Introduction
Despite substantial advances in surgical,
cardioprotective and anesthetic techniques, the incidence of
myocardial infarction (MI) following cardiac surgery remains
between 3 and 15% and is associated with reduced, long-term
survival rates (1). Postoperative
MI is a predominant and severe complication in patients undergoing
aortocoronary bypass surgery and is a multifactorial disorder with
significant inter-patient variability, which is poorly predicted by
clinical procedures (1). The
development of MI following cardiac surgery has been associated
with high morbidity, mortality and cost (2).
The renin-angiotensin system (RAS) is crucial in
cardiovascular regulation (3). In
the RAS, angiotensin-converting enzyme (ACE) metabolizes
angiotensin (Ang) I to form Ang II, which exerts a direct trophic
effect upon cardiovascular cells (3). Local Ang II production is important
in the pathophysiology of the RAS in the cardiovascular system
(4) and recently, ACE2, a novel
member of the RAS, was identified to function as a negative
regulator of the Ang system by metabolizing Ang II into a
putatively protective peptide Ang (1–7)
exhibiting high efficiency (5–7).
ACE2 is present in the heart and a reduction in its expression is
associated with enhanced cardiac hypertrophy and reduced pumping
ability (8,9). Although ACE2 was initially localized
exclusively in the cardiac endothelial cells, more recent studies
have demonstrated ACE2 immunoreactivity in the endothelial and
smooth muscle cells of the myocardial vessels, as well as within
cardiomyocytes (8,9). Following MI, significant activation
of cardiac ACE2 occurs in rats and humans, which combats the
adverse effect of an activated cardiac RAS (8). Further evidence of the
cardioprotective role of ACE2 arises from studies conducted on
ACE2-knockout mice, where the loss of ACE2 facilitated adverse,
post-MI ventricular remodeling (10); furthermore, previous studies have
indicated that ACE2 overexpression in MI rats improved cardiac
contractility and remodeling (10,11).
It has been identified that ACE2 serum activity increases during
the first week following an acute MI and that ACE2 activation may
be a compensatory mechanism in MI (12).
In the present study, the association between serum
ACE2 levels and postoperative MI, following coronary artery bypass
grafting (CABG), was investigated.
Patients and methods
Patients
Between March 2008 and April 2013, 136 Han Chinese
patients, who underwent CABG with a cardiopulmonary bypass (CPB) at
the Department of Cardiothoracic Surgery of the Second Xiangya
Hospital, Central South University (Hunan, China) were enrolled in
the present study. The exclusion criteria were a history of renal
failure, active liver disease, bleeding disorders, autoimmune
diseases or immunosuppressive therapy; patients with a family
history of coronary artery disease were also excluded. The present
study was approved by the Ethics Committee of the Second Xiangya
Hospital, Central South University and the participants provided
written informed consent prior to commencing the study.
Definition of postoperative MI
According to definitions for periprocedural necrosis
and periprocedural infarction, collectively established by the
American College of Cardiology Foundation, American Heart
Association, European Society of Cardiology and the World Heart
Federation taskforce, cardiac troponin I (cTnI) is the preferred
biomarker for MI. Post-CABG biomarker values that exceed the
99th percentile of the normal reference range represent
myocardial necrosis (13) and in
the present study, a postoperative MI was defined as an increase of
cTnI to greater than five times the 99th percentile of
the normal reference range, during the first 72 h following CABG.
This was in addition to manifestations of novel pathological
Q-waves, a left bundle branch block, an angiographically documented
novel graft of native coronary artery occlusion or imaging evidence
of a novel loss of a viable MI (13).
Data collection
Patients that were undergoing CABG ceased all
antiplatelet agent usage at least two days prior to surgery.
Intraoperative anesthetic, perfusion and cardioprotective
management was standardized, using fentanyl-isoflurane anesthesia,
nonpulsatile CPB (32–35°C), crystalloid prime, pump flow rates
>2.4 l/min/m2, cold blood cardioplegia, α-stat blood
gas management, heparin (to maintain activated clotting times
>450 secs), ɛ-aminocaproic acid infusion and serial hematocrit
levels were maintained at ≥0.18 during the CPB. The serum ACE2
levels were measured using an ACE2 (human) ELISA kit (K4918-100;
BioVision, Milpitas, CA, USA) according to the manufacturer’s
instructions on preoperative day 3 and 1, 1 h post surgery and
postoperative days 1, 2, 6, 9 and 12. Serum creatine kinase (CK)-MB
levels were measured via a human cTnI ELISA kit (EA-0301; Signosis,
Sunnyvale, CA, USA) according the manufacturer’s instructions on
preoperative day 1, 1 h post surgery and postoperative days 1, 2, 6
and 9.
Genotyping
Three single nucleotide polymorphisms (SNPs),
1075A/G (rs1978124), 8790A/G (rs2285666) and 16854G/C (rs4646142)
were selected as proxies to investigate the ACE2 polymorphisms as
previously described (14).
Quality control was performed by sequencing the three SNPs in 80
randomly selected subjects from the study cohort; the discrepancy
rate was 1.25%.
Statistical analysis
Serum ACE2 levels were divided into quartile
categories; ≤1.06, 1.07–1.42, 1.43–1.85 and ≥1.86 ng/ml. The
adjusted hazard ratios (HRs) and the 95% confidence intervals (CIs)
were calculated using the Cox proportional hazard model. The
continuous variable values were expressed as the mean ± standard
deviation and comparisons between the means of the two groups were
performed using Student’s t-test. The categorical variables were
expressed as n (%) and analyzed using the χ2 test.
Statistical analysis was performed using SPSS version 10.0 (SPSS
Inc., Chicago, IL, USA). The statistical significance level of this
study was set at a two-tailed α=0.05.
Results
Serum ACE2
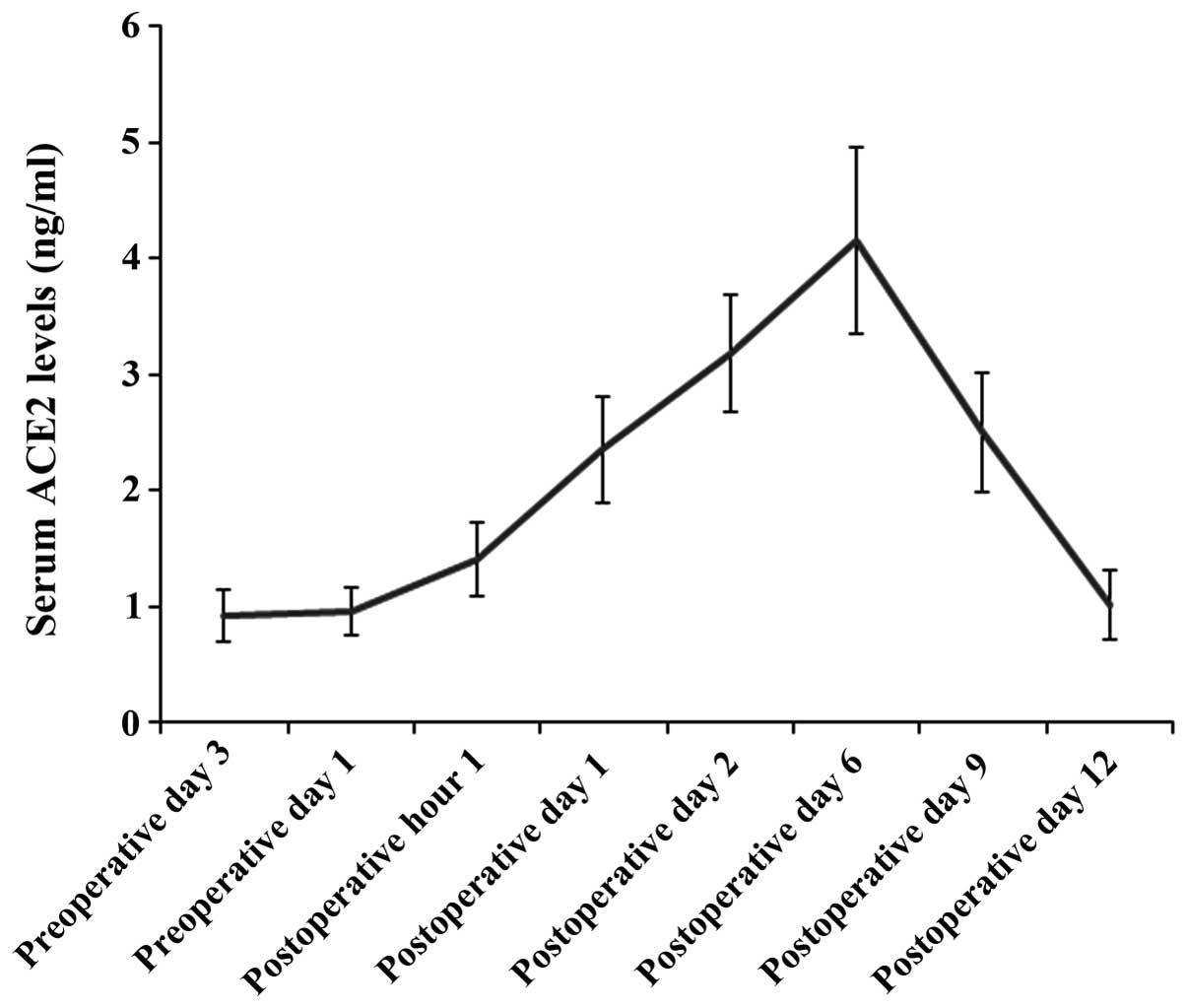
The serum ACE2 levels were measured in the blood
samples collected on preoperative days 3 and 1, 1 h post surgery,
and postoperative days 1, 2, 6, 9 and 12. The serum ACE2 level
observed in the patients who underwent CABG with CPB, was at
baseline level prior to surgery and began to rise 1 h post CABG;
six days after surgery, the serum ACE2 levels peaked and
subsequently returned to baseline 12 days post surgery (Fig. 1). To investigate the prognostic
value of the serum ACE2 level for postoperative MI following CABG,
the serum ACE2 level was analyzed 1 h post surgery; this was the
earliest time point at which the serum ACE2 level began to rise
above baseline following CABG. The serum ACE2 levels that were
observed 1 h post surgery, were divided into quartile categories;
≤1.06, 1.07–1.42, 1.43–1.85 and ≥1.86 ng/ml. No statistically
significant differences were identified between the quartile
categories in age, gender, body mass index (BMI), current smoking
status, unstable angina, prior MI and prevalence of hyperlipidemia,
hypertension and diabetes mellitus (Table I). Furthermore, there were no
statistically significant differences observed between the quartile
categories regarding disease pattern, procedural characteristics
and pre-CABG medication use (Table
II).
 | Table IGeneral characteristics of study
subjects. |
Table I
General characteristics of study
subjects.
| Serum ACE2 quartile
categories, ng/ml (n=34) | |
|---|
|
| |
|---|
| ≤1.06 | 1.07–1.42 | 1.43–1.85 | ≥1.86 | P-value |
|---|
| Age (years) | 63.5±4.5 | 64.2±3.8 | 63.2±3.4 | 63.7±4.2 | 0.29 |
| Gender | | | | | 0.83 |
| Male, n (%) | 27 (79.4) | 29 (85.3) | 26 (76.5) | 27 (79.4) | |
| Female, n (%) | 7 (20.6) | 5 (14.7) | 8 (23.5) | 7 (20.6) | |
| Unstable angina | 14 (41.2) | 15 (44.1) | 13 (38.2) | 11 (32.4) | 0.78 |
| Hyperlipidemia, n
(%) | 31 (91.2) | 31 (91.2) | 32 (94.1) | 30 (88.2) | 0.87 |
| Hypertension, n
(%) | 22 (64.7) | 21 (61.8) | 23 (67.6) | 18 (52.9) | 0.63 |
| Diabetes mellitus, n
(%) | 6 (17.6) | 5 (14.7) | 7 (20.6) | 5 (14.7) | 0.90 |
| Prior MI | 9 (26.5) | 10 (29.4) | 8 (23.5) | 8 (23.5) | 0.94 |
| BMI
(kg/m2) | 28.7±4.8 | 27.9±6.0 | 29.3±5.2 | 28.1±4.9 | 0.27 |
| Current smoker | 8 (23.5) | 12 (35.3) | 9 (26.5) | 11 (32.4) | 0.70 |
 | Table IIDisease and procedural characteristics
of study subjects. |
Table II
Disease and procedural characteristics
of study subjects.
| Serum ACE2 quartile
categories, ng/ml (n=34) | |
|---|
|
| |
|---|
| ≤1.06 | 1.07–1.42 | 1.43–1.85 | ≥1.86 | P-value |
|---|
| Disease pattern |
| LM or LM+1 vessel, n
(%) | 3 (8.8) | 4 (11.8) | 3 (8.8) | 2 (5.9) | 0.87 |
| LM+2 or 3 vessels, n
(%) | 5 (14.7) | 4 (11.8) | 4 (11.8) | 3 (8.8) | 0.90 |
| Two vessel, n
(%) | 8 (23.5) | 9 (26.5) | 8 (23.5) | 6 (17.6) | 0.85 |
| Three vessel, n
(%) | 18 (52.9) | 17 (50.0) | 19 (55.9) | 23 (67.6) | 0.48 |
| Lesions (n) | 3.1±0.2 | 3.0±0.3 | 3.0±0.3 | 3.2±0.4 | 0.83 |
| Gensini score | 37.5±4.6 | 37.3±5.1 | 37.4±4.8 | 37.0±3.9 | 0.92 |
| Procedural
characteristics |
| On-pump, n (%) | 34 (100.0) | 34 (100.0) | 34 (100.0) | 34 (100.0) | 1.00 |
| CPB time (mins) | 49.5±3.9 | 52.3±4.2 | 54.2±3.9 | 53.6±4.5 | 0.41 |
| Aortic clamp time
(mins) | 32.5±1.9 | 31.9±2.0 | 33.2±2.1 | 32.9±2.7 | 0.53 |
| Grafts (n) | 2.5±0.2 | 2.4±0.2 | 2.5±0.1 | 2.5±0.2 | 0.95 |
| LIMA use, n (%) | 30 (88.2) | 31 (91.2) | 31 (91.2) | 30 (88.2) | 0.96 |
| SVG use, n (%) | 30 (88.2) | 31 (91.2) | 31 (91.2) | 30 (88.2) | 0.96 |
| Free arterial
graft use, n (%) | 4 (11.8) | 3 (8.8) | 3 (8.8) | 4 (11.8) | 0.96 |
| Pre-CABG
medication |
| Aspirin, n
(%) | 31 (91.2) | 32 (94.1) | 30 (88.2) | 31 (91.2) | 0.87 |
| Clopidogrel, n
(%) | 4 (11.8) | 6 (17.6) | 5 (14.7) | 5 (14.7) | 0.93 |
| Statin, n (%) | 31 (91.2) | 31 (91.2) | 30 (88.2) | 32 (94.1) | 0.87 |
| β-blocker, n
(%) | 26 (76.5) | 28 (82.4) | 27 (79.4) | 25 (73.5) | 0.84 |
| ACE inhibitor/ARB,
n (%) | 21 (61.8) | 19 (55.9) | 19 (55.9) | 20 (58.8) | 0.95 |
Postoperative MI increases following a
decrease in the levels of serum ACE2
Postoperative MI increased as the serum ACE2 level
decreased and the risk was identified to be significantly higher in
the first quartile group when compared with the second, third and
fourth quartile groups (Model 1; Table III). Following adjustment for
age, gender, BMI, hypertension, prior MI, current smoking status,
hyperlipidemia, diabetes mellitus, Gensini score, aortic clamp
time, number of grafts and pre-CABG medications, the risk of
developing postoperative MI following CABG was significantly higher
in the lowest serum ACE2 level quartile group, compared with the
highest quartile group (hazard ratio, 2.94; 95% CI, 1.85–4.16;
P=0.009). Analysis of serum ACE2 levels on postoperative days 1, 2,
6 and 9 resulted in values that were not statistically significant
(data not shown). All of the subjects exhibited significant
negative correlation between serum ACE2 and cTnI levels 1 h after
surgery, and on days 1, 2, 6 and 9 post surgery, ranging between
r=−0.525 (1 h prior to surgery; P<0.001) and r=−462
(postoperative day 9; P<0.001).
 | Table IIIAdjusted HRs of postoperative MI by
serum ACE2 levels. |
Table III
Adjusted HRs of postoperative MI by
serum ACE2 levels.
| Serum ACE2 quartile
categories, ng/ml (n=34) | P-value for trend
(across categories) | Continuous log
scalea | P-value for trend
(continuous) |
|---|
|
|---|
| ≤1.06 | 1.07–1.42 | 1.43–1.85 | ≥1.86 |
|---|
| Postoperative MI, n
(%) | 9 (26.5) | 5 (14.7) | 4 (11.8) | 2 (5.9) | | | |
| Model 1b HR (95% CI) | 5.76
(1.14–29.08) | 2.76
(0.50–15.33) | 2.13
(0.36–12.51) | 1 (Reference) | <0.001 | 2.51
(2.06–2.92) | <0.001 |
| Model 2c HR (95% CI) | 2.94
(1.85–4.16) | 1.49
(0.92–2.58) | 1.19
(0.57–2.11) | 1 (Reference) | 0.009 | 1.92
(1.53–2.27) | 0.007 |
Association between low serum ACE2 levels and
typical morbidities postCABG. The association between the lowest
serum ACE2 level quartile group (≤1.06 ng/ml) and the incidence of
typical morbidities post CABG, including postoperative MI,
arrhythmia, low cardiac output, pulmonary complications,
neurological complications and excessive bleeding was investigated.
In the analysis, the second, third and fourth quartiles of serum
ACE2 levels were combined into a single category (>1.06 ng/ml;
n=102) to enable comparison with the first serum ACE2 level
quartile group (≤1.06 ng/ml; n=34). The subjects exhibiting a serum
ACE2 level ≤1.06 ng/ml indicated significantly higher rates of
postoperative MI, arrhythmia and reduced cardiac output, as well as
greater in-hospital mortality following CABG, compared with those
exhibiting a serum ACE2 level >1.06 ng/ml (Table IV).
 | Table IVACE2 levels with postoperative
morbidities and in-hospital mortality. |
Table IV
ACE2 levels with postoperative
morbidities and in-hospital mortality.
| | Serum ACE2 quartile
categories (ng/ml) | |
|---|
| |
| |
|---|
| Total (n=136) | ≤1.06 (n=34) | >1.06
(n=102) | P-value |
|---|
| Postoperative
MI | 20 (14.7) | 9 (26.5) | 11 (10.8) | 0.046a |
| Arrhythmia | 37 (27.2) | 14 (41.2) | 23 (22.5) | 0.045a |
| Low cardiac
output | 17 (12.5) | 8 (23.5) | 9 (8.8) | 0.036a |
| Pulmonary
complications | 21 (15.4) | 8 (23.5) | 13 (12.7) | 0.170 |
| Neurologic
complications | 6 (4.4) | 2 (5.9) | 4 (3.9) | 0.640 |
| Excessive
bleeding | 3 (2.2) | 1 (2.9) | 2 (2.0) | 1.000 |
| In-hospital
mortality | 6 (4.4) | 4 (11.8) | 2 (2.0) | 0.034a |
ACE2 polymorphisms
ACE2 polymorphisms are reportedly associated with
MI; Yang et al (14)
reported that ACE2 SNPs, 1075A/G (rs1978124), 8790A/G (rs2285666)
and 16854G/C (rs4646142) were associated with MI (14). Association analysis that was
conducted with female subjects indicated that the 1075AA and
16854GG genotypes were significantly associated with MI (P<0.05)
and that the 8790AA genotype was associated with MI at a
non-significant level (P=0.058). In the male subjects, the
1075A/G-8790A/G and 16854G/C haplotype GGC was significantly
associated with MI, when compared with the most common haplotype
AAG (P<0.05) (14). To
determine whether those ACE2 polymorphisms were associated with the
serum ACE2 level in the present study, the association between the
serum ACE2 level and the ACE2 genotypes and haplotypes, reportedly
associated with MI, were examined in all of the subjects. As the
ACE2 gene is located on the X chromosome, the association analysis
was conducted by gender. There was no significant association
observed between the serum ACE2 level quartile groups and the ACE2
1075AA, 16854GG or 8790AA genotypes in the female subjects, or the
ACE2 1075A/G-8790A/G and 16854G/C haplotype GGC in the male
subjects (Table V). The results
indicated that the observed association between the serum ACE2
level and postoperative MI following CABG, in the present study,
was not a result of ACE2 gene polymorphisms.
 | Table VACE2 polymorphisms and serum ACE2
levels. |
Table V
ACE2 polymorphisms and serum ACE2
levels.
| A, Female
(n=27) |
|---|
|
|---|
| Serum ACE2 quartile
categories (ng/ml) | | |
|---|
|
| | |
|---|
| ACE2
polymorphisms | ≤1.06 | 1.07–1.42 | 1.43–1.85 | ≥1.86 | Total | P-value |
|---|
| 1075A/G
(rs1978124) | | | | | | 0.87 |
| AA | 3 | 2 | 2 | 2 | 9 | |
| Non-AA | 4 | 3 | 6 | 5 | 18 | |
| Total | 7 | 5 | 8 | 7 | 27 | |
| 8790A/G
(rs2285666) | | | | | | 0.85 |
| AA | 2 | 1 | 3 | 3 | 9 | |
| Non-AA | 5 | 4 | 5 | 4 | 18 | |
| Total | 7 | 5 | 8 | 7 | 27 | |
| 16854G/C
(rs4646142) | | | | | | 0.73 |
| GG | 3 | 3 | 4 | 2 | 12 | |
| Non-GG | 4 | 2 | 4 | 5 | 15 | |
| Total | 7 | 5 | 8 | 7 | 27 | |
|
| B, Male
(n=109) |
|
| Serum ACE2 quartile
categories (ng/ml) | | |
|
| | |
| ACE2
polymorphisms | ≤1.06 | 1.07–1.42 | 1.43–1.85 | ≥1.86 | Total | P-value |
|
| 1075A/G-8790A/G and
16854G/C haplotype | | | | | | 0.57 |
| GGC | 3 | 7 | 4 | 6 | 20 | |
| Non-GGC | 24 | 22 | 22 | 21 | 89 | |
| Total | 27 | 29 | 26 | 27 | 109 | |
Discussion
MI is a predominant and severe complication in
patients undergoing aortocoronary bypass surgery. Early diagnosis
and prediction of MI is an important condition for optimal
postoperative patient management. Previous studies have indicated
that ACE2 activation is a protective mechanism in MI and that
supplementing ACE2 may be a potential therapy for MI/ischemic heart
disease (8–11,15).
In the present study, the results indicated that the serum ACE2
level was associated with postoperative MI and in-hospital
mortality following CABG.
The serum ACE2 level remained at baseline level
preoperatively, began to rise 1 h post CABG, peaked on day 6 post
surgery and returned to baseline level on day 12, post surgery. As
MI can occur within 24 h of CABG, the serum ACE2 level 1 h post
surgery was analyzed to evaluate the potential prognostic value of
the serum ACE2 level for postoperative MI following CABG. The
association analysis results 1 h post surgery were significant
compared with those at other time points. According to previous
studies, the duration of aortic cross-clamping, number of coronary
grafts and history of previous cardiac surgery were independent
predictors of postoperative MI (1). In addition, ACE inhibitors and
angiotensin receptor blockers are capable of enhancing ACE2
expression (16,17). Thus, the hazard ratio was adjusted
for prior MI, aortic clamp time, number of grafts and pre-CABG
medications, in addition to a variety of other confounding factors
that may have affected the incidence of postoperative MI and/or
serum ACE2 levels, including age, gender, BMI, hypertension,
current smoking status, hyperlipidemia, diabetes mellitus and
Gensini score. After adjusting for the confounding factors, the
risk of developing postoperative MI remained significantly higher
in the lowest serum ACE2 level quartile group than in the highest
quartile group, 1 h post surgery, indicating that the serum ACE2
level 1 h post surgery may be an independent risk factor for
postoperative MI following CABG. Thus, the serum ACE2 level 1 h
post surgery may be a potential novel biomarker or prognostic
factor for postoperative MI following CABG; its value in clinical
applications may be investigated in future studies using a larger
patient population.
Postoperative MI, following cardiac surgery is
associated with reduced long-term survival rates (1). In the present study, the serum ACE2
level in the lowest quartile group was observed to be associated
with the increased rate of postoperative MI, arrhythmia, low
cardiac output and in-hospital mortality, indicating that the serum
ACE2 level may be a potential novel prognostic factor for
short-term survival following cardiac surgery. A prospective study,
using the same patient cohort, is being conducted to investigate
the association between the serum ACE2 level, postoperative MI and
long-term survival rate following CABG.
cTnI, a contractile protein unique to the heart
muscle, is a sensitive biomarker, which was introduced
predominantly for risk stratification in patients exhibiting acute
coronary syndrome and is the gold standard for identifying MI
(18,19); in addition, ACE2 is expressed in
the heart and has been observed to exhibit a protective effect
during MI (8–11). Increasing evidence indicates that
the expression of cardiac ACE2 is higher following MI, which
combats the adverse effects of an activated cardiac RAS and,
therefore, may be a compensatory mechanism in MI (8–11,15,20).
In agreement with this, a significant increase in the ACE2 serum
level following MI was observed in the present study. Notably,
postoperative serum ACE2 and cTnI levels exhibited significant
negative correlation, which confirmed that ACE2 protects against
MI; therefore, a relatively low serum ACE2 level shortly after
CABG, may indicate a deficient protective mechanism against MI.
This may explain why the risk of developing postoperative MI was
significantly greater in the lowest serum ACE2 level quartile group
following CABG. The troponin system has been observed to be
extremely sensitive, however, not definitive (21). Therefore, future investigations are
required to identify whether the serum ACE2 level may be a more
specific marker than cardiac troponin levels for detecting
postoperative MI.
Polymorphisms in the ACE2 gene are associated with
the development of pathological myocardial hypertrophy and heart
disease in humans (14). No
significant associations were identified between the serum ACE2
level quartile groups and the ACE2 genotypes and haplotypes, in the
present study, which were identified to be associated with MI in
previous studies (14). Although
the sample size may not be adequate for a definitive answer to
address this issue (particularly in the female group), the findings
reduced the possibility that the observed association between the
serum ACE2 level and postoperative MI following CABG was a result
of ACE2 polymorphisms.
The strength of the present study was due to the use
of a relatively large sample size of patients who underwent CABG
and the analysis results were adjusted for multiple relevant
factors, including aortic clamp time, number of grafts and pre-CABG
medication use. The findings indicated that post surgery, specific
attention should be given to CABG patients exhibiting a relatively
low serum ACE2 level (when based on an established
population-specific normal referencing range) shortly after
undergoing CABG, as there is a tendency to develop postoperative MI
following CABG. In addition, the findings indicate that
supplementing ACE2 levels may be a potential novel therapy for
postoperative MI following CABG. The limitation of the present
study was that it was conducted only with patients undergoing CABG
with a CPB, and patients undergoing off-pump CABG was not included.
This was because CABG with a CPB was the predominant type of CABG
surgery with which we were able to recruit for an adequate sample
size. The association between the serum ACE2 level and
postoperative MI following off-pump CABG will be investigated in
future studies, based on accumulating a sample of appropriate
patients.
In conclusion, the present study indicated that the
serum ACE2 level 1 h post CABG was independently associated with an
increased risk of postoperative MI. Thus, observations of the serum
ACE2 level may be a potential novel prognostic factor for
postoperative MI, following CABG.
References
|
1
|
Podgoreanu MV, White WD, Morris RW, et al;
Perioperative Genetics and Safety Outcomes Study (PEGASUS)
Investigative Team. Inflammatory gene polymorphisms and risk of
postoperative myocardial infarction after cardiac surgery.
Circulation. 114(1 Suppl): I275–I281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nathoe HM, van Dijk D, Jansen EW, et al;
Octopus Study Group. A comparison of on-pump and off-pump coronary
bypass surgery in low-risk patients. N Engl J Med. 348:394–402.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Domenighetti AA, Wang Q, Egger M, Richards
SM, Pedrazzini T and Delbridge LM: Angiotensin II-mediated
phenotypic cardiomyocyte remodeling leads to age-dependent cardiac
dysfunction and failure. Hypertension. 46:426–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazzolai L, Pedrazzini T, Nicoud F,
Gabbiani G, Brunner HR and Nussberger J: Increased cardiac
angiotensin II levels induce right and left ventricular hypertrophy
in normotensives mice. Hypertension. 35:985–991. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vickers C, Hales P, Kaushik V, Dick L,
Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, et al:
Hydrolysis of biological peptides by human angiotensin-converting
enzyme-related carboxypeptidase. J Biol Chem. 277:14838–14843.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Der Sarkissian S, Huentelman MJ, Stewart
J, Katovich MJ and Raizada MK: ACE2: A novel therapeutic target for
cardiovascular diseases. Prog Biophys Mol Biol. 91:163–198.
2006.PubMed/NCBI
|
|
7
|
Raizada MK and Ferreira AJ: ACE2: a new
target for cardiovascular disease therapeutics. J Cardiovasc
Pharmacol. 50:112–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burrell LM, Risvanis J, Kubota E, et al:
Myocardial infarction increases ACE2 expression in rat and humans.
Eur Heart J. 26:369–375; discussion 322–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamming I, Cooper ME, Haagmans BL, et al:
The emerging role of ACE2 in physiology and disease. J Pathol.
212:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Der Sarkissian S, Grobe JL, Yuan L,
Narielwala DR, Walter GA, Katovich MJ and Raizada MK: Cardiac
overexpression of angiotensin converting enzyme 2 protects the
heart from ischemia-induced pathophysiology. Hypertension.
51:712–718. 2008.PubMed/NCBI
|
|
11
|
Zhao YX, Yin HQ, Yu QT, et al: ACE2
overexpression ameliorates left ventricular remodeling and
dysfunction in a rat model of myocardial infarction. Hum Gene Ther.
21:1545–1554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ortiz Pérez JT, Riera M, Genover XB, De
Caralt TM, et al: Serum ACE2 activity correlates with infarct size
and left ventricular dysfunction during acute myocardial
infarction. J Cardiovasc Magn Reson. 13(Suppl 1): P1422011.
|
|
13
|
Thygesen K, Alpert JS and White HD; Joint
ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial
Infarction. Universal definition of myocardial infarction. J Am
Coll Cardiol. 50:2173–2195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang W, Huang W, Su S, Li B, Zhao W, Chen
S and Gu D: Association study of ACE2 (angiotensin I-converting
enzyme 2) gene polymorphisms with coronary heart disease and
myocardial infarction in a Chinese Han population. Clin Sci (Lond).
111:333–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kassiri Z, Zhong J, Guo D, et al: Loss of
angiotensin-converting enzyme 2 accelerates maladaptive left
ventricular remodeling in response to myocardial infarction. Circ
Heart Fail. 2:446–455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keidar S, Gamliel-Lazarovich A, Kaplan M,
et al: Mineralocorticoid receptor blocker increases
angiotensin-converting enzyme 2 activity in congestive heart
failure patients. Circ Res. 97:946–953. 2005. View Article : Google Scholar
|
|
17
|
Ferrario CM, Jessup J, Chappell MC, et al:
Effect of angiotensin.converting enzyme inhibition and angiotensin.
II receptor blockers on cardiac angiotensin.converting enzyme.2.
Circulation. 111:2605–2610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alyanakian MA, Dehoux M, Chatel D, et al:
Cardiac troponin I in diagnosis of perioperative myocardial
infarction after cardiac surgery. J Cardiothorac Vasc Anesth.
12:288–294. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim CC, Cuculi F, van Gaal WJ, et al:
Early diagnosis of perioperative myocardial infarction after
coronary bypass grafting: a study using biomarkers and cardiac
magnetic resonance imaging. Ann Thorac Surg. 92:2046–2053. 2011.
View Article : Google Scholar
|
|
20
|
Burchill LJ, Velkoska E, Dean RG, Griggs
K, Patel SK and Burrell LM: Combination renin-angiotensin system
blockade and angiotensin-converting enzyme 2 in experimental
myocardial infarction: implications for future therapeutic
directions. Clin Sci (Lond). 123:649–658. 2012. View Article : Google Scholar
|
|
21
|
Mahajan VS and Jarolim P: How to interpret
elevated cardiac troponin levels. Circulation. 124:2350–2354. 2011.
View Article : Google Scholar : PubMed/NCBI
|