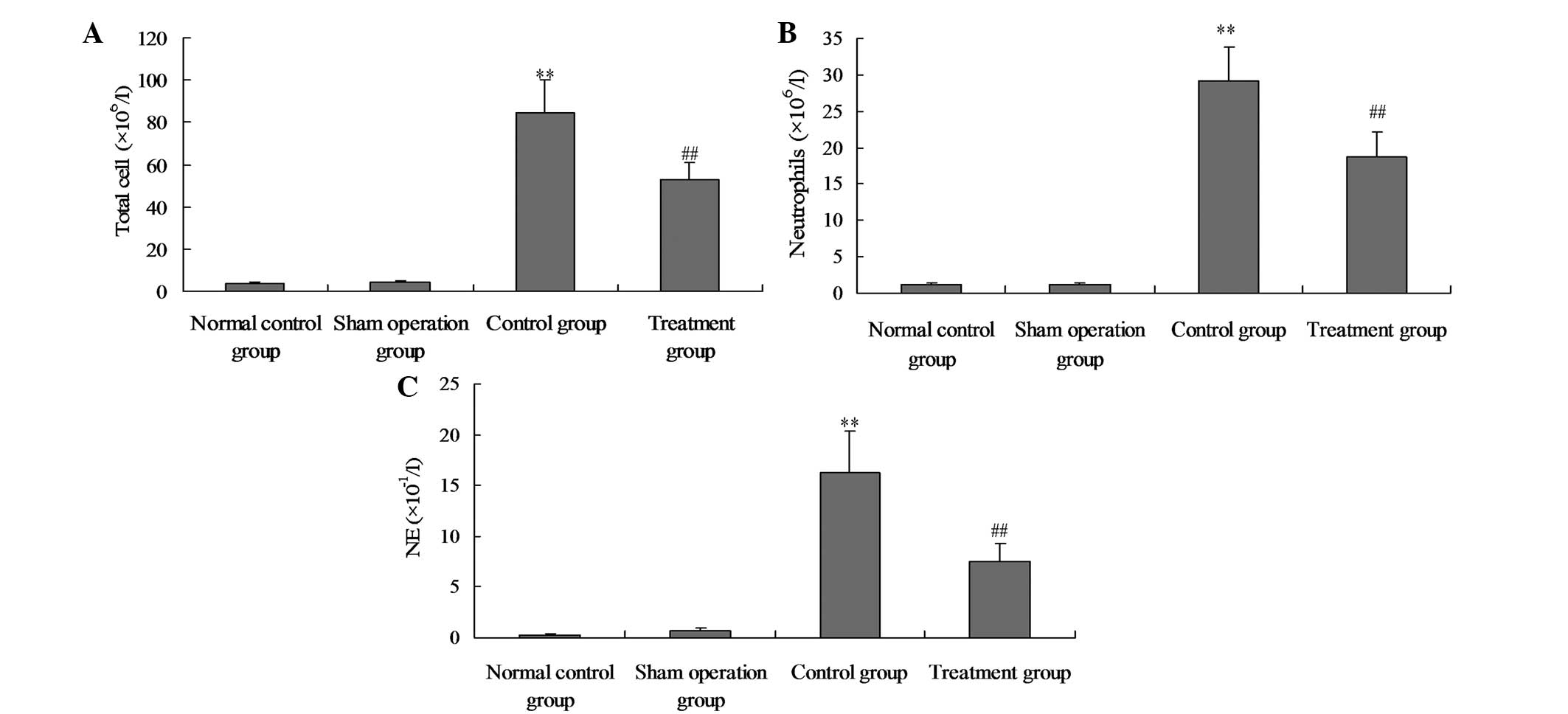
Salidroside attenuates the acute
CLP-induced pulmonary inflammation
To investigate the possible mechanism underlying the
protective effect of salidroside on CLP-induced ALI, the total cell
counts, neutrophil counts and neutrophil elastase levels were
analysed in the BALF of the rats treated with CLP with or without
salidroside treatment. As shown in Fig. 8, the total numbers of inflammatory
cells, neutrophils and NE in the BALF were markedly increased
following the administration of CLP. Following the administration
of salidroside, the total numbers of inflammatory cells,
neutrophils and NE in the BALF were significantly reduced.
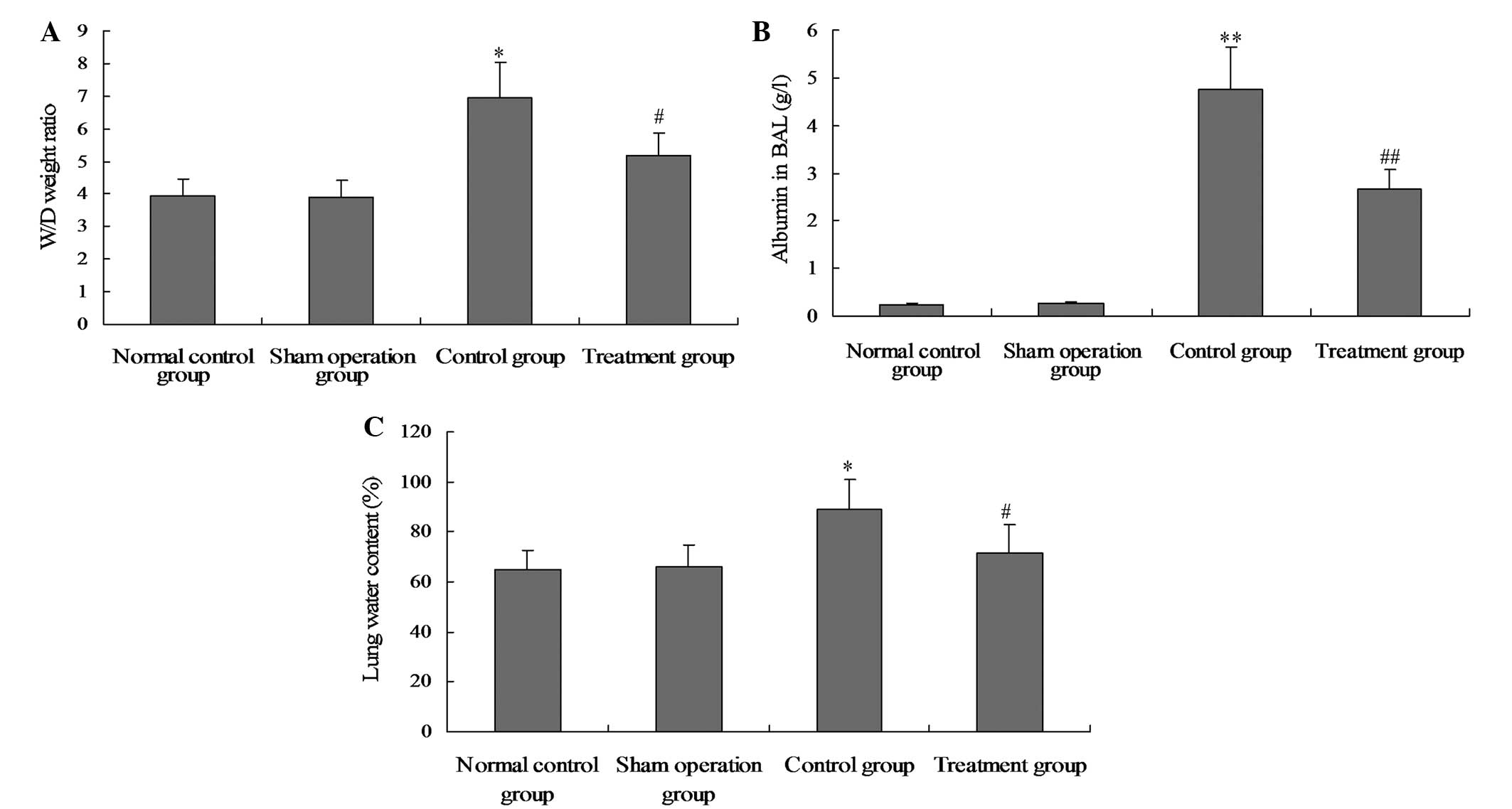
Salidroside reduces CLP-induced lung
permeability
Total protein concentration in the BALF, the W/D
lung weight ratio and the water content in the lung tissue were
determined to evaluate the integrity of the alveolar-capillary
membrane barrier and assess the pulmonary vascular leakage as a
marker of ALI. As shown in Fig. 9,
BALF albumin concentration, W/D lung weight ratio and the water
content in the lung tissue of the model group were markedly
increased compared with the normal control and sham groups.
However, following salidroside treatment, the albumin concentration
in the BALF, the W/D lung weight ratio and the water content
decreased significantly.
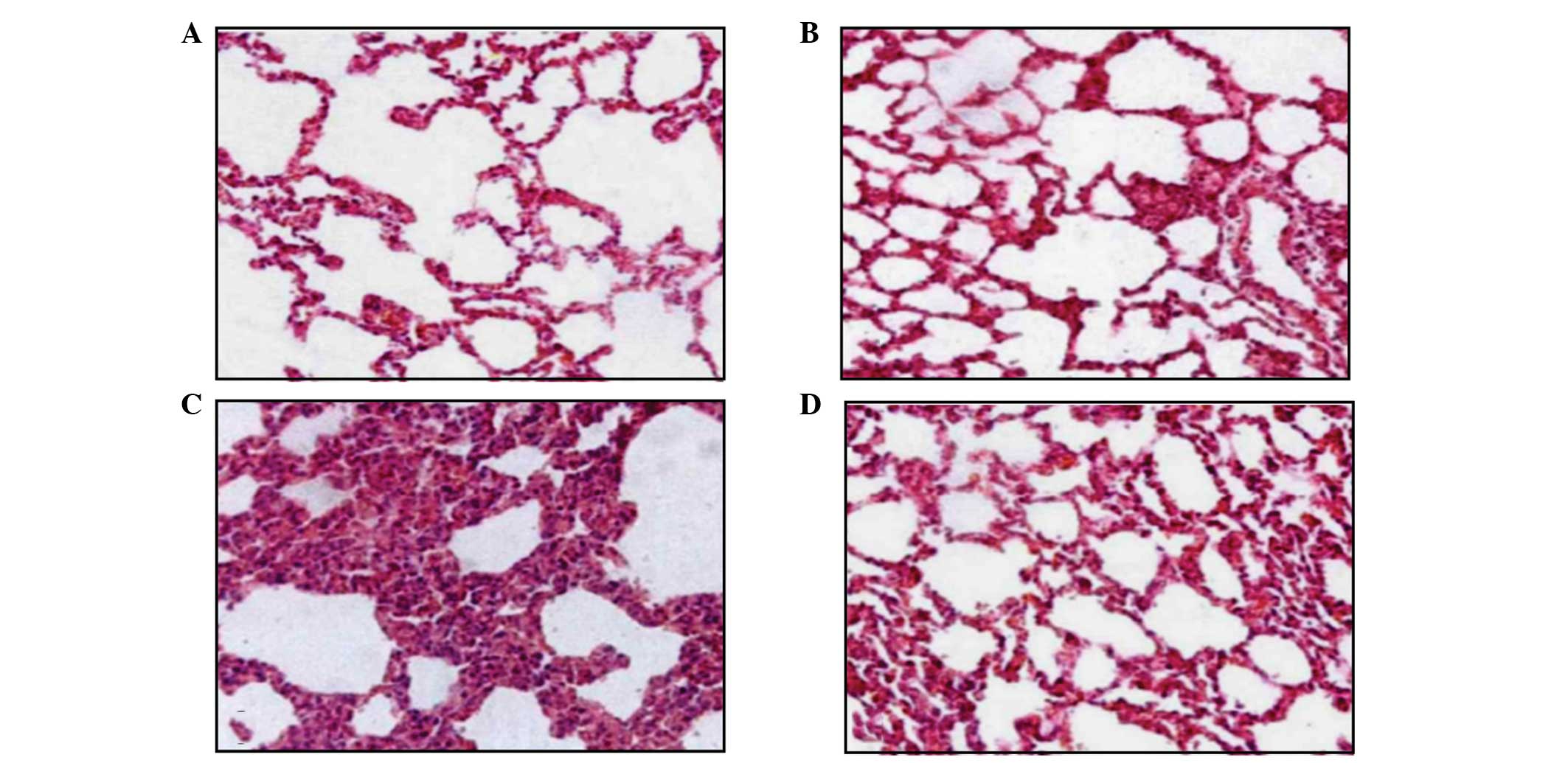
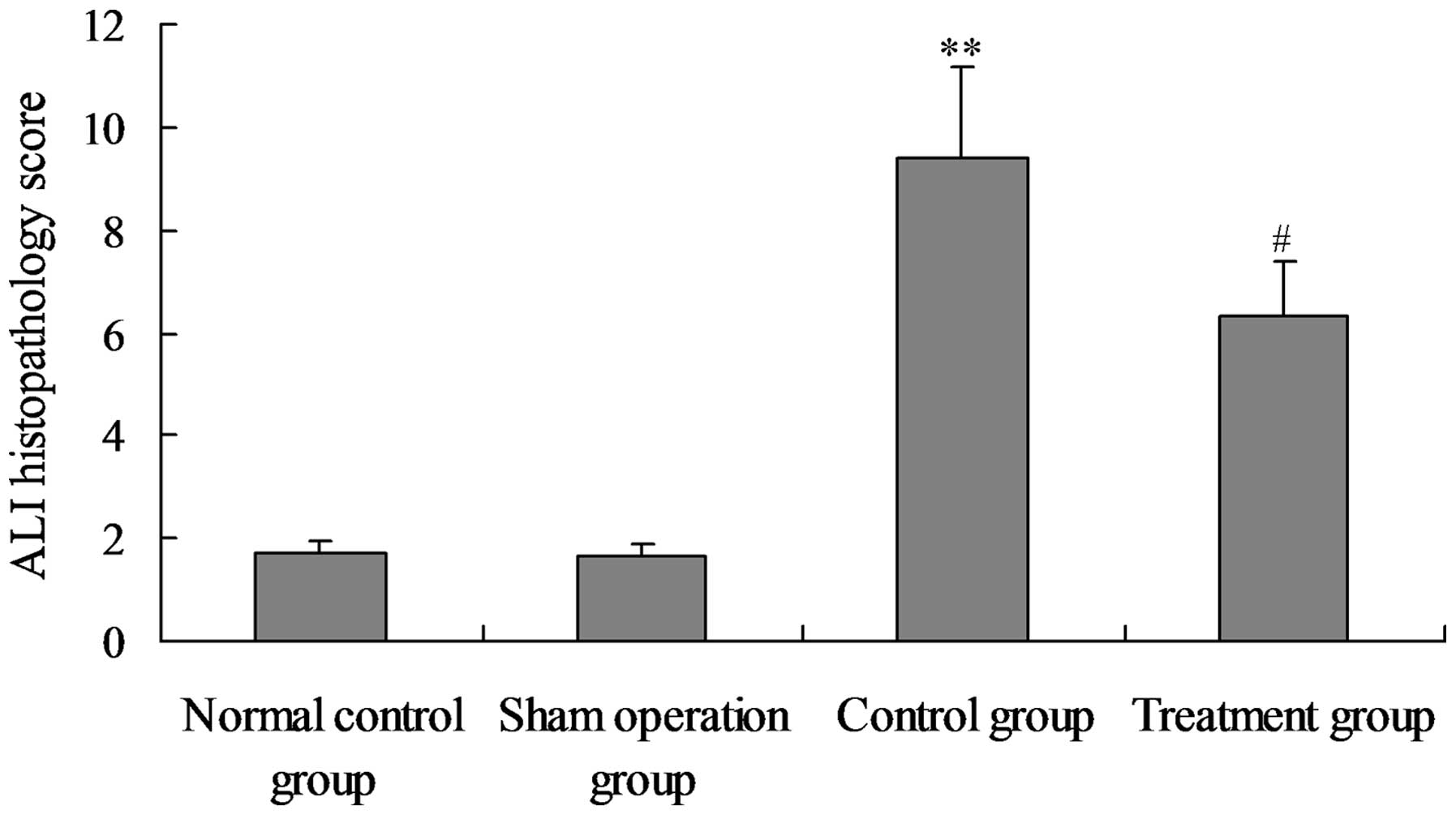
Salidroside ameliorates the
histopathological changes in the lungs of CLP-ALI rats
To determine the effect of salidroside on
histological lung injury, histopathological analysis was performed
on the lung sections stained with haematoxylin and eosin.
Histological analyses of the lungs following CLP exposure revealed
a damaged alveolar structure with evident concretions and liquid
draining within the bleeding inflammatory cells. In addition, the
perivascular gap was widened and there were numerous alveolar stoma
that were infiltrated by mononuclear inflammatory cells, including
macrophages, plasma cells and neutrophils. The ALI pathology score
also increased significantly compared with that in the normal
control and sham groups. The lungs of the rats in the treatment
group exhibited less severe damage without significant bleeding and
the ALI pathology score was significantly reduced compared the
model group (Figs. 10 and
11).
Discussion
CLP-induced sepsis with acute suppurative
peritonitis has been demonstrated to be a typical sepsis model with
Gram-negative bacteria being the predominant source of infection
(20). Gram-negative bacteria
release numerous endotoxins and severe endotoxemia may activate the
inflammatory cells and cause inflammatory reactions that lead to
tissue and organ injury, dysfunction, and possibly mortality. Lung
tissue is one of the most vulnerable tissues to endotoxemia. LPS
causes ALI, which further develops into ARDS (21,22).
In the present study, an endotoxemia rat model was created via the
use of CLP to simulate sepsis-related lung injury in order to
observe the effect of salidroside on ALI. The experimental results
revealed that the rats exhibited varying degrees of lung tissue
hyperaemia, haemorrhage, alveolar septal thickening, infiltration
of the inflammatory cells and neutrophil accumulation, which are
all pathological changes associated with ALI. These observations
indicated that the model was successful.
ALI is an uncontrollable pulmonary inflammation
caused by large amounts of inflammatory cells and cytokines. Under
the effects of CLP, lung macrophages and neutrophils produce
proinflammatory cytokines, including TNF-α and IL-1β, triggering
the inflammatory reaction cascade (23). In the present study, the plasma
levels of TNF-α, IL-1β, and IL-6 significantly increased 24 h after
CLP surgery. When 800 mg/kg salidroside (i.v.) was administered 24
h after the CLP-induced injury, the plasma levels of the
proinflammatory cytokines and lung inflammation decreased
significantly. In vitro experiments demonstrated that 800
mg/kg salidroside (i.v.) reduced the levels of TNF-α, IL-1β and
IL-6 secretion by lung macrophages. IL-10 is one of the most
important anti-inflammatory cytokines and salidroside
administration markedly increased the IL-10 concentration in the
CLP-induced ALI rats. The administration of salidroside clearly
inhibited the production of the proinflammatory cytokines, TNF-α,
IL-1β and IL-6, and increased the IL-10 levels. Thus, salidroside
improves the homeostasis of the cytokine network and the balance
between the inflammatory and anti-inflammatory reactions associated
with ALI.
PPARs are members of the nuclear receptor
superfamily with three isomers existing in mammals: α, β and γ
(24). Steroid, thyroid and
retinoid hormones are ligands for the receptors. PPAR-γ is highly
expressed in adipose tissue and its activation plays a key role in
increasing systemic insulin sensitivity. PPAR-γ agonists are
clinically used in the treatment of type 2 diabetes mellitus and
metabolic syndrome. PPAR-γ has been shown to be constitutively
expressed in numerous types of tissue, including lung tissue, where
it has been hypothesised to play a protective role (25). In addition, PPAR-γ expression in
macrophages and lymphocytes suppresses inflammatory responses, and
PPAR-γ agonists inhibit the production of proinflammatory cytokines
and regulate the process of inflammation by activating this nuclear
receptor (20). PPAR-γ is a
ligand-activated transcription factor, whose activation plays a
role in controlling the inflammatory response. Several studies have
demonstrated that the activation of PPAR-γ by specific ligands
significantly improves survival rates in clinically relevant models
of septic shock (26). The
beneficial effect of PPAR-γ activation is likely to be secondary to
the inhibition of the production of several inflammatory mediators,
as has been shown in vivo in septic rodents (26) and in vitro in activated
macrophages and monocytes (27).
Sepsis and other inflammatory states affect the PPAR-γ expression
levels and correlate with the inflammatory response. The expression
levels of PPAR-γ are downregulated in the lung and vascular
endothelium in rodent models of septic shock, and treatment with
PPAR-γ ligands reverses the sepsis-induced reduction (26). In adipose tissue, the expression
levels of PPAR-γ decreased after the rats were challenged in
vivo with endotoxins and the cytokine-induced suppression of
PPAR-γ was reversed with synthetic agonists (28). However, the mechanisms that lead to
a reduction in the levels of PPAR-γ activity in the presence of
sepsis remain unclear. In the present study, CLP blocked PPAR-γ
expression in the lung tissue, increasing the levels of
proinflammatory cytokines; however, the administration of
salidroside enhanced the PPAR-γ expression levels in the lung
tissue and inhibited the inflammatory response.
To further characterise the inhibitory effect of
salidroside on cytokine production, the present study examined the
effects of salidroside on the activation of the transcription
factor NF-κβ, which regulates the expression of numerous immune and
inflammatory genes and the production of cytokines. NF-κβ is
essential for host defence and the inflammatory responses to
microbial and viral infections (29), as it is an important transcription
factor required for the expression of a number of proinflammatory
cytokines (30). In the majority
of cells, NF-κβ exists in an inactive form in the cytoplasm as it
is bound to inhibitory Iκβ proteins. Following CLP challenge, NF-κβ
is translocated to the nucleus to drive the expression of a variety
of inflammatory genes that are involved in the pathogenesis of ALI.
Therefore, a blockage of NF-κβ activation and an increase in the
Iκβ expression levels is expected to attenuate ALI (30). This is supported by the results of
the present study, which demonstrated that salidroside treatment
following the CLP challenge inhibited NF-κβ activation, and the
release of inflammatory cytokines promoted the expression of
PPAR-γ. This is consistent with the theory that salidroside
prevents the release of LPS-induced inflammatory cytokines via its
anti-NF-κβ activity, which upregulates PPAR-γ expression
levels.
CLP stimulates macrophages, neutrophils and other
types of immune cell to produce different mediators, including
cytokines such as TNF-α and IL-6, that recruit polymorphonuclear
neutrophils to the injured site and contribute to the pathogenesis
of ALI and ARDS (31). Activated
neutrophils that release various types of mediators and secrete the
elastase enzyme, whose activity is an indicator of neutrophil
accumulation in tissues (32), are
recognised to be a primary mechanism in the development of ALI. In
the present study, the interstitial space was shown to be filled
with activated alveolar macrophages and neutrophils following the
LPS challenge. These pathological changes were reversed by
salidroside treatment following the challenge, indicating that
salidroside may inhibit LPS-induced leukocyte rolling and
transmigration into the lung tissue.
In addition, vascular leakage is a critical
pathological process in sepsis (33). Leakage permits plasma protein and
leukocyte extravasation, leading to oedema and inflammatory
reactions in the affected tissues (34). Oedema causes tissue hypoxia, and
leukocytes, including neutrophils, cause tissue damage through
their excessive production of free radicals and proteases. Thus,
vascular leakage is a promising target for the therapeutic
treatment of sepsis. In the present study, CLP was found to
markedly increase the albumin concentration in BALF, the W/D lung
weight ratio and the water content in lung tissue. Histological
analyses of the lungs following CLP exposure revealed a damaged
alveolar structure with evident concretions leaking liquid within
the bleeding inflammatory cells. In addition, the perivascular gap
was widened and there were numerous alveolar stoma infiltrated by
mononuclear inflammatory cells. These observations indicated that
CLP exacerbates the lung leakage permeability; however, the
exacerbated lung leakage permeability was ameliorated by
salidroside. These results provide supporting evidence that
salidroside post-treatment is effective in reversing LPS-induced
lung permeability and injuries.
In conclusion, the observations of the present study
clearly demonstrate the anti-inflammatory activity of salidroside
via increasing PPAR-γ activation. Furthermore, PPAR-γ was shown to
block CLP-induced NF-κβ expression, which consequently upregulated
the expression levels of Iκβ. The upregulation of Iκβ expression
levels inhibited the accumulation of inflammatory cells within the
lungs, improving CLP-induced lung permeability and alleviating the
pathological injury of lung tissue in rats with sepsis. Therefore,
the present study has provided an experimental basis involving
animals for the treatment of sepsis by the administration of
salidroside.
Acknowledgements
The authors thank Professor Mei-Xian Sun and
Professor Lan-Fang Qin for their kind and excellent technical
assistance. The study was supported by a grant from the Yunnan
Science and Technology Foundation of China (no. 2010C093).
Abbreviations:
|
PPAR-γ
|
peroxisome proliferator-activated
receptor γ
|
|
NF-κβ
|
nuclear factor-κβ
|
|
IL
|
interleukin
|
|
TNF-α
|
tumour necrosis factor-α
|
|
LPS
|
lipopolysaccharide
|
|
CLP
|
cecal ligation and puncture
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
ALI
|
acute lung injury
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
ELISA
|
enzyme-linked immunosobent assay
|
|
Iκβ
|
inhibitor-κβ
|
|
RT
|
room temperature
|
|
W/D
|
wet to dry
|
|
ANOVA
|
analysis of variance
|
References
|
1
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berthiaume Y, Folkesson HG and Matthay MA:
Lung edema clearance: 20 years of progress: invited review:
alveolar edema fluid clearance in the injured lung. J Appl Physiol
(1985). 93:2207–2213. 2002.PubMed/NCBI
|
|
4
|
Eaton DC, Helms MN, Koval M, Bao HF and
Jain L: The contribution of epithelial sodium channels to alveolar
function in health and disease. Annu Rev Physiol. 71:403–423. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morty RE, Eickelberg O and Seeger W:
Alveolar fluid clearance in acute lung injury: what have we learned
from animal models and clinical studies? Intensive Care Med.
33:1229–1240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Magnotti LJ, Upperman JS, Xu DZ, Lu Q and
Deitch EA: Gut-derived mesenteric lymph but not portal blood
increases endothelial permeability and promotes lung injury after
hemorrhagic shock. Ann Surg. 228:518–527. 1998. View Article : Google Scholar
|
|
7
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong H, Xin H, Wu LX and Zhu YZ:
Salidroside attenuates apoptosis in ischemic cardiomyocytes: a
mechanism through a mitochondria-dependent pathway. J Pharmacol
Sci. 114:399–408. 2010. View Article : Google Scholar
|
|
9
|
Zhao Y, Ling Y, Zhao J, Yuan Y, Guo Y, Liu
Q, Wu B, Ding Z and Yang Y: Synthesis and protective effects of
novel salidroside analogues on glucose and serum depletion induced
apoptosis in PC12 cells. Arch Pharm (Weinheim). 346:300–307. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu X, Lin S, Yu D, Qiu S, Zhang X and Mei
R: A preliminary study: the anti-proliferation effect of
salidroside on different human cancer cell lines. Cell Biol
Toxicol. 26:499–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin SS, Chin LW, Chao PC, Lai YY, Lin LY,
Chou MY, Chou MC, Wei JC and Yang CC: In vivo Th1 and Th2 cytokine
modulation effects of Rhodiola rosea standardised solution
and its major constituent, salidroside. Phytother Res.
25:1604–1611. 2011.PubMed/NCBI
|
|
12
|
Lu L, Yuan J and Zhang S: Rejuvenating
activity of salidroside (SDS): dietary intake of SDS enhances the
immune response of aged rats. Age (Dordr). 35:637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guan S, He J, Guo W, Wei J, Lu J and Deng
X: Adjuvant effects of salidroside from Rhodiola rosea L. on
the immune responses to ovalbumin in mice. Immunopharmacol
Immunotoxicol. 33:738–743. 2011.
|
|
14
|
Straus DS, Pascual G, Li M, Welch JS,
Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G and Glass CK:
15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in
the NF-kappa B signaling pathway. Proc Natl Acad Sci USA.
97:4844–4849. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okada M, Yan SF and Pinsky DJ: Peroxisome
proliferator-activated receptor-gamma (PPAR-gamma) activation
suppresses ischemic induction of Egr-1 and its inflammatory gene
targets. FASEB J. 16:1861–1868. 2002. View Article : Google Scholar
|
|
16
|
Ma X, Chang W, Zhang C, Zhou X and Yu F:
Staphylococcal Panton-Valentine leukocidin induces pro-inflammatory
cytokine production and nuclear factor-kappa B activation in
neutrophils. PLoS One. 7:e349702012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Genovese T, Cuzzocrea S, Di Paola R,
Mazzon E, Mastruzzo C, Catalano P, Sortino M, Crimi N, Caputi AP,
Thiemermann C and Vancheri C: Effect of rosiglitazone and
15-deoxy-delta 12,14-prostaglandin J2 on bleomycin-induced lung
injury. Eur Respir J. 25:225–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lipke AB, Matute-Bello G, Herrero R,
Kurahashi K, Wong VA, Mongovin SM and Martin TR: Febrile-range
hyperthermia augments lipopolysaccharide-induced lung injury by a
mechanism of enhanced alveolar epithelial apoptosis. J Immunol.
184:3801–3813. 2010. View Article : Google Scholar
|
|
19
|
Repine JE and Elkins ND: Effect of
ergothioneine on acute lung injury and inflammation in cytokine
insufflated rats. Prev Med. 54(Suppl): S79–S82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kabir K, Gelinas JP, Chen M, Chen D, Zhang
D, Luo X, Yang JH, Carter D and Rabinovici R: Characterization of a
murine model of endotoxin-induced acute lung injury. Shock.
17:300–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Idriss HT and Naismith JH: TNF alpha and
the TNF receptor superfamily: structure-function relationship(s).
Microsc Res Tech. 50:184–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perl M, Lomas-Neira J, Chung CS and Ayala
A: Epithelial cell apoptosis and neutrophil recruitment in acute
lung injury - a unifying hypothesis? What we have learned from
small interfering RNAs. Mol Med. 14:465–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bernard GR, Artigas A, Brigham KL, Carlet
J, Falke K, Hudson L, Lamy M, Legall JR, Morris A and Spragg R: The
American-European Consensus Conference on ARDS. Definitions,
mechanisms, relevant outcomes, and clinical trial coordination. Am
J Respir Crit Care Med. 149:818–824. 1994. View Article : Google Scholar
|
|
24
|
Petty JM, Sueblinvong V, Lenox CC, Jones
CC, Cosgrove GP, Cool CD, Rai PR, Brown KK, Weiss DJ, Poynter ME
and Suratt BT: Pulmonary stromal-derived factor-1 expression and
effect on neutrophil recruitment during acute lung injury. J
Immunol. 178:8148–8157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ryu SL, Shim JW, Kim DS, Jung HL, Park MS,
Park SH, Lee J, Lee WY and Shim JY: Expression of peroxisome
proliferator-activated receptor (PPAR)-α and PPAR-γ in the lung
tissue of obese mice and the effect of rosiglitazone on
proinflammatory cytokine expressions in the lung tissue. Korean J
Pediatr. 56:151–158. 2013.
|
|
26
|
Hunter RL, Choi DY, Ross SA and Bing GY:
Protective properties afforded by pioglitazone against
intrastriatal LPS in Sprague-Dawley rats. Neurosci Lett.
432:198–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ricote M, Li AC, Willson TM, Kelly CJ and
Glass CK: The peroxisome proliferator-activated receptor-gamma is a
negative regulator of macrophage activation. Nature. 391:79–82.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Standage SW, Caldwell CC, Zingarelli B and
Wong HR: Reduced peroxisome proliferator-activated receptor α
expression is associated with decreased survival and increased
tissue bacterial load in sepsis. Shock. 37:164–169. 2012.
|
|
29
|
Christman JW, Sadikot RT and Blackwell TS:
The role of nuclear factor-kappa β in pulmonary diseases. Chest.
117:1482–1487. 2000.
|
|
30
|
Delerive P, Gervois P, Fruchart JC and
Staels B: Induction of Iκβalpha expression as a mechanism
contributing to the anti-inflammatory activities of peroxisome
proliferator-activated receptor-alpha activators. J Biol Chem.
275:36703–36707. 2000.
|
|
31
|
Hahn I, Klaus A, Janze AK, Steinwede K,
Ding N, Bohling J, Brumshagen C, Serrano H, Gauthier F, Paton JC,
Welte T and Maus UA: Cathepsin G and neutrophil elastase play
critical and nonredundant roles in lung-protective immunity against
Streptococcus pneumoniae in mice. Infect Immun.
79:4893–4901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Warner EA, Kotz KT, Ungaro RF, Abouhamze
AS, Lopez MC, Cuenca AG, Kelly-Scumpia KM, Moreno C, O’Malley KA,
Lanz JD, Baker HV, Martin LC, Toner M, Tompkins RG, Efron PA and
Moldawer LL: Microfluidics-based capture of human neutrophils for
expression analysis in blood and bronchoalveolar lavage. Lab
Invest. 91:1787–1795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou G, Kamenos G, Pendem S, Wilson JX and
Wu F: Ascorbate protects against vascular leakage in cecal ligation
and puncture-induced septic peritonitis. Am J Physiol Regul Integr
Comp Physiol. 302:R409–R416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumpers P, Gueler F, David S, Slyke PV,
Dumont DJ, Park JK, Bockmeyer CL, Parikh SM, Pavenstadt H, Haller H
and Shushakova N: The synthetic tie2 agonist peptide vasculotide
protects against vascular leakage and reduces mortality in murine
abdominal sepsis. Crit Care. 15:R2612011. View Article : Google Scholar : PubMed/NCBI
|