Introduction
Immunoglobulin A nephropathy (IgAN), also termed
Berger’s disease, is the most common glomerulopathy worldwide and
accounts for between 10 and 40% of cases of glomerulonephritis. It
is a renal-limited form of glomerulonephritis, characterized by the
deposition of IgA-containing immune deposits in the glomerular
mesangium. In many of these conditions, IgA is deposited in the
glomerulus without inducing inflammation, and this may be a
clinically insignificant consequence of perturbed IgA homeostasis.
The disease is particularly common in southern Europe and Asia and
appears to be more common in Caucasians compared with individuals
of African descent. The disease has also been reported in Native
Americans, however, rarely. Patients with IgAN typically present
with gross hematuria, often between 24 and 48 h following a
pharyngeal or gastrointestinal infection, vaccination or strenuous
exercise. Other cases are diagnosed upon detection of microscopic
hematuria during routine physical examinations. Hypertension and
nephrotic syndrome are unusual at presentation. Light microscopy of
renal biopsy specimens typically shows mesangial expansion due to
an increase in the size of the matrix and cells.
Previous studies have demonstrated that
erythropoietin (EPO), which is primarily produced and released by
peritubular capillary lining cells within the kidney, is able to
regulate erythrocyte generation. Furthermore, EPO protects renal
cells, however, the mechanism by which it does this remains to be
elucidated. Although EPO is considered to be a promising candidate
for the treatment of nephrological disorders, the half-life of EPO
is too short to be effective for the treatment of IgAN. EPO
production is stimulated by the availability of O2 for
tissue metabolism. EPO facilitates the delivery of O2 by
increasing the production of red blood cells. Impaired
O2 delivery to the kidney, liver and brain may result
from an increase in EPO production (1). In addition, EPO reduces apoptosis and
oxidative stress in numerous pathological processes (2,3). It
has been demonstrated that pretreated EPO has a marked protective
effect against organ injuries, including the heart, brain and
kidney (4–6). However, over-treatment with EPO
results in uncontrolled proliferation of red blood cells and high
blood viscosity. Attenuating the side effects of EPO treatment is
an important problem in clinical studies.
Due to advances in nanotechnology, the half-life of
the polypeptide drug may be increased and the side-effects
attenuated. It has been previously demonstrated by Fayed et
al (7) that poly
lactic-co-glycolic acid (PLGA) nanoparticles containing EPO may
significantly prolong its activity. It has also been demonstrated
in the treatment of hypoxia and anemia in a newborn rat model, that
the effect of treatment with EPO nanoparticles is 10 times greater
compared with regular EPO treatment (8), suggesting that nanotechnology with
EPO delivery is able to significantly enhance its therapeutic
effects.
Chitosan (CS) is a common biodegradable multimer
that exists widely in nature. It shows a high bioactivity. Previous
studies have used CS containing peptides, proteins and
water-soluble small molecules in numerous disease models (9–11).
In the present study, CS and tripolyphosphate (TPP) nanoparticles
containing EPO using an ionotropic gelation system were developed.
The effect of CS-TPP-EPO nanoparticles in a rat IgAN model was then
investigated.
Materials and methods
Preparation and characterization of the
CS-TPP nanoparticles
A 1% CS solution (Mw, 550,000; deacetylation degree,
90%; Haidebei Co., Jinan, Shandong, China) with acetic acid was
prepared at room temperature. Using magnetic stirring, TPP solution
(China National Pharmaceutical Group Shanghai Chemical Reagent Co.,
Shanghai, China) was added dropwise into the CS solution using a 1
ml syringe, and the pH was adjusted to 5.7. Following 1 h, the
solution was centrifuged at 30,000 × g for 10 min. The solution was
washed with ethanol using a gradient concentration and then
lyophilized to obtain CS/TPP nanoparticles. The CS-TPP
nanoparticles were observed using a scanning electron microscope
(SEM; Hitachi 2s100; Hitachi, Tokyo, Japan). The CS/TPP
nanoparticles were dispersed in ethanol and the capsule size and
distribution of the CS/TPP nanoparticles were measured using a
particle size analyzer (Siemens, Munich, Germany).
Determination of the encapsulation
efficiency (EE) in the CS microcapsule and in vitro release
assay
To develop EPO-containing nanoparticles, EPO powder
(Sigma, St. Louis, MO, USA) was dissolved in CS solution (EPO and
CS mass ratio of 1:1) prior to the addition of TPP. A total of 10
mg encapsulated dried CS-TPP-EPO nanoparticles were placed in 20 ml
phosphate-buffered saline (pH 7.2) and agitated at 120 × g at 37
°C. A total of 0.5 ml supernatant was aspirated at 5 min intervals
and the EPO levels were measured using the Coomassie blue protein
assay kit. All experiments were repeated three times.
The CS/TPP nano microcapsules encapsulating rate was
calculated using the following formula: EE% = (TEPO -
SEPO) / TEPO × 100% (where EE is the
encapsulation efficiency, TEPO is the total content and
SEPO is the EPO content in the supernatant).
Establishment of the rat IgAN model
A total of 30 female Sprague-Dawley rats, weighing
between 160 and 200 g were purchased from the Experimental Animal
Center of Xinxiang Medical University (Xinxiang, Henan, China). The
present study was performed in accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals from the
National Institutes of Health (Bethesda, MD, USA). The animal
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of the Third Affiliated Hospital of Xinxiang
Medical University. To generate an IgAN model, rats were
administered 200 mg/kg bovine serum albumin (BSA) every other day
for a total of 14 weeks. In addition, a total of 0.2 ml complete
Freund’s adjuvant (including 2 mg BSA) was subcutaneously injected
on day 1. A total of 0.2 ml incomplete Freund’s adjuvant (including
2 mg BSA) was intraperitoneally injected on days 14 and 28.
Staphylococcal enterotoxin B (SEB; Academy of Military Medical
Sciences Institute of the PLA microbial production, lot number
061030) was then intravenously injected (0.4 mg/kg) following 8–10
weeks. The alterations in histopathological staining, BUN and Cr in
the IgAN model were then analyzed.
In vivo experiments of CS-TPP-EPO
A total of 30 IgAN rats were randomly divided into
three groups: the CS-TPP group treated without EPO loading, the
CS-TPP-EPO group treated with packaged EPO (3,000 IU/kg) delivery
nanoparticles and the EPO group treated with EPO directly. Each
group was treated every other day for 2 weeks. Serum was collected
every week to analyze the changes in blood urea nitrogen (BUN) and
creatinine (Cr) levels using a Biochemical Analyzer (Hitachi,
Tokyo, Japan).
Statistical analysis
SPSS 16.0 (SPSS, Inc, Chicago, IL, USA) was used for
statistical analysis and the data were analyzed using analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification and characterization of
nanoparticles
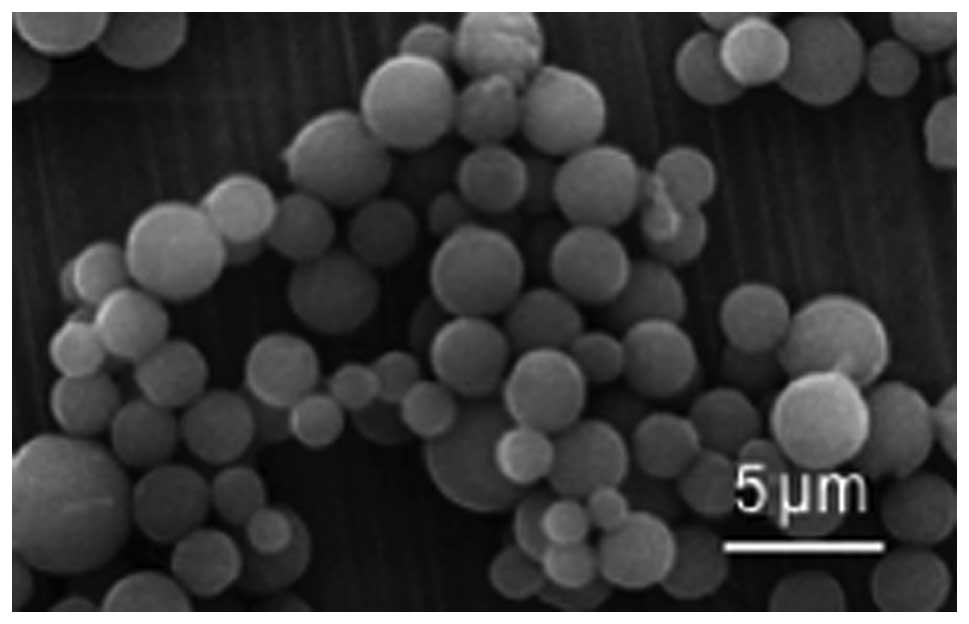
The nanoparticles were observed using SEM. It was
found that the CS-TPP-EPO nanoparticles had a smooth surface with a
relatively uniform particle diameter of 485±12 nm (Fig. 1). According to the data in Table I, the amount of EPO in CS-TPP
nanoparticles was raised with increasing concentrations of CS.
However, the EE decreased when the concentration of CS exceeded a
certain value. The release properties of CS-TPP-EPO nanoparticles
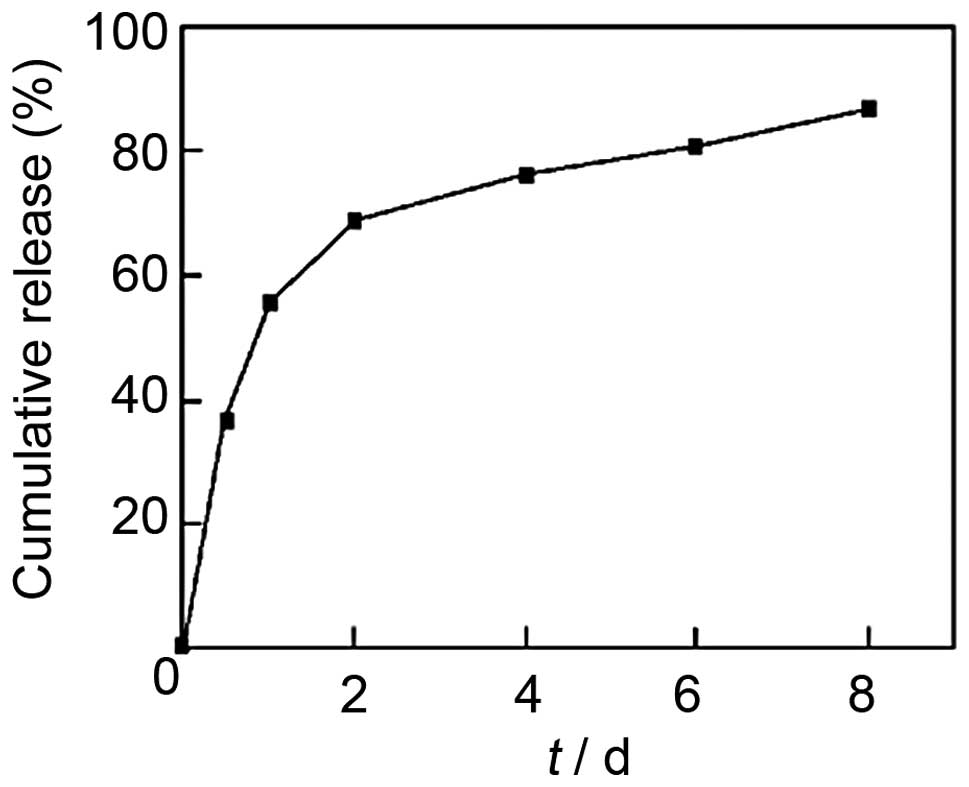
with 78.45% EE were then investigated. As shown in Fig. 2, the release curve of CS-TPP-EPO
microcapsules demonstrated a biphasic release: an early violent
release phase and a slow release phase. Furthermore, the release of
EPO from nanoparticles was sustained for up to seven days.
 | Table IEE of bovine serum albumin-loading in
CS/TPP nanoparticles prepared with different concentrations of CS
and EPO (0.5% TPP; pH 5.7; weight ratio of CS to EPO, 1:1). |
Table I
EE of bovine serum albumin-loading in
CS/TPP nanoparticles prepared with different concentrations of CS
and EPO (0.5% TPP; pH 5.7; weight ratio of CS to EPO, 1:1).
| Concentration of CS
(% w/v) | Weight of EPO | EE (%) |
|---|
| 0.125 | 0.0375 | 25.10 |
| 0.25 | 0.75 | 78.45 |
| 0.5 | 0.15 | 74.63 |
| 1.0 | 0.3 | 63.22 |
Effect of CS-TPP-EPO nanoparticles in the
rat IgAN model
The BUN and Cr levels in the EPO and CS-TPP-EPO
groups were significantly decreased compared with the untreated
rats, whilst the hemoglobin (Hb) levels were increased compared
with the untreated rats. By contrast, these alterations were not
observed in the CS-TPP group. The treatment of nanoparticles loaded
with EPO (CS-TPP-EPO group) was more effective compared with direct
EPO injection (EPO group). Although these differences were all
detected between the first and third week, the changes in
concentrations showed a gradient accumulation. Following the end of
the treatments, the levels of BUN, Cr and Hb in the EPO group
decreased slightly, whilst the levels were maintained at a stable
level in the CS-TPP-EPO group (Table
II).
 | Table IIEvaluation of BUN, Cr and Hb during
the treatment at different time points. |
Table II
Evaluation of BUN, Cr and Hb during
the treatment at different time points.
| Week 0 | Week 1 | Week 2 | Week 3 |
|---|
|
|
|
|
|
|---|
| Group | BUN (mmol/l) | Cr (μmol/l) | Hb (g/dl) | BUN (mmol/l) | Cr (μmol/l) | Hb (g/dl) | BUN (mmol/l) | Cr (μmol/l) | Hb (g/dl) | BUN (mmol/l) | Cr (μmol/l) | Hb (g/dl) |
|---|
| EPO | 255.66±3.46 | 51.04±15.84 | 13.75±1.57 | 135.78±4.23 | 32.08±6.35 | 15.23±0.37 | 118.25±4.24 | 25.07±3.14 | 16.57±2.16 | 165.38±4.28 | 40.12±8.26 | 14.58±0.45 |
| CS-TPP | 238.35±4.11 | 49.67±12.38 | 13.47±1.28 | 252.43±4.32 | 53.26±5.85 | 12.82±3.25 | 258.28±3.39 | 55.21±6.21 | 11.85±0.87 | 264.32±3.92 | 57.34±10.3 | 11.05±0.65 |
| CS-TPP-EPO | 240.45±4.37 | 49.87±14.58 | 12.86±1.08 | 126.42±3.72a | 29.92±7.04b | 14.78±0.44c | 120.20±3.22a | 23.59±11.48b | 15.25±0.58c | 115.46±3.27a | 22.37±12.6b | 15.30±0.58c |
Discussion
Previous studies and clinical evidence have
demonstrated that EPO and its derivatives are important in renal
protection during chronic kidney disease. Eren et al
(12) applied EPO treatment in
rats with sepsis. The results demonstrated that EPO had a renal
protective effect by reducing apoptosis (12). Another study confirmed that the
synthesis of nitric oxide as a result of EPO treatment is able to
protect rats with sepsis by inhibiting the nuclear factor-κB
pathway (13). However, treatment
with higher levels of EPO stimulates the proliferation of red blood
cells, leading to an increase in blood viscosity. This results in
high blood pressure and increases the risk of embolic stroke. Using
an alternative mechanism of delivery of EPO, particularly via
nanoparticle loading, EPO may potentially have a greater
therapeutic effect (14). It has
previously been demonstrated that poly lactic-co-glycolic acid
(PLGA) nanoparticles containing EPO may be maintained in
vivo for ≤14 days (7).
Furthermore, EPO with PLGA nanoparticles has been used for the
treatment of hypoxia and ischemia in neonatal rats (8), and improved results were found using
this technology compared with traditional EPO treatment (15).
In the present study, CS and sodium TPP were used to
encapsulate EPO. The size of the nanoparticles was ~485 nm and the
EPO EE was 78.45%. The in vitro release profile of
CS-TPP-EPO showed biphasic distribution, the day after the release
was 50%, after a slow release, on the eighth day release was 80%.
This indicates that CS-TPP nanoparticles exhibit good release
properties. Since EPO is a protein with biological activity, the
preparation process requires relatively mild conditions. In the
present study, the condition of crosslinking reaction between CS
and TPP was mild, did not react with EPO, and had the
characteristic of biocompatible, sustained release resistance, low
toxicity and biodegradability. Notably, in the present study the
entrapment efficiency achieved a peak value at 78.45%. It was
hypothesized that when the CS concentration exceeds the equilibrium
value, the viscosity is also increased in parallel. Therefore,
regular or spherical capsules are not formed as usual, resulting in
a decrease in the EPO encapsulation rate. In addition, sustained
EPO release is an important indicator for drug loading
microcapsules.
In the present study, an SEB and BSA prepared rat
model of IgAN was used to determine the therapeutic effect of
CS-TPP-EPO on IgAN. The results demonstrated that in the CS-TPP-EPO
treated group, BUN and Cr levels were significantly lower compared
with the CS-TPP group, whilst the quantity of Hb increased
significantly during the CS-TPP-EPO treatment. One week after
treatment, BUN, Cr and blood Hb in the CS-TPP-EPO group remained at
a stable concentration during treatment, however, in the EPO group
the values fluctuated. This indicated that CS-TPP-EPO may sustain
the release of nanoparticles in vivo, maintaining a high
concentration of EPO in the blood and improving renal function in a
rat model of IgAN.
In conclusion, to achieve an effective therapeutic
effect, CS-TPP nanoparticles may be novel protein or polypeptide
nanocarriers for EPO and other drugs.
References
|
1
|
Weidemann A and Johnson RS: Nonrenal
regulation of EPO synthesis. Kidney Int. 75:682–688. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toba H, Nakashima K, Oshima Y, et al:
Erythropoietin prevents vascular inflammation and oxidative stress
in subtotal nephrectomized rat aorta beyond haematopoiesis. Clin
Exp Pharmacol Physiol. 37:1139–1146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malgorzewicz S, Lichodziejewska-Niemierko
M, Lizakowski S, Liberek T, Lysiak-Szydlowska W and Rutkowski B:
Oxidative stress, inflammation and nutritional status during
darbepoetin alpha treatment in peritoneal dialysis patients. Clin
Nephrol. 73:210–215. 2010. View
Article : Google Scholar
|
|
4
|
Ozawa T, Toba K, Suzuki H, et al;
EPO/AMI-I Pilot Study Researchers. Single-dose intravenous
administration of recombinant human erythropoietin is a promising
treatment for patients with acute myocardial infarction -
randomized controlled pilot trial of EPO/AMI-1 study. Circ J.
74:1415–1423. 2010. View Article : Google Scholar
|
|
5
|
Haljan G, Maitland A, Buchan A, et al: The
Erythropoietin NeuroProtective Effect: Assessment in CABG Surgery
(TENPEAKS): a randomized, double-blind, placebo controlled,
proof-of-concept clinical trial. Stroke. 40:2769–2775. 2009.
View Article : Google Scholar
|
|
6
|
Song YR, Lee T, You SJ, et al: Prevention
of acute kidney injury by erythropoietin in patients undergoing
coronary artery bypass grafting: a pilot study. Am J Nephrol.
30:253–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fayed BE, Tawfik AF and Yassin AE: Novel
erythropoietin-loaded nanoparticles with prolonged in vivo
response. J Microencapsul. 29:650–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen H, Spagnoli F, Burris M, et al:
Nanoerythropoietin is 10-times more effective than regular
erythropoietin in neuroprotection in a neonatal rat model of
hypoxia and ischemia. Stroke. 43:884–887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagarwal RC, Singh PN, Kant S, Maiti P and
Pandit JK: Chitosan nanoparticles of 5-fluorouracil for ophthalmic
delivery: characterization, in-vitro and in-vivo study. Chem Pharm
Bull (Tokyo). 59:272–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohammadpourdounighi N, Behfar A, Ezabadi
A, Zolfagharian H and Heydari M: Preparation of chitosan
nanoparticles containing Naja naja oxiana snake venom.
Nanomedicine. 6:137–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Hu Y, Zhang L, Jiang L, Chen Z and
He N: Erythropoietin nanoparticles: therapy for cerebral ischemic
injury and metabolize in kidney and liver. Nano Biomed Eng.
2:31–39. 2010. View Article : Google Scholar
|
|
12
|
Eren Z, Coban J, Ekinci ID, Kaspar C and
Kantarci G: Evaluation of the effects of a high dose of
erythropoietin-beta on early endotoxemia using a rat model. Adv
Clin Exp Med. 21:321–329. 2012.PubMed/NCBI
|
|
13
|
Souza AC, Volpini RA, Shimizu MH, et al:
Erythropoietin prevents sepsis-related acute kidney injury in rats
by inhibiting NF-κB and upregulating endothelial nitric oxide
synthase. Am J Physiol Renal Physiol. 302:F1045–F1054.
2012.PubMed/NCBI
|
|
14
|
Bulmer C, Margaritis A and Xenocostas A:
Encapsulation and controlled release of recombinant human
erythropoietin from chitosan-carrageenan nanoparticles. Curr Drug
Deliv. 9:527–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khachane P, Date AA and Nagarsenker MS:
Eudragit EPO nanoparticles: application in improving therapeutic
efficacy and reducing ulcerogenicity of meloxicam on oral
administration. J Biomed Nanotechnol. 7:590–597. 2011. View Article : Google Scholar : PubMed/NCBI
|