Introduction
Brain natriuretic peptide (BNP) is a 32-amino acid
polypeptide containing a 17-amino acid ring structure common to all
natriuretic peptides (1). BNP is
stored in human cardiac tissue as BNP-32 with a lesser amount of
the precursor preproBNP, and in the circulating plasma as BNP-32
and the N-terminal proBNP (NT-proBNP) (2). BNP is a cardiac neurohormone that is
secreted into the plasma from the ventricles in response to
ventricular volume expansion and pressure overload. BNP levels are
useful for the diagnosis of left ventricular (LV) systolic and
diastolic dysfunction and have been shown to correlate with the
severity and prognosis (3). BNP
provides an easy method for the early detection of heart failure
(HF), and for assessing the severity of HF and the effectiveness of
treatment (4).
A previous study identified that BNP and NT-proBNP
are the prognostic importance in patients with HF and with acute
coronary syndromes, and both markers have been shown to be strong
predictors of morbidity and mortality (5).
Diastolic wall stress has been shown to exhibit a
stronger correlation with the levels of NT-proBNP than that of
systolic wall stress (6). The
estimation of BNP values may be accepted as a fast and reliable
blood test in the diagnosis of asymptomatic diastolic dysfunction
in patients with hypertension, diabetes and hypertrophic
cardiomyopathy (HCM) (7–9). Measurement of BNP levels, which is
simple and noninvasive, can be easily and rapidly conducted in
emergency departments to guide therapy, follow the response to
therapy and predict the exercise capacity of patients (10). However, the role of BNP as a
predictor of morbidity and mortality in patients with diastolic
dysfunction is unknown. In 2011, a small sample size study
(11) hypothesized that the
increase in BNP levels over time directly reflected LV diastolic
dysfunction and impairment of exercise tolerance. HF patients with
reserved left ventricular systolic function (RLVSF) are considered
to be patients with diastolic dysfunction. Therefore, the present
study was conducted to evaluate the effect of BNP levels on the
survival times of HF patients with RLVSF.
Patients and methods
Study subjects and procedures
This was an observational study. Consecutive
inpatients with cardiovascular disease, admitted to the Division of
Cardiology at Jinshan Hospital of Fudan University (Shanghai,
China) between June 2006 and December 2009, underwent follow-up
examinations. The Ethics Committee of Jinshan Hospital approved the
study protocol and written informed consent was obtained from each
patient. The patients were classified according to the initial BNP
cutoff point of 100 pg/ml. LV systolic dysfunction was defined by
an ejection fraction of <45%, while systolic function was
considered to be normal when the left ventricular ejection fraction
(LVEF) was ≥45%. Evaluations of the patients with HF were performed
using the New York Heart Association (NYHA) classification system.
HF was defined as NYHA classes II, III or IV (12). Key events included cardiovascular
mortality, readmission due to cardiovascular disease or mortality
due to any other reasons.
Measurement of plasma BNP
concentration
Blood samples for the analysis of plasma BNP levels
were collected at the time of admission and were obtained from the
antecubital vein. BNP levels were analyzed using a
Triage® BNP test (Biosite Diagnostics, Inc., San Diego,
CA, USA), which is a single-use fluorescence immunoassay device
designed to determine the concentration of BNP in
EDTA-anticoagulated whole blood or plasma specimens. The specimen
was added to the sample port of the test device with a transfer
pipette that is designed to deliver the appropriate amount of
specimen (250 μl) to the test device. Following the addition of the
specimen, the device was inserted into the Triage®
MeterPro (Biosite Diagnostics, Inc.). The MeterPro was programmed
to automatically perform the BNP analysis following the reaction of
the sample with the reagents within the BNP device. The reaction
and analysis process occurred over ~15 min. BNP analysis was based
on the amount of fluorescence that the MeterPro detected within a
measurement zone on the device. A greater amount of fluorescence
detected by the MeterPro indicated a higher BNP concentration in
the specimen.
Echocardiography
M-mode and two-dimensional images, as well as
spectral and color flow Doppler recordings, were obtained by Vivid
7 ultrasound (GE Healthcare, Andover, MA, USA) with Vivid 7
ultrasound operating at 2.0 to 3.5 MHz. The LVEF was calculated
from the four-chamber view images using the formula of Simpson’s
rule.
Statistical analysis
Baseline characteristics of the patients are
presented as percentages for dichotomous variables and medians with
interquartile ranges for continuous variables such as age. The
baseline characteristics were compared between the groups using the
Wilcoxon rank-sum test for continuous variables and the
χ2 test for discrete variables. Survival curves were
generated by Kaplan-Meier estimates and differences in the survival
rates were compared between groups using the log-rank test. The
incidence of endpoint events was compared between the groups by
means of relative risk. Spearman’s correlation was used to analyze
the correlation between the levels of BNP and the survival times of
the patients. BNP levels were evaluated by receiver operating
characteristic (ROC) and area under the curve (AUC) analyses for
predicting the incidence of clinical compound endpoints. To
determine the optimal value of specificity and sensitivity, the
closest value to the best specificity and sensitivity point on the
ROC curve was identified. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The study population consisted of 1,415 patients.
The mean age was 65.7 years and almost half the patients were male
(790/1,415, 55.8%). The duration of the follow-up period ranged
between 21 and 63 months (average duration, 35.8 months).
Characteristics of the overall patient population are shown in
Table I. Risk factors of the
patients included hypertension (953/1415, 67.35%), diabetes
(291/1415, 32.33%), dyslipidemia (622/1415, 43.96%), renal
dysfunction (115/1415, 8.13%; serum creatinine >84 μmol/l in
females; serum creatinine >104 μmol/l in males), myocardial
infarction (190/1415, 13.53%) and intervention with medication,
including β-blockers, calcium antagonists, diuretics, nitrates,
antiplatelet agents, statins, angiotensin converting enzyme
inhibitors and angiotensin receptor blockers. The numbers of
patients with BNP levels of ≤100 and >100 pg/ml were 900 and
515, respectively. A total of 336 endpoint events occurred,
including 143 and 193 in the two BNP groups, respectively. Among
the 1,415 patients, 1,312 underwent echocardiographic detection at
the same time as admission, including 395 (30.11%) patients with
NYHA classes II–IV and a LVEF of ≥45% and 123 (9.38%) patients with
systolic dysfunction. The incidence of compound endpoint events was
significantly higher in the BNP >100 pg/ml group than in the BNP
≤100 pg/ml group (86/232, 37.07 vs. 39/163, 23.93%; relative
risk=1.55) in 395 patients with NYHA classes II–IV and a LVEF of
≥45%.
 | Table IBaseline clinical characteristics
according to the levels of BNP. |
Table I
Baseline clinical characteristics
according to the levels of BNP.
| Characteristics | Patients with BNP
≤100 pg/ml (n=900) | Patients with BNP
>100 pg/ml (n=515) | P-value |
|---|
| Age, years
(range) | 63 (50.0–76.0) | 70 (56.4–83.6) | <0.05 |
| Male gender, n
(%) | 513/900
(57.00) | 277/515
(53.79) | 0.242 |
| Mortality, n
(%) | 7 (0.77) | 24 (4.66) | <0.05 |
| Readmission, n
(%) | 136 (15.11) | 169 (32.82) | <0.05 |
| Key events, n
(%) | 143 (15.89) | 193 (37.47) | <0.05 |
| Systolic
dysfunction, n (%) | 18/841 (2.14) | 105/470
(22.34) | <0.05 |
| Non-systolic
dysfunction, n (%) | 163/841
(19.38) | 232/470
(49.36) | <0.05 |
| Hypertension, n
(%) | 638 (70.89) | 315 (61.16) | <0.05 |
| Diabetes, n
(%) | 176 (19.55) | 115 (22.33) | 0.214 |
| Dyslipidemia, n
(%) | 477 (53.00) | 145 (28.15) | <0.05 |
| Renal dysfunction,
n (%) | 31 (3.44) | 84 (16.31) | <0.05 |
| Myocardial
infarction, n (%) | 96 (10.67) | 94 (18.25) | <0.05 |
| Medication, n
(%) |
| β-blockers | 480 (53.33) | 250 (48.54) | 0.083 |
| Calcium
antagonists | 413 (45.89) | 186 (36.12) | <0.05 |
| Diuretics | 230 (25.56) | 378 (73.40) | <0.05 |
| Nitrates | 386 (42.89) | 230 (44.66) | 0.518 |
| Antiplatelet
agents | 713 (79.22) | 379 (73.59) | 0.015 |
| Statins | 325 (36.11) | 135 (26.21) | <0.05 |
| ACEIs or ARBs | 638 (70.88) | 375 (72.82) | 0.439 |
| ACEIs | 302 (33.56) | 187 (36.31) | 0.294 |
| ARBs | 336 (37.33) | 188 (36.50) | 0.756 |
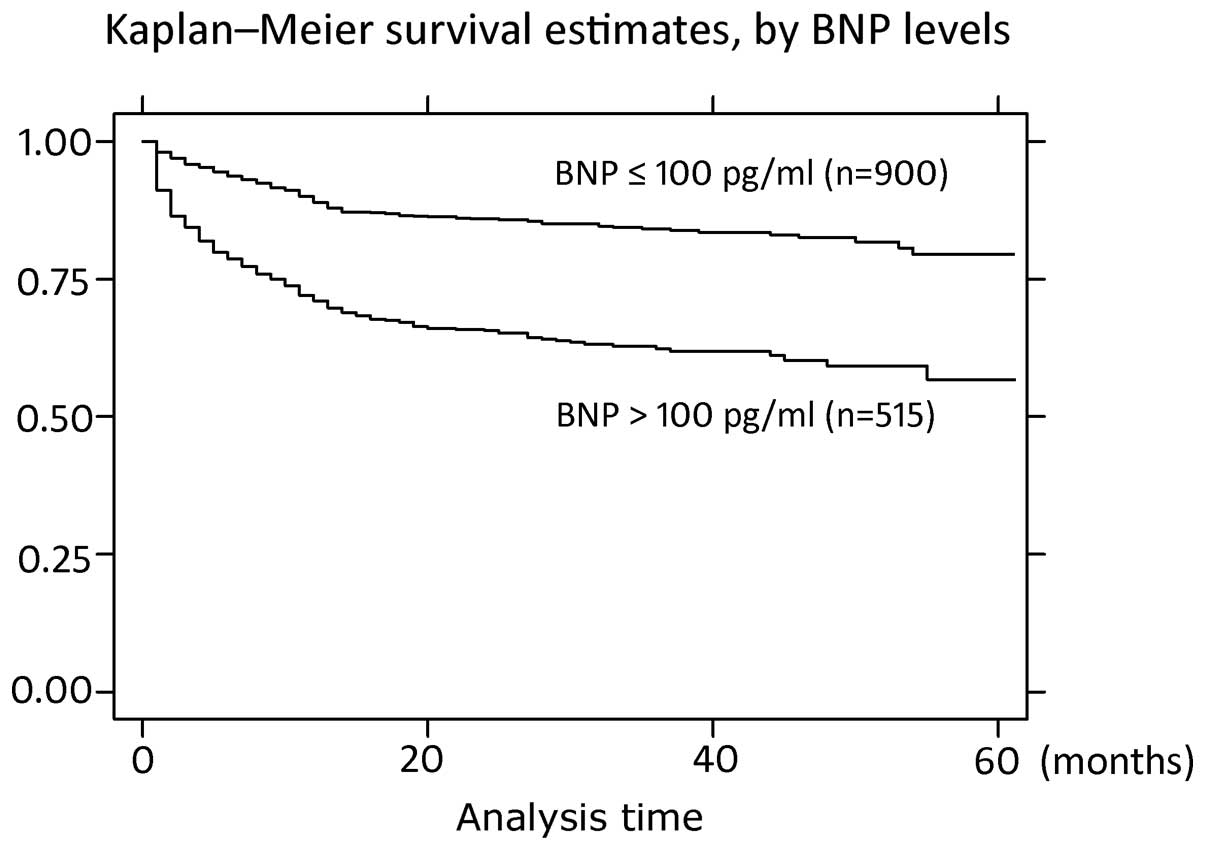
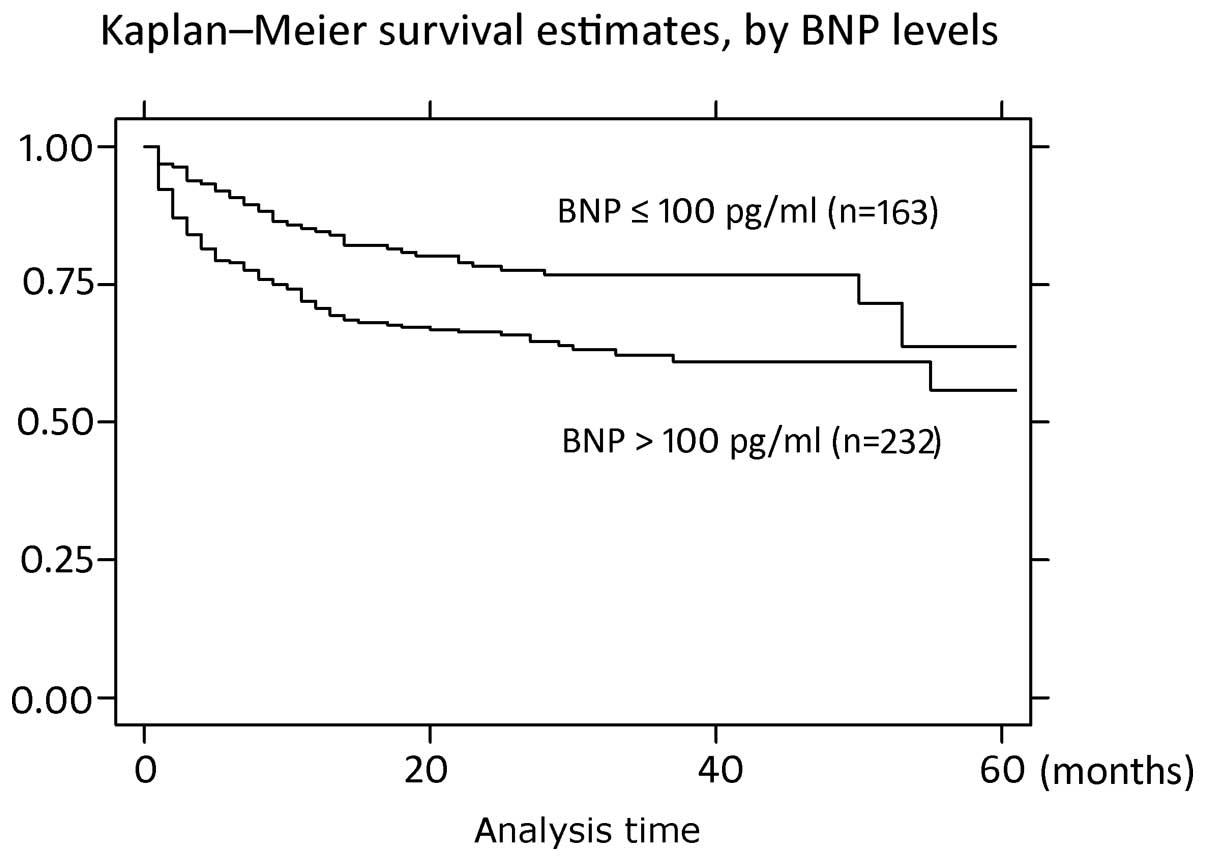
Survival curves
Kaplan-Meier survival curves of all the patients and
specifically the HF patients with RLVSF, according to the BNP
levels, are shown in Figs. 1 and
2. The survival curves were
constructed in the two groups to predict the survival times of the
patients. Survival times were longer in the BNP ≤100 pg/ml group
when compared with the BNP >100 pg/ml group and statistically
significant differences were observed (P<0.0001,
χ2=94.11 and P=0.0039, χ2=8.33, for all
patients and the HF patients with RLVSF, respectively).
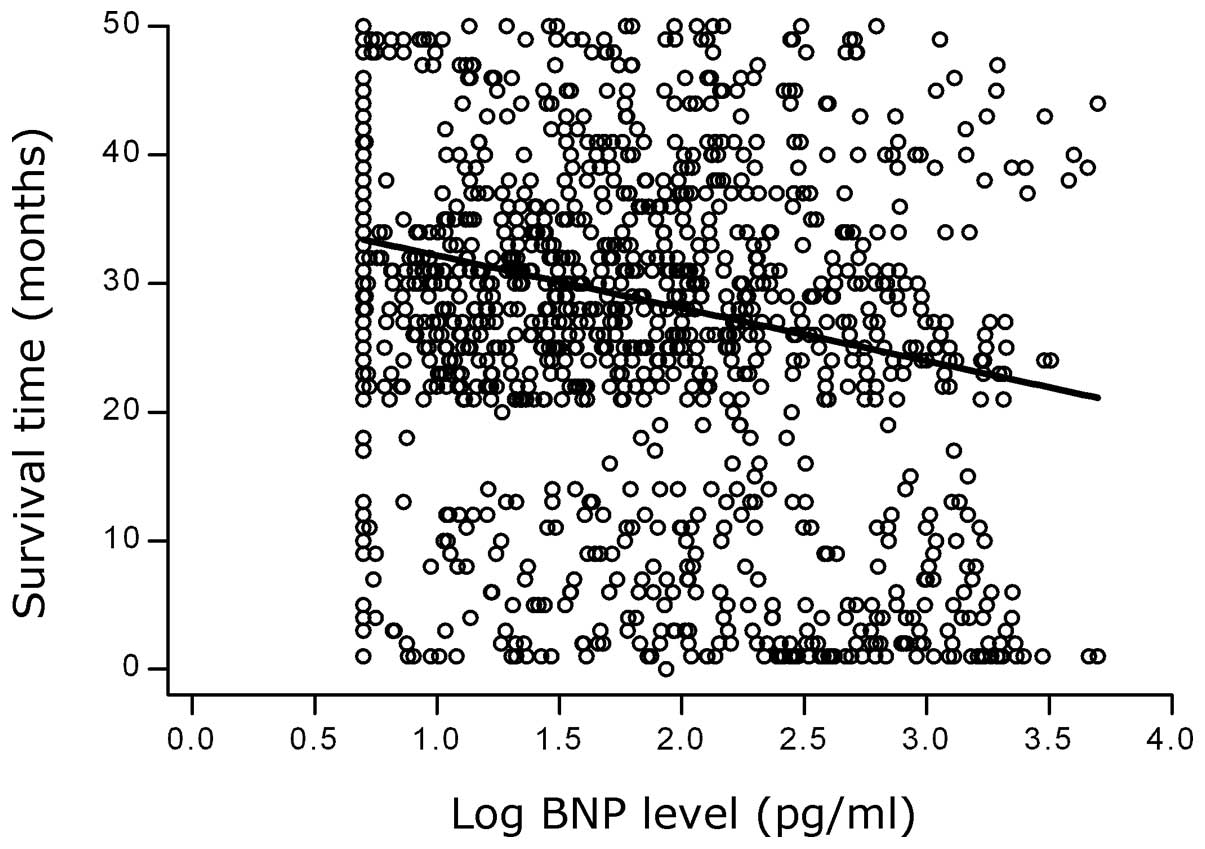
Correlation analysis
Spearman correlation analysis demonstrated that the
survival times decreased as the BNP levels increased (Spearman’s ρ,
−0.1877; P=0.0000). A negative correlation between the logBNP
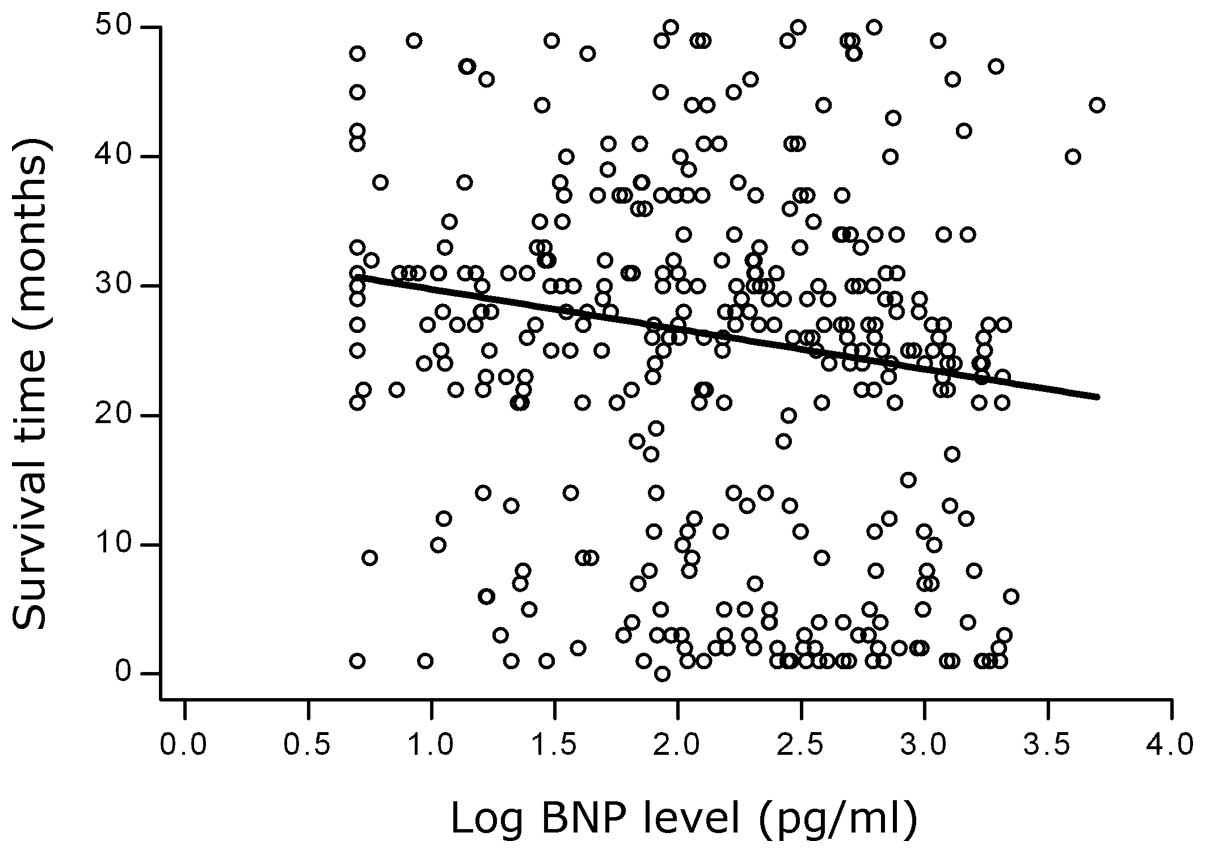
levels and the survival times is shown in Fig. 3. A negative correlation was also
observed in the 395 HF patients with RLVSF (Spearman’s ρ, −0.1738;
P=0.0005). A scatter plot demonstrating the correlation between the
logBNP levels and the survival times of the HF patients with RLVSF
is shown in Fig. 4.
BNP levels as a predictor for clinical
endpoints
The predictive utility of plasma BNP levels in all
the patients for determining compound clinical endpoints was
calculated with ROC analysis. Plasma BNP levels has diagnostic
value in the incidence of clinical compound endpoint events in all
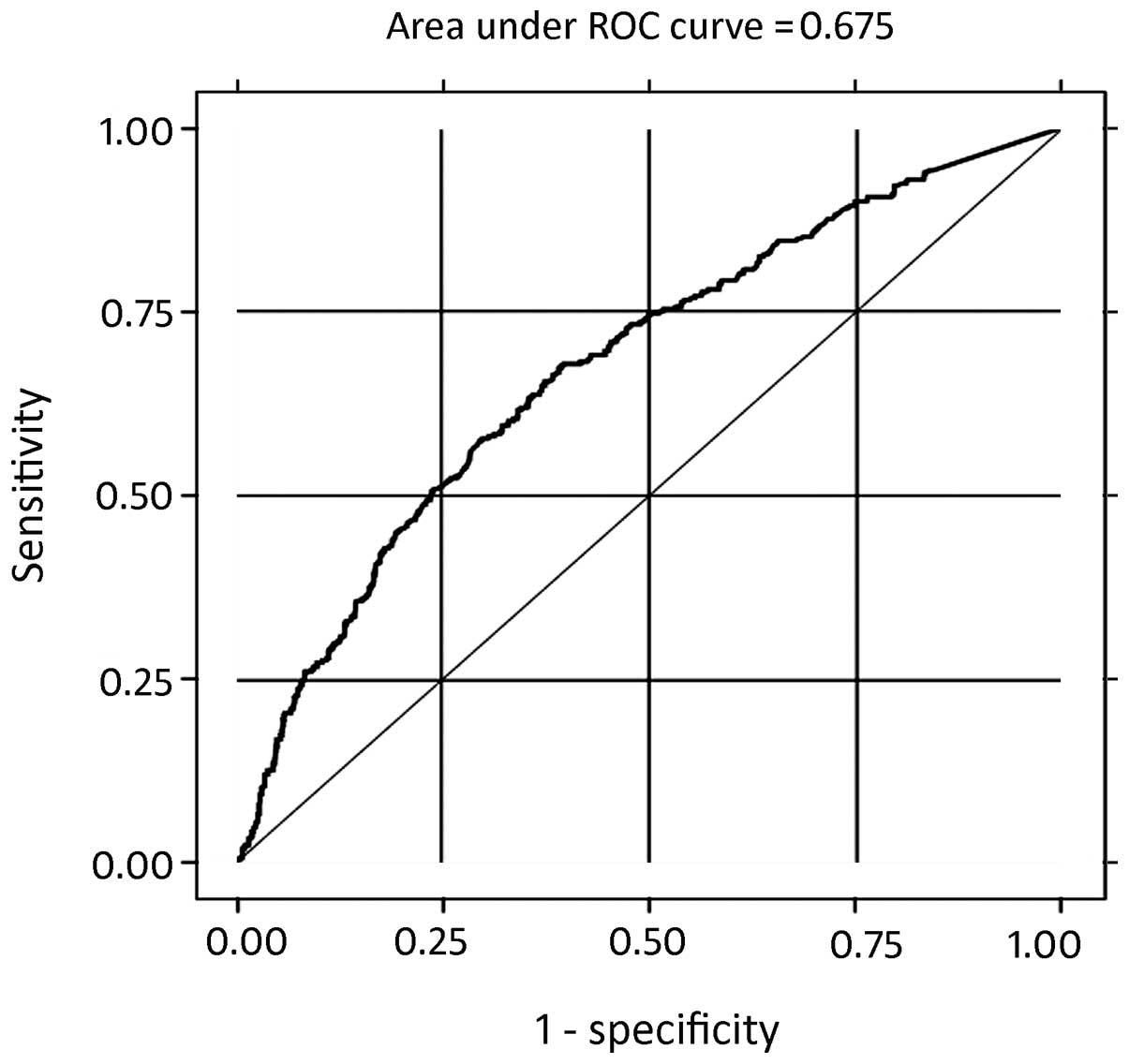
the patients and in patients with diastolic dysfunction. The AUROC
was 0.6752 with a standard error of 0.01698 (95% confidence
interval, 0.64198–0.70835) and the cut-off value for the plasma BNP
levels was 100 pg/ml (sensitivity and specificity, 57.44 and
70.16%, respectively; Fig. 5 and
Table II). The predictive utility
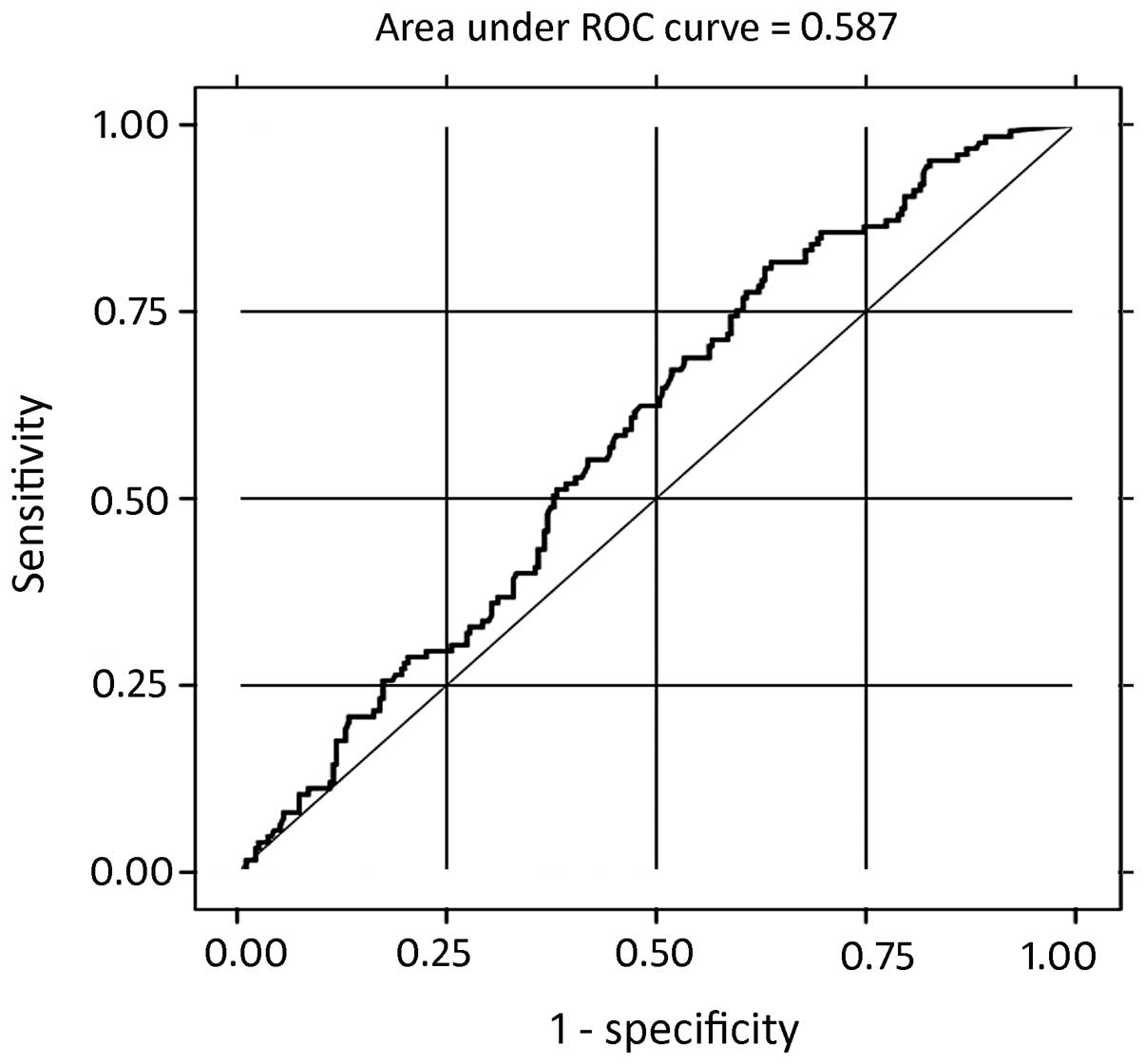
of plasma BNP levels in the HF patients with RLVSF for determining
compound clinical endpoints was also calculated with ROC analysis.
The AUROC was 0.5877 with a standard error of 0.0296 (95%
confidence interval, 0.52965–0.64573) and the cut-off value for the
plasma BNP levels was 100 pg/ml (sensitivity and specificity, 68.8
and 45.93%, respectively; Fig. 6
and Table III).
 | Table IICorrelation between the logBNP levels
and the incidence of compound endpoint events in all the
patients. |
Table II
Correlation between the logBNP levels
and the incidence of compound endpoint events in all the
patients.
| Compound endpoint
events, n | |
|---|
|
| |
|---|
| Yes | No | Total |
|---|
| BNP >100
pg/ml | 193 | 322 | 515 |
| BNP ≤100 pg/ml | 143 | 757 | 900 |
| Total | 336 | 1079 | 1415 |
 | Table IIICorrelation between the logBNP levels
and the incidence of compound endpoint events in the patients with
diastolic dysfunction. |
Table III
Correlation between the logBNP levels
and the incidence of compound endpoint events in the patients with
diastolic dysfunction.
| Compound endpoint
events, n | |
|---|
|
| |
|---|
| Yes | No | Total |
|---|
| BNP >100
pg/ml | 86 | 146 | 232 |
| BNP ≤100 pg/ml | 39 | 124 | 163 |
| Total | 125 | 270 | 395 |
Discussion
BNP is a cardiac neurohormone that is secreted into
the plasma from the ventricles in response to ventricular volume
expansion and pressure overload (3). BNP plasma levels have been shown to
be significantly higher in patients with decompensated chronic HF
as compared with those in a control group (13). BNP levels provide an easy method
for the early detection of HF and for assessing the severity of HF
and the effectiveness of treatment (14). A number of previous studies have
demonstrated that the levels of BNP and NT-proBNP are powerful
prognostic markers across a spectrum of acute coronary syndromes
(15), from unstable angina and
non-ST elevation myocardial infarction to ST elevation myocardial
infarction (16–18), as well as in patients with stable
angina pectoris (19,20) and even in the absence of
significant necrosis (21). BNP
and NT-proBNP are present in human coronary arteries (22) and are associated with the extent
and severity of coronary atherosclerotic lesions (23). The observations of the present
study revealed a similar correlation; the proportion of patients
with myocardial infarction was significantly higher in the BNP
>100 pg/ml group as compared with the BNP ≤100 pg/ml group
(P=0.039). Ischemia per se may function as a stimulus for
the release of BNP and NT-proBNP (24). Overactivity of the sympathetic
nervous system in the left ventricle appears to be an important
mechanism for the induction of elevated BNP levels in chronic
ischemic HF (4). BNP gene
expression levels are upregulated in the ventricular wall by acute
myocardial hypoxia, resulting in augmented plasma concentrations of
BNP and proBNP (25,26).
NT-proBNP is independent of invasive measurements of
LV function and the severity of coronary artery disease (20). The prognostic importance of BNP and
NT-proBNP has been extensively studied in patients with HF, as well
as in patients with acute coronary syndromes, with both markers
having been demonstrated to be strong and independent predictors of
morbidity and all-cause mortality (20,27,28).
The predictors were also evident in the subgroup of patients with a
LVEF of >60% and in patients with diabetes mellitus (29). A previous study (20) demonstrated that measuring NT-proBNP
levels immediately prior to coronary angiography in patients with
stable coronary heart disease provided prognostic information on
all-cause mortality. The present study also demonstrated the same
prognostic value. The incidence of cardiovascular mortality,
readmission due to cardiovascular disease or mortality through
other causes was significantly higher in the BNP >100 pg/ml
group than in the BNP ≤100 pg/ml group. Kaplan-Meier analysis was
performed to predict the survival times of the patients and the
results indicated that the survival times were longer in the BNP
≤100 pg/ml group than in the BNP >100 pg/ml group. The results
also demonstrated a negative correlation between the logBNP levels
and the survival times of patients with cardiovascular disease,
with survival times decreasing as the BNP levels increased. A
plasma BNP level of 100 pg/ml was selected as a cut-off value for
the prediction of cardiovascular morbidity and all-cause mortality,
with a sensitivity of 57.44% and a specificity of 70.16% in all
patients. BNP (or NT-proBNP) has been shown to have high negative
predictive values as a single test (30). The observations of the present
study revealed a similar outcome. The subjects of the present study
included inpatients with various types of disease, including
hypertension, diabetes, dyslipidemia, renal dysfunction and
myocardial infarction. Therefore, this study demonstrates that the
BNP level is correlated to the prediction of most cardiovascular
diseases, not only one or several specific diseases. Thus, the
application of BNP is wider than previously considered.
A previous study demonstrated that 40–50% of
individuals with HF have a normal ejection fraction, and diastolic
dysfunction is the presumed cause of diastolic HF (DHF) (31). Since abnormalities in diastolic
function may not always produce symptoms of HF, the conditions are
often missed and patients are predisposed to symptomatic HF due to
the delay in treatment (31).
Furthermore, the prognosis of patients suffering from DHF is as
ominous as that of patients suffering from systolic HF. Diastolic
dysfunction without symptoms (preclinical diastolic dysfunction) is
common and is independently predictive of the future development of
HF and cardiac mortality (32).
Early diagnosis of LV diastolic dysfunction in an initial phase
enables the start of effective treatment, which functions by
stopping the progress of the disease and delaying the development
of symptomatic HF (33). Analysis
of the diastole by means of echocardiography, using Doppler
measurements of transmitral and pulmonary vein blood flow
velocities and tissue Doppler imaging, is widely accepted for
clinical purposes (34). However,
this type of assessment is expensive as it requires complex
equipment, time-consuming as it involves the analysis of numerous
variables and difficult as it must be performed by a skilled and
trained operator (35). Thus, a
simple and objective method to quantify diastole function with high
sensitivity and specificity is required. An association between the
levels of BNP and the indexes of diastolic function has been
described in patients with reduced LVEF and in those with preserved
LVEF (36). A previous study has
shown that estimating BNP levels may be accepted as a fast and
reliable blood test for the diagnosis of asymptomatic diastolic
dysfunction. The BNP test may be used for the prediction of
asymptomatic diastolic dysfunction in patients with hypertension
(7). In addition, BNP levels may
be used for the repeat evaluation of an occult LV dysfunction in
patients who are periodically assessed for diabetic complications
(8). Thus, BNP may be used as an
adjunctive, reliable and objective method of estimating cardiac
dysfunction in HCM (9).
Measurement of BNP levels is simple and noninvasive, and can be
easily and rapidly conducted in emergency departments to guide
therapy, follow the response to therapy and predict the exercise
capacity of patients (10). The
observations of the present study indicated that the prognoses of
patients with higher BNP levels were worse compared with those with
lower BNP levels. A negative correlation between the levels of BNP
and the survival times was identified in 395 HF patients with
RLVSF; survival times of the HF patients with RLVSF decreased with
increasing BNP levels. Furthermore, the predictive utility of
plasma BNP levels in HF patients with RLVSF for determining the
incidence of compound clinical endpoints was also demonstrated.
Morbidity and mortality rates from cardiovascular diseases are
increased in patients with high plasma BNP levels. However, a
plasma BNP level cut-off value of 100 pg/ml may be used for the
prediction of cardiovascular morbidity and all-cause mortality,
with a sensitivity of 68.8% and a specificity of 45.93% in HF
patients with RLVSF. The predictive utility of plasma BNP levels in
HF patients with RLVSF is lower than in all the patients.
BNP has been shown to have a higher sensitivity (85
vs. 63%) and positive predictive value (69 vs 55%) than NT-proBNP.
The negative predictive values of BNP and NT-proBNP were similar
(70 and 71%, respectively) (37).
The level of BNP appears to have a higher sensitivity and higher
positive predictive value for the accurate diagnosis of severe LVSD
than the level of NT-proBNP (38).
The plasma half-life of BNP in humans is ~20 min, while the
circulating half-life of NT-proBNP is ~120 min (38). Therefore, BNP levels may used to
assess the current severity of LV dysfunction, guide therapy and
follow the immediate response to therapy. However, NT-proBNP is
unable to this since it has an assessment lag of ~10 h. Clearance
of BNP is hypothesized to occur via two main mechanisms: Binding to
clearance receptors and enzymatic degradation by the enzyme neutral
endopeptidase (39). Clearance of
NT-proBNP occurs predominantly via the kidney, thus, in patients
with mild renal dysfunction, utility of diagnosis is seriously
affected (40,41). Approximately 29% of HF patients
have renal failure (42). BNP
levels are a more useful diagnostic indicator for cardiogenic
pulmonary edema than proBNP in patients aged ≥65 years (40). The estimated glomerular filtration
rate has independent effects on the plasma BNP and NT-proBNP
concentrations in patients with chronic kidney disease. However,
NT-proBNP appears to be affected more than BNP by declining kidney
function (43). Therefore, in the
present study, the use of plasma BNP levels may have produced
reliable, accurate and effective results.
There are several relevant limitations of the
present study. Firstly, the number of patients in the study was
small and the follow-up period was relatively short. Further
studies with a larger number of patients that are conducted over a
longer time period are required to assess the predictive value of
BNP levels in patients with cardiovascular-related disease,
particularly in patients with RLVSF. Secondly, the
echocardiographic parameters should be interpreted with caution as
the ejection fraction may be affected by different sections and
atrial fibrillation. Further studies with myocardial perfusion
imaging are required to calculate the LVEF, which is likely to
provide more precise results. Thirdly, the sensitivity and
specificity values for predicting the utility of plasma BNP levels
in determining the incidence of compound clinical endpoints are not
very high for either groups of patients. A combination of NT-BNP
(or BNP) with LVEF has been shown to substantially improve the risk
stratification for mortality, HF and new ischemic events (44).
In conclusion, the prognoses were worse for patients
with higher levels of BNP. Furthermore, a significant correlation
was observed between BNP levels and survival times in HF patients
with RLVSF. BNP can predict the prognosis of patients with
cardiovascular disease, particularly in HF patients with RLVSF.
References
|
1
|
Epshteyn V, Morrison K, Krishnaswamy P, et
al: Utility of B-type natriuretic peptide (BNP) as a screen for
left ventricular dysfunction in patients with diabetes. Diabetes
Care. 26:2081–2087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magnusson M, Melander O, Israelsson B, et
al: Elevated plasma levels of NT-proBNP in patients without overt
cardiovascular disease. Diabetes Care. 27:1929–1935. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kremastinos DT, Tsiapras DP, Kostopoulou
AG, et al: NT-proBNP levels and diastolic dysfunction in
beta-thalassaemia major patients. Eur J Heart Fail. 9:531–536.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakata K, Iida K, Mochiduki N and Nakaya
Y: Brain natriuretic peptide (BNP) level is closely related to the
extent of left ventricular sympathetic overactivity in chronic
ischemic heart failure. Intern Med. 48:393–400. 2009. View Article : Google Scholar
|
|
5
|
McKie PM, Rodeheffer RJ, Cataliotti A, et
al: Amino-terminal pro-B-type natriuretic peptide and B-type
natriuretic peptide: biomarkers for mortality in a large
community-based cohort free of heart failure. Hypertension.
47:874–880. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krittayaphong R, Boonyasirinant T,
Saiviroonporn P, et al: Correlation between NT-pro BNP levels and
left ventricular wall stress, sphericity index and extent of
myocardial damage: a magnetic resonance imaging study. J Card Fail.
14:687–694. 2008. View Article : Google Scholar
|
|
7
|
Karaca II, Gülcü E, Yavuzkir M, et al:
B-type natriuretic peptide level in the diagnosis of asymptomatic
diastolic dysfunction. Anadolu Kardiyol Derg. 7:262–267.
2007.PubMed/NCBI
|
|
8
|
Kremastinos DT, Hamodraka E, Parissis J,
et al: Predictive value of B-type natriuretic peptides in detecting
latent left ventricular diastolic dysfunction in beta-thalassemia
major. Am Heart J. 159:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panou FK, Kotseroglou VK, Lakoumentas JA,
et al: Significance of brain natriuretic peptide in the evaluation
of symptoms and the degree of left ventricular diastolic
dysfunction in patients with hypertrophic cardiomyopathy. Hellenic
J Cardiol. 47:344–351. 2006.PubMed/NCBI
|
|
10
|
Eroglu S, Yildirir A, Bozbas H, et al:
Brain natriuretic peptide levels and cardiac functional capacity in
patients with dyspnea and isolated diastolic dysfunction. Int Heart
J. 48:97–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romano S, di Mauro M, Fratini S, et al:
Serial BNP assay in monitoring exercise tolerance in patients with
diastolic dysfunction. Int J Cardiol. 147:312–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hunt SA, Abraham WT, Chin MH, et al:
ACC/AHA 2005 Guideline Update for the Diagnosis and Management of
Chronic Heart Failure in the Adult: a report of the American
College of Cardiology/American Heart Association Task Force on
Practice Guidelines (Writing Committee to Update the 2001
Guidelines for the Evaluation and Management of Heart Failure):
developed in collaboration with the American College of Chest
Physicians and the International Society for Heart and Lung
Transplantation: endorsed by the Heart Rhythm Society. Circulation.
112:e154–e235. 2005.
|
|
13
|
Teicholz LE, Kreulen T, Herman MV and
Gorlin R: Problems in echocardiographic volume determinations:
echocardiographic-angiographic correlations in the presence of
absence of asynergy. Am J Cardiol. 37:7–11. 1976.
|
|
14
|
Speranza L, Franceschelli S, Riccioni G,
et al: BNP and iNOS in decompensated chronic heart failure: a
linear correlation. Front Biosci (Elite Ed). 4:1255–1262. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He Q, Wu G and Lapointe MC: Isoproterenol
and cAMP regulation of the human brain natriuretic peptide gene
involves Src and Rac. Am J Physiol Endocrinol Metab.
278:E1115–E1123. 2000.PubMed/NCBI
|
|
16
|
Suo M, Hautala N, Földes G, et al:
Posttranscriptional control of BNP gene expression in angiotensin
II-induced hypertension. Hypertension. 39:803–808. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
James SK, Lindahl B, Siegbahn A, et al:
N-terminal pro-brain natriuretic peptide and other risk markers for
the separate prediction of mortality and subsequent myocardial
infarction in patients with unstable coronary artery disease: a
Global Utilization of Strategies To Open occluded arteries
(GUSTO)-IV substudy. Circulation. 108:275–281. 2003.
|
|
18
|
Morrow DA, de Lemos JA, Blazing MA, et al;
Investigators. Prognostic value of serial B-type natriuretic
peptide testing during follow-up of patients with unstable coronary
artery disease. JAMA. 294:2866–2871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morrow DA, de Lemos JA, Sabatine MS, et
al: Evaluation of B-type natriuretic peptide for risk assessment in
unstable angina/non-ST-elevation myocardial infarction: B-type
natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll
Cardiol. 41:1264–1272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ceriani L and Giovanella L: Cardiac
natriuretic peptides after myocardial infarction: relationship with
infarct size, left ventricular function and remodelling assessed by
99mTc-sestamibi gated-single photon emission tomography. Clin Chem
Lab Med. 45:226–231. 2007. View Article : Google Scholar
|
|
21
|
Kragelund C, Grønning B, Køber L, et al:
N-terminal pro-B-type natriuretic peptide and long-term mortality
in stable coronary heart disease. N Engl J Med. 352:666–675. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barbosa MM, Nunes Mdo C, Castro LR, et al:
Correlation between NT-pro BNP levels and early mitral annulus
velocity (E′) in patients with non-ST-segment elevation acute
coronary syndrome. Echocardiography. 25:353–359. 2008.PubMed/NCBI
|
|
23
|
Casco VH, Veinot JP, Kuroski de Bold ML,
et al: Natriuretic peptide system gene expression in human coronary
arteries. J Histochem Cytochem. 50:799–809. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weber M, Dill T, Arnold R, et al:
N-terminal B-type natriuretic peptide predicts extent of coronary
artery disease and ischemia in patients with stable angina
pectoris. Am Heart J. 148:612–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Omland T: B-type natriuretic peptides:
prognostic markers in stable coronary artery disease. Expert Rev
Mol Diagn. 8:217–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goetze JP, Christoffersen C, Perko M, et
al: Increased cardiac BNP expression associated with myocardial
ischemia. FASEB J. 17:1105–1107. 2003.PubMed/NCBI
|
|
27
|
Goetze JP, Gore A, Møller CH, et al: Acute
myocardial hypoxia increases BNP gene expression. FASEB J.
18:1928–1930. 2004.PubMed/NCBI
|
|
28
|
Omland T, Persson A, Ng L, et al:
N-terminal pro-B-type natriuretic peptide and long-term mortality
in acute coronary syndromes. Circulation. 106:2913–2918. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Lemos JA, Morrow DA, Bentley JH, et al:
The prognostic value of B-type natriuretic peptide in patients with
acute coronary syndromes. N Engl J Med. 345:1014–1021. 2001.
|
|
30
|
Kragelund C, Gustafsson I, Omland T, et
al: Prognostic value of NH2-terminal pro B-type
natriuretic peptide in patients with diabetes and stable coronary
heart disease. Diabetes Care. 29:1411–1413. 2006.
|
|
31
|
Kelder JC, Rutten FH and Hoes AW:
Clinically relevant diagnostic research in primary care: the
example of B-type natriuretic peptides in the detection of heart
failure. Fam Pract. 26:69–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Redfield MM, Jacobsen SJ, Burnett JC Jr,
et al: Burden of systolic and diastolic ventricular dysfunction in
the community: appreciating the scope of the heart failure
epidemic. JAMA. 289:194–202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimabukuro M, Higa N, Oshiro Y, et al:
Diagnostic utility of brain-natriuretic peptide for left
ventricular diastolic dysfunction in asymptomatic type 2 diabetic
patients. Diabetes Obes Metab. 9:323–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Görmüş U, Ozmen D, Ozmen B, et al: Serum
N-terminal-pro-brain natriuretic peptide (NT-pro-BNP) and
homocysteine levels in type 2 diabetic patients with asymptomatic
left ventricular diastolic dysfunction. Diabetes Res Clin Pract.
87:51–56. 2010.PubMed/NCBI
|
|
35
|
Metra M, Ponikowski P, Dickstein K, et al;
Heart Failure Association of the European Society of Cardiology.
Advanced chronic heart failure: A position statement from the Study
Group on Advanced Heart Failure of the Heart Failure Association of
the European Society of Cardiology. Eur J Heart Fail. 9:684–694.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nagueh SF, Appleton CP, Gillebert TC, et
al: Recommendations for the evaluation of left ventricular
diastolic function by echocardiography. J Am Soc Echocardiogr.
22:107–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grewal J, McKelvie R, Lonn E, et al: BNP
and NT-proBNP predict echocardiographic severity of diastolic
dysfunction. Eur J Heart Fail. 10:252–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kotaska K, Popelova J, Tiserova M, et al:
NT-proBNP and BNP values in cardiac patients with different degree
of left ventricular systolic dysfunction. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 150:125–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mair J, Hammerer-Lercher A and Puschendorf
B: The impact of cardiac natriuretic peptide determination on the
diagnosis and management of heart failure. Clin Chem Lab Med.
39:571–588. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ruskoaho H: Cardiac hormones as diagnostic
tools in heart failure. Endor Rev. 24:341–356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ray P, Arthaud M, Birolleau S, et al:
Comparison of brain natriuretic peptide and probrain natriuretic
peptide in the diagnosis of cardiogenic pulmonary edema in patients
aged 65 and older. J Am Geriatr Soc. 53:643–648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sykes E, Karcher RE, Eisenstadt J, et al:
Analytical relationships among Biosite, Bayer, and Roche methods
for BNP and NT-proBNP. Am J Clin Pathol. 123:584–590. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fonarow GC; ADHERE Scientific Advisory
Committee. The Acute Decompensated Heart Failure National Registry
(ADHERE): opportunities to improve care of patients hospitalized
with acute decompensated heart failure. Rev Cardiovasc Med. 4(Suppl
7): S21–S30. 2003.
|
|
44
|
Vickery S, Price CP, John RI, et al:
B-type natriuretic peptide (BNP) and amino-terminal proBNP in
patients with CKD: relationship to renal function and left
ventricular hypertrophy. Am J Kidney Dis. 46:610–620. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Richards AM, Nicholls MG, Espiner EA, et
al: B-type natriuretic peptides and ejection fraction for prognosis
after myocardial infarction. Circulation. 107:2786–2792. 2003.
View Article : Google Scholar : PubMed/NCBI
|