Introduction
The mitogen-activated protein kinase (MAPK) pathways
are important signal transduction pathways that are key in many
metabolic processes (1,2). In mammals, three predominant MAPK
family members have been identified; extracellular signal-regulated
kinase (ERK), c-Jun N-terminal kinase (JNK; also known as
stress-activated protein kinase) and the p38 group of protein
kinases (3). The ERK pathway
participates in regulating cell differentiation, invasion,
metastasis and opposing apoptosis. p38 MAPK has been identified to
regulate microtubule polymerization and depolymerization (4). In addition, JNK and p38 predominantly
regulate apoptosis, differentiation, growth and inflammatory
responses (3).
JWA is a tumor suppressor gene that is commonly
present in a variety of human tissues and cultured cells. The
expression levels of JWA have been observed to be lower in
malignant tumor tissues compared with those in non-tumor tissues
(5–9). Furthermore, previous studies in mice
and cervical carcinoma HeLa cells in vitro have shown that
the expression level of JWA affects tumor proliferation, invasion
and apoptosis via the MAPK pathway (9,10).
However, to the best of our knowledge, the association between JWA
and MAPK pathways in human esophageal cell lines has not been
identified.
In the present study, the apoptosis, proliferation,
migration and invasion of Eca109 human esophageal squamous cell
carcinoma (ESCC) and normal human esophageal cells were observed
following JWA gene knockdown. In addition, the levels of JWA
protein and proteins associated with three major MAPK pathways were
detected to analyze the association between MAPK, JWA and
esophageal cancer.
Materials and methods
Materials
The Eca109 human ECSS and HET-1A human esophageal
epithelial cell lines were purchased from American Type Culture
Collection (Manassas, VA, USA) and cultured in RPMI-1640 culture
medium containing 10% fetal bovine serum (FBS), 100 μg/ml
streptomycin and 100 U/ml penicillin, in a 5% CO2
humidified atmosphere at 37°C. ERK1/2, JNK, p38, phosphorylated
(p)-ERK1/2, p-JNK, and p-p38 monoclonal antibodies were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). JWA, BAX
and Bcl-2 antibodies were purchased from Abcam (Cambridge, MA, USA)
and anti-mouse IgG (H+L) alkaline phosphatase (AP) conjugate was
purchased from Promega GmbH (Mannheim, Germany). JWA-small
interfering (si)RNA was purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA).
JWA-siRNA transfection
The cells were seeded in 6-well plates, with
antibiotic-free medium containing 10% FBS for 24 h, using
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) and siRNA to transfect the cells. Transfection
was conducted according to the instructions provided with
Lipofectamine 2000. Groups were established corresponding to 50,
100 and 150 nM concentrations of siRNA. A scrambled siRNA sequence
served as the negative control (NC) group, and there was also an
untreated cell group. The serum and antibiotic medium was replaced
after 6 h and the total protein was extracted after 48 h. Western
blot analysis was performed to identify the most effective
concentration of siRNA, which was selected for subsequent
experiments.
Thiazolyl blue tetrazolium bromide (MTT;
M2128; Sigma, St
Louis, MO, USA) assay. Eca109 and HET-1A cells
transfected with 150 nM (the most effective concentration)
JWA-siRNA were seeded in 96-well plates following 24 h of cell
growth. NC and untreated groups of Eca109 and HET-1A were
simultaneously seeded. After 48 h, an MTT assay was performed and
the absorbance of each well was detected at a wavelength of 570 nm
using a microplate reader (infinite F50; Tecan, Männedorf,
Switzerland).
Transwell assay
A Transwell assay (PIHT12R48; Millipore, Billerica,
MA, USA) was performed without a Matrigel™ basement membrane to
detect migration and with a Matrigel basement membrane to detect
invasion of Eca109 and HET-1A cells. Twenty-four-well plates and
Boyden chambers (diameter, 8 μm) were used in the present study;
the cells (2×105) were added to the upper chamber in a
serum-free medium and the culture medium, containing 10% FBS, was
added to the lower chamber. After incubating for 40 h at 37°C, the
cells in the upper chamber were carefully removed and the cells
from the reverse face of the membranes were harvested, fixed in
methanol, stained with Giemsa and counted.
Western blot assay
Equal amounts of protein were extracted from each
group, separated by SDS-PAGE electrophoresis and transferred to
nitrocellulose membranes. The membranes were blocked for 1 h with
5% bovine serum albumin in Tris-buffered saline/Tween-20 and
incubated with primary antibodies overnight at 4°C. Primary
antibodies included ERK1/2 (9102s), JNK (9252s), p38 (9212s),
phosphorylated (p)-ERK1/2 (9101s), p-JNK (9251s), and p-p38 (9211s)
monoclonal antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) and JWA (ab173223), BAX
(ab7977) and Bcl-2 (ab7973) antibodies were purchased from Abcam
(Cambridge, MA, USA). Secondary antibodies [AP-conjugated
anti-mouse IgG (S372B; Promega, Fitchburg, WI, USA) and
AP-conjugated anti-rabbit IgG (S372B; Promega)] were added and the
membranes were incubated for 2 h at room temperature. Enhanced
chemiluminescence (Lumi-Phos WB; 34150; Thermo Fisher Scientific,
Tewksbury, MA, USA) was used for film development, observations and
radiography. Protein levels were quantified by relative to tubulin,
the software used was Gel-Pro analyzer (Media Cybernetics Inc.,
Rockville,. MD, USA).
Statistical analysis
Statistical significance was analyzed using SPSS
software, version 14.0 (SPSS Inc., Chicago, IL, USA). The results
of the quantitative analysis were expressed as mean ± standard
deviation. The samples were compared using Student’s t-test or one
way analysis of variance and P<0.05 was considered to indicate a
statistically significant difference.
Results
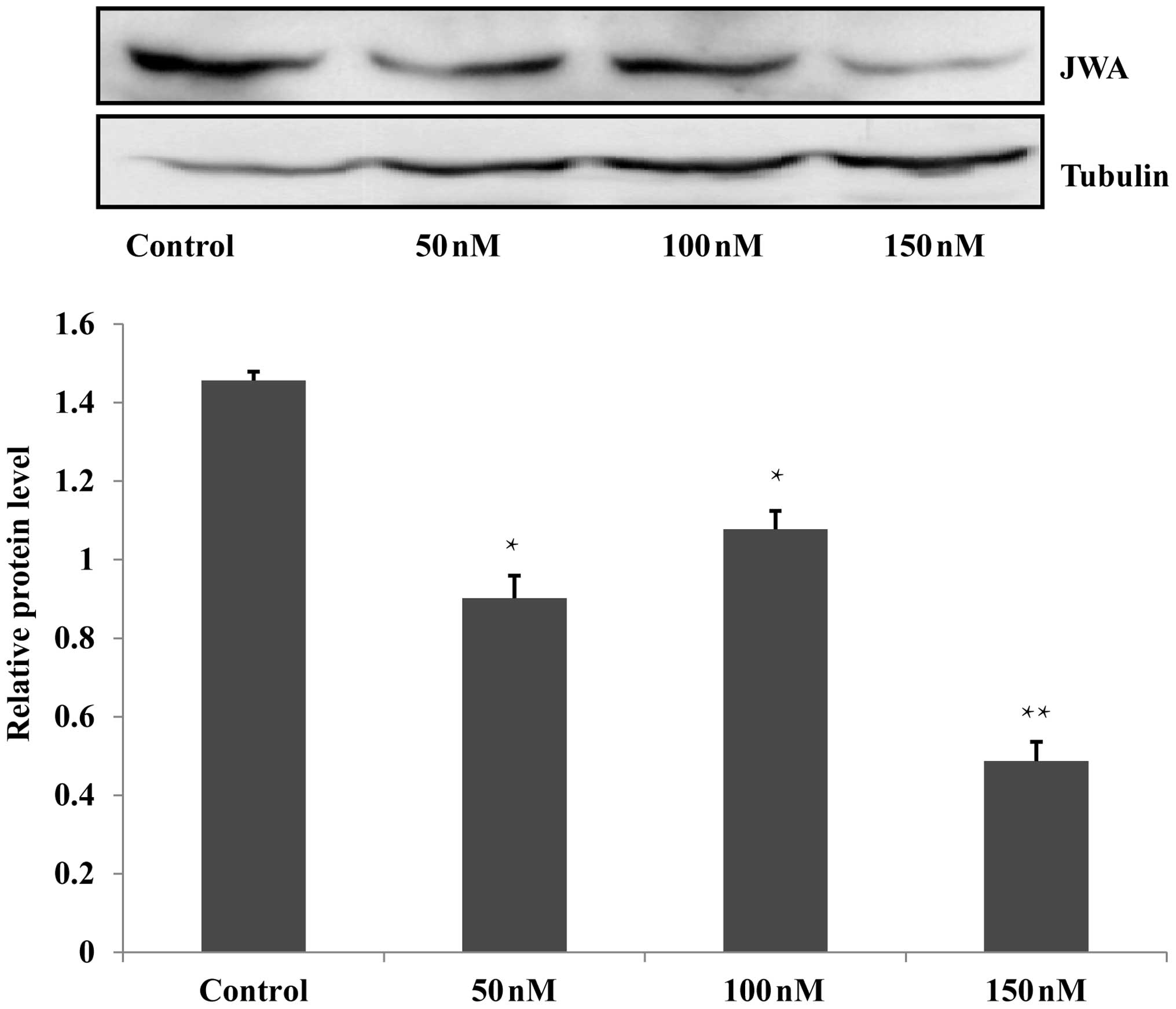
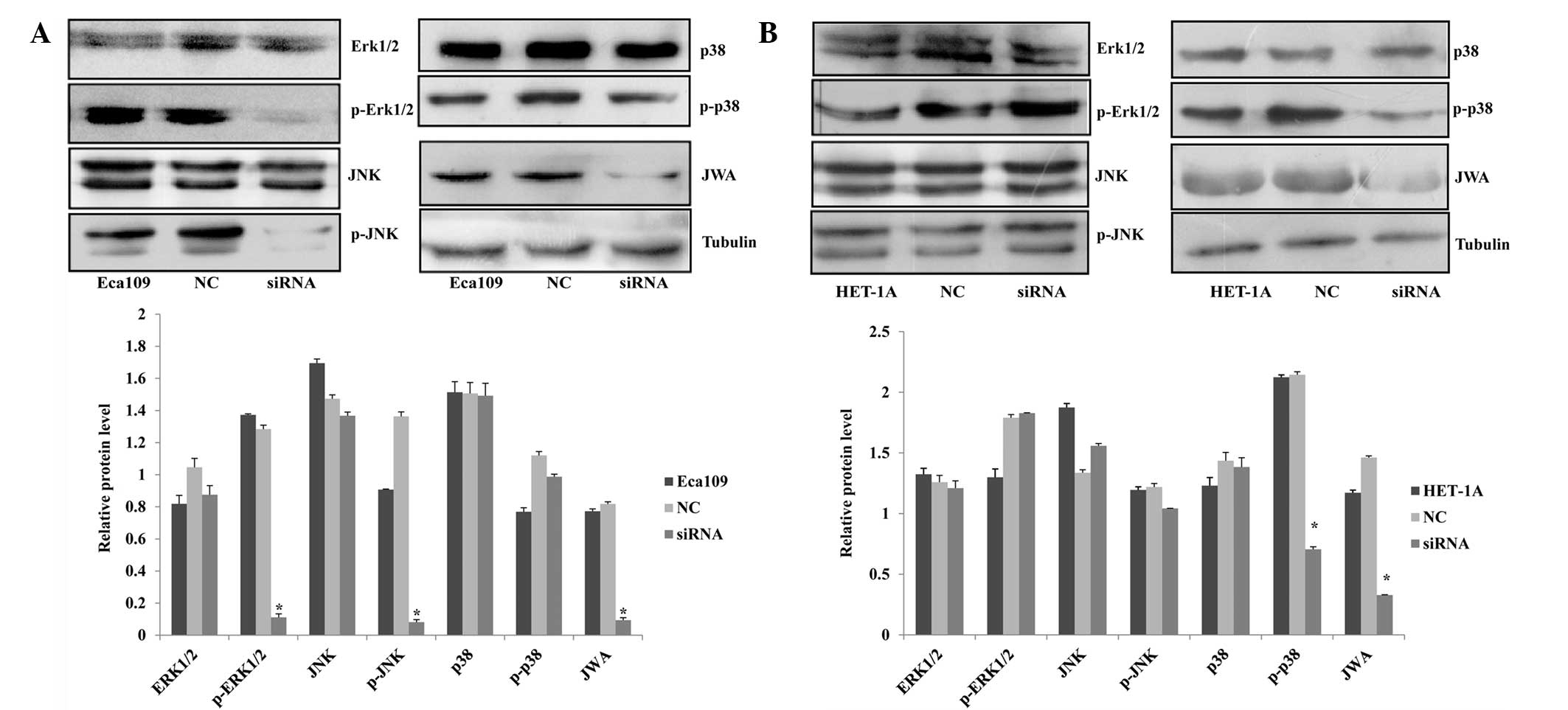
siRNA decreases the expression of
JWA
In the present study, RNA interference was used to
interfere with the JWA gene in the Eca109 and HET-1A cells. For
each cell line, the siRNA groups were treated with 50, 100 or 150
nM siRNA, a scrambled siRNA sequence was used to establish an NC
group and untreated wild-type cells were included as a blank
control group. Following Lipofectamine 2000-mediated transfection
of the Eca109 and HET-1A cells, western blot analysis identified
that 150 nM siRNA was the most effective concentration, which was
selected for subsequent analysis (Fig.
1; data not shown for HET-1A cells).
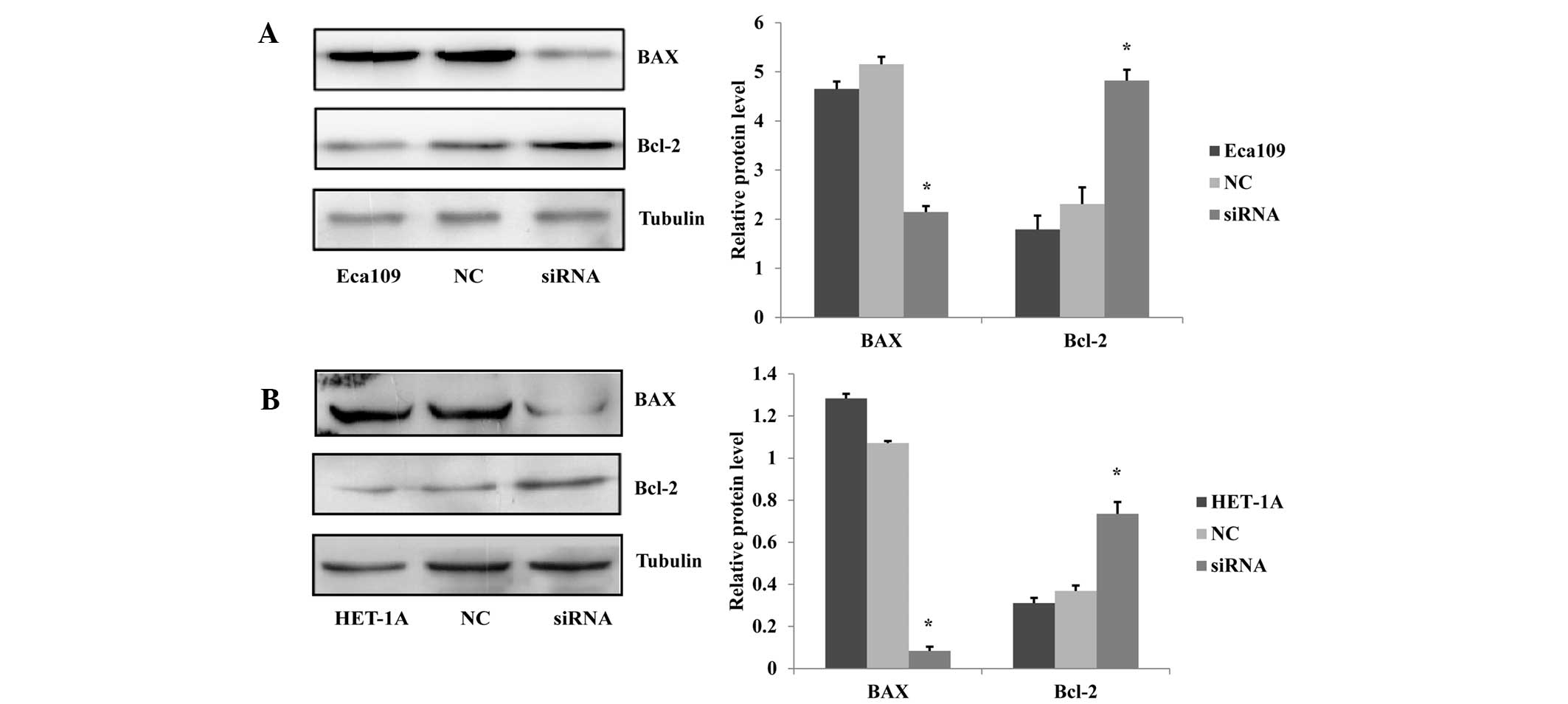
Knockdown of the JWA gene reduces cell
apoptosis
The protein regulators Bcl-2 and BAX are known to
independently regulate apoptosis. The Bcl-2 gene is a member of the
Bcl-2 family of regulator proteins, which inhibit cell death while
BAX overexpression promotes apoptosis (11). To exhibit the effect of JWA on the
apoptosis of esophageal cell lines, the expression levels of the
apoptosis-related proteins BAX and Bcl-2 were analyzed via western
blotting. The results showed that the BAX expression levels were
decreased and the Bcl-2 expression levels were increased in the
siRNA-treated Eca109 cells compared with those in the NC and
untreated Eca109 groups (Fig. 2A).
Furthermore, in the HET-1A cells, the effect of the siRNA treatment
on the BAX and Bcl-2 expression levels was similar to that observed
in the Eca109 cells (Fig. 2B).
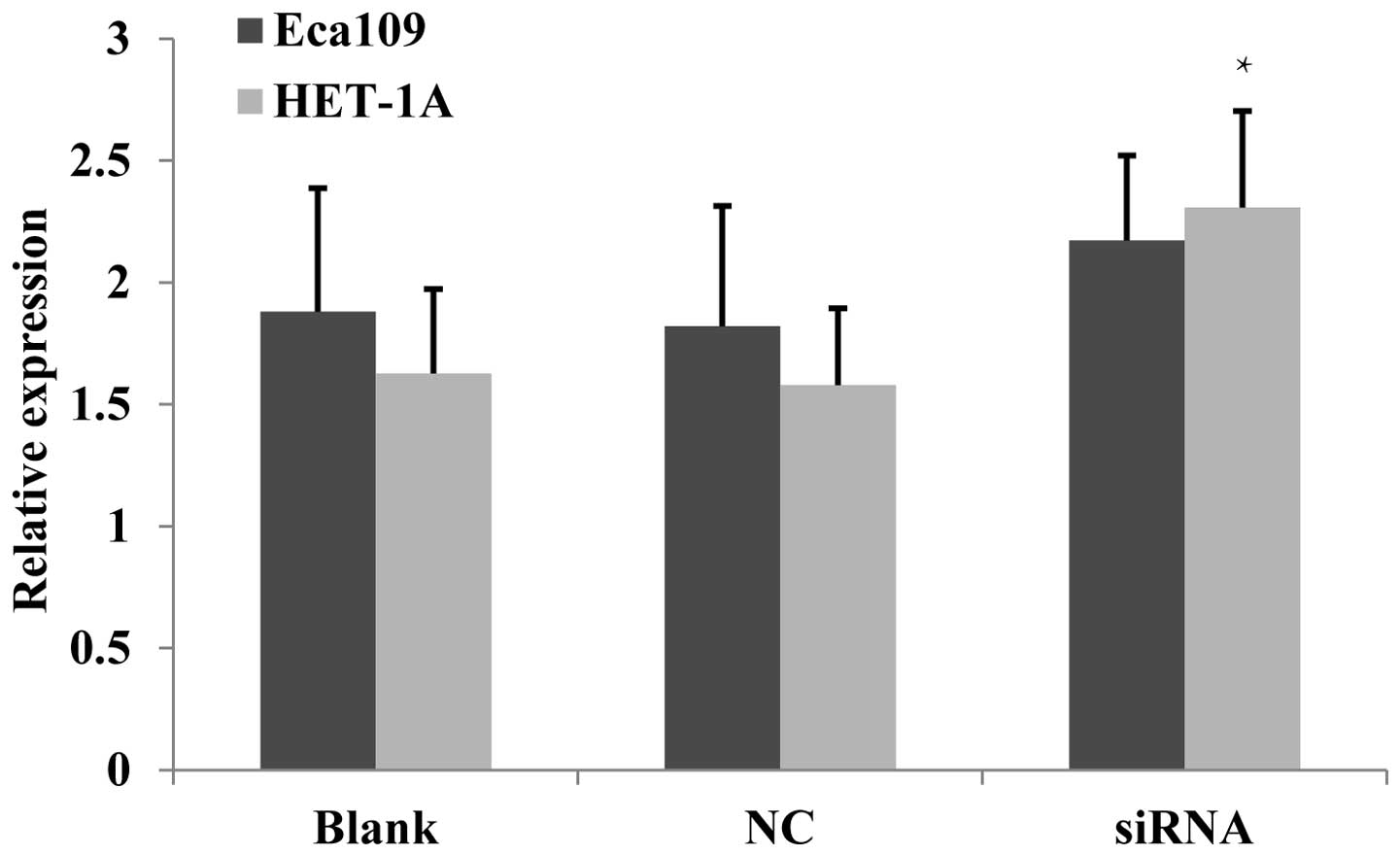
Proliferative activity increases
following JWA-siRNA transfection
Chen et al (12) identified that the overexpression of
JWA inhibited the proliferation of HeLa cells, whereas a low
expression of JWA promoted their proliferation. In the present
study, Eca109 and HET-1A cells were employed to evaluate the effect
of JWA on cell proliferation following JWA-siRNA transfection. The
MTT assay identified that in the Eca109 cells, the proliferation
activity of the siRNA group was significantly increased compared
with that in the NC and untreated cell groups (Fig. 3).
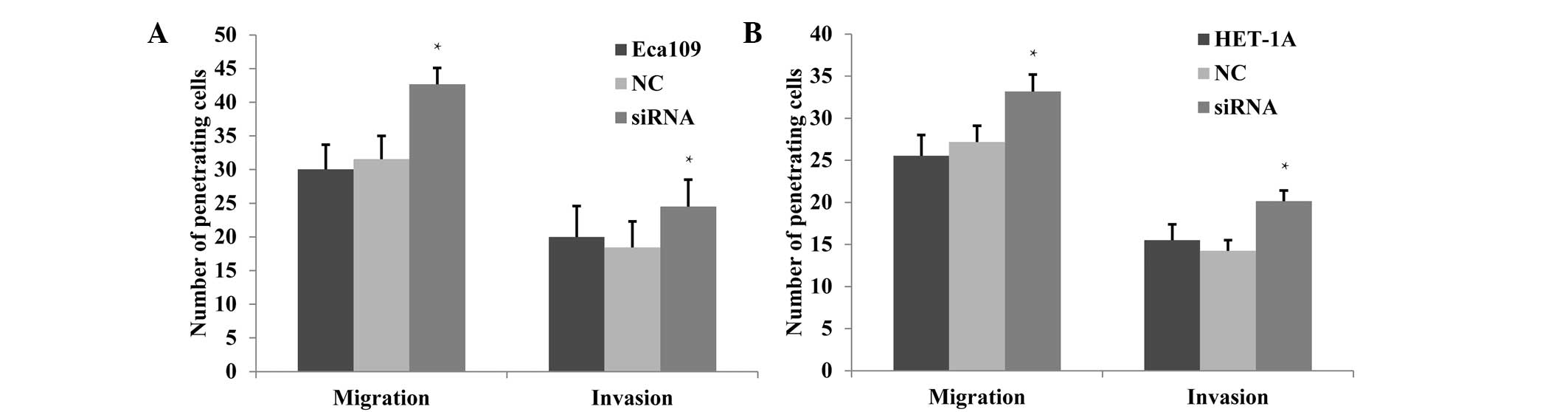
Cell migration and invasion increase
following JWA gene knockdown
Previous studies have shown a high metastatic
ability for tumor cell lines when the expression level of the JWA
gene is low, whereas a low metastatic ability was accompanied by a
greater level of JWA expression. Bai et al (13) demonstrated that the migration and
invasion abilities of melanoma cells were inhibited following JWA
knockdown. In the present study, changes in invasion and migration
capabilities following JWA-siRNA transfection were observed. The
migration and invasion effects were detected via Transwell
experiments, which demonstrated that in Eca109 cells, these effects
were significantly enhanced in the siRNA group compared with those
in the NC and untreated groups (P<0.05; Fig. 4A). In the HET-1A cells, the
migration and invasion were also observed to increase significantly
in the siRNA group (P<0.05; Fig.
4B).
Protein expression of p-ERK1/2 and p-JNK
in Eca109 cells and p-p38 in HET-1A cells decreases following
JWA-siRNA transfection
In previous studies, JWA has been identified as a
common signaling molecule of the cell signal transduction pathways
induced by cancer-promoting or tumor suppressor agents; moreover,
it is significant in the regulation of the MAPK pathways (14–16).
Chen et al (12) showed
that in HeLa cells, phorbol 12-myristate 13-acetate (PMA) and
arsenic trioxide (AS2O3) activate MEK and ERK
phosphorylation. To observe the association between JWA and the
MAPK pathways, the expression of proteins associated with three
MAPK pathways in the esophageal cell lines were analyzed by western
blot assay. In the Eca109 cells, the protein expression level of
p-ERK1/2 exhibited the most marked reduction in the siRNA group;
however, the protein level of the ERK1/2 demonstrated no clear
change. In another MAPK pathway, p-JNK expression was significantly
decreased while the expression of JNK was not. In the third MAPK
pathway, the protein levels of p38 and p-p38 did not change
(Fig. 5A). However, in the HET-1A
cells, the expression levels of ERK1/2, p-ERK1/2, JNK, p-JNK and
p-38 did not indicate any changes. By contrast, p-p38 expression
decreased following siRNA interference. These data demonstrate that
the JWA regulatory mechanisms differed between the two cell lines
(Fig. 5B).
Discussion
Esophageal cancer is a predominant type of cancer
worldwide. As with other malignant tumors, esophageal carcinomas
are associated with changes occurring to genes. The development of
esophageal cancer is a complex process involving multiple factors
and stages, and numerous oncogenes and tumor suppressor genes,
which alternate at the molecular level. However, the mechanism of
the occurrence and development of esophageal cancer remains
unclear. An insight into the mechanisms of progression and
metastasis of human esophageal cell lines may provide important
information for the development of therapeutic treatments.
Although the role of JWA has been investigated in
various tumor cell lines (5–9),
there are few studies relating to the JWA gene, which regulates
proliferation, apoptosis, migration and invasion in human
esophageal cells. In the present study, siRNA was used to
investigate the association between JWA and MAPK signaling pathways
in human esophageal cell lines.
Bcl-2 and BAX regulate cell apoptosis;
overexpression of Bcl-2 inhibits cell apoptosis, which is one of
the mechanisms of anticancer agents (17) and BAX overexpression promotes
apoptosis to ensure that the malignant transformation of normal
cells does not occur (11).
Moreover, the expression of Bcl-2 and a lack of BAX generates a
synergistic effect on apoptosis (13,18).
In the present study, the expression of BAX was observed to
decrease, whereas Bcl-2 expression increased significantly in the
siRNA group. The results indicate that JWA knockdown has inhibitory
effects on apoptosis in both normal and cancer cells.
Cancer cells are characterized by their rapid
proliferation. In the present study, following knockdown of the
tumor suppressor gene JWA, the proliferation of Eca109 and HET-1A
cells accelerated. This may be associated with the cytoskeletal
proteins and cell cycle characteristic of JWA (12).
JWA has been demonstrated to be a functional
molecule that regulates cancer cell migration via MAPK cascades and
actin filaments (12). The
transfection of melanoma cells with JWA inhibited their invasive
ability (13). Chen et al
(12) indicated that the
overexpression of JWA in HeLa, B16 and HCCLM3 cancer cells
effectively inhibited cell migration; however, cell migration was
significantly accelerated as a result of JWA knockdown. The present
results indicate that migration and invasion were significantly
enhanced following transfection with JWA-siRNA compared with those
in untreated cells. These findings indicate that JWA inhibits the
migration and invasion of normal and cancer human esophageal
cells.
MAPK pathways are significant signal transduction
pathways in numerous metabolic processes (1,2).
Chen et al (12)
demonstrated that in HeLa cells, PMA and
As2O3 led to the phosphorylation of MEK and
ERK, whereas JWA knockdown blocked MEK and ERK phosphorylation. The
authors concluded that JWA regulated the cell migration via
inhibition of MEK/ERK phosphorylation. Ye et al identified
that low expression levels of JWA inhibited the RAF proto-oncogene
serine/threonine-protein kinase (c-Raf)/MEK/ERK signaling pathway
in MCF-7 breast cancer cells (14,19).
In the present study, the levels of proteins associated with three
MAPK pathways were examined in Eca109 human esophageal cancer
cells; the expression level of p-ERK1/2 decreased significantly in
the siRNA group, however, the protein level of ERK1/2 exhibited no
clear change. These results indicate that JWA performs a regulatory
role in the c-Raf/MEK/ERK signaling pathway in ESCC cells.
Zhang et al demonstrated that in JAr human
choriocarcinoma cells, high expression levels of p-JNK and p-p38
were accompanied by increased levels of apoptosis. However, in a
JWA-knockout JAr cell model, VP16 (etoposide phosphate), was unable
to activate the phosphorylation of JNK and p38, thus apoptosis
decreased (20). However, in the
present study, a significant reduction in the level of p-JNK was
observed in Eca109 cells following JWA knockdown, whereas the JNK
expression did not decrease. In the third MAPK pathway, the protein
levels of p38 and p-p38 did not change in the Eca109 cells;
however, the p-p38 level decreased in the HET-1A cells following
JWA knockdown. This revealed that there were two different
regulatory mechanisms within the two cell lines.
In conclusion, it may be hypothesized that JWA
regulates cell proliferation, migration and invasion via ERK1/2 and
JNK pathways in human esophageal cancer cells and via p-38 in human
esophageal cells. The results indicate that JWA has effects on the
mechanisms that lead to the development of esophageal cancer.
Acknowledgements
This study was supported by the Natural Science
Foundation of Jiangsu Province (grant no. BK2012563) and the
Medical Research Project of the Health Department of Jiangsu
Province (grant no. Z201218).
References
|
1
|
Yang SH, Sharrocks AD and Whitmarsh AJ:
MAP kinase signalling cascades and transcriptional regulation.
Gene. 513:1–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu JY, Chu ZG, Han J, Dang YM, Yan H,
Zhang Q, Liang GP and Huang YS: The p38/MAPK pathway regulates
microtubule polymerization through phosphorylation of MAP4 and Op18
in hypoxic cells. Cell Mol Life Sci. 67:321–333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Wu X, Chen Y, Zhang J, Ding J,
Zhou Y, He S, Tan Y, Qiang F, Bai J, et al: Prognostic and
predictive role of JWA and XRCC1 expressions in gastric cancer.
Clin Cancer Res. 18:2987–2996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu YQ, Li AP, Chen R and Zhou JW: The role
of JWA in N-methyl-N′-nitro-N-nitrosoguanidine induced human
bronchial epithelial cell apoptosis. Zhonghua Lao Dong Wei Sheng
Zhi Ye Bing Za Zhi. 24:205–208. 2006.(In Chinese).
|
|
7
|
Zhou J, Ge Z, Tan Y, Jiang G, Feng J, Wang
H and Shi G: Downregulation of JWA expression in human esophageal
squamous cell carcinoma and its clinical significance. Oncol Res.
20:157–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li CP, Zhu YJ, Chen R, Wu W, Li AP, Liu J,
Liu QZ, Wei QY, Zhang ZD and Zhou JW: Functional polymorphisms of
JWA gene are associated with risk of bladder cancer. J Toxicol
Environ Health A. 70:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi GZ, Yuan Y, Jiang GJ, Ge ZJ, Zhou J,
Gong DJ, Tao J, Tan YF and Huang SD: PRAF3 induces apoptosis and
inhibits migration and invasion in human esophageal squamous cell
carcinoma. BMC Cancer. 12:972012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen L, Xu W, Li A, Ye J and Zhou J: JWA
enhances As2O3-induced tubulin polymerization
and apoptosis via p38 in HeLa and MCF-7 cells. Apoptosis.
16:1177–1193. 2011.
|
|
11
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Bai J, Ye J, Liu Z, Chen R, Mao W,
Li A and Zhou J: JWA as a functional molecule to regulate cancer
cells migration via MAPK cascades and F-actin cytoskeleton. Cell
Signal. 19:1315–1327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai J, Zhang J, Wu J, Shen L, Zeng J, Ding
J, Wu Y, Gong Z, Li A, Xu S, et al: JWA regulates melanoma
metastasis by integrin alphaVbeta3 signaling. Oncogene.
29:1227–1237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Ye J, Zhao X, Li A and Zhou J: JWA
is required for arsenic trioxide induced apoptosis in HeLa and
MCF-7 cells via reactive oxygen species and mitochondria linked
signal pathway. Toxicol Appl Pharmacol. 230:33–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao WG, Li AP, Ye J, Huang S, Li AQ and
Zhou JW: Effect of differentiation inducer and heat stress on the
expression of JWA protein and Hsp70 of K562 cells. Zhonghua Lao
Dong Wei Sheng Zhi Ye Bing Za Zhi. 21:253–256. 2003.(In
Chinese).
|
|
16
|
Mao WG, Liu ZL, Chen R, Li AP and Zhou JW:
JWA is required for the antiproliferative and pro-apoptotic effects
of all-trans retinoic acid in Hela cells. Clin Exp Pharmacol
Physiol. 33:816–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye J, Li A, Liu Q, Wang X and Zhou J:
Inhibition of mitogen-activated protein kinase kinase enhances
apoptosis induced by arsenic trioxide in human breast cancer MCF-7
cells. Clin Exp Pharmacol Physiol. 32:1042–1048. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Zhou J, Xu W, Li A, Zhou J and Xu
S: JWA sensitizes P-glycoprotein-mediated drug-resistant
choriocarcinoma cells to etoposide via JNK and
mitochondrial-associated signal pathway. J Toxicol Environ Health
A. 72:774–781. 2009. View Article : Google Scholar
|