Introduction
Hyperthermia may be defined as overheating of the
body, including heatstroke caused by an over-high ambient
temperature, or as an abnormally high body temperature (1). The potential causes of hyperthermia
include infection, certain drugs and medications, and brain trauma
(1–3). High temperature may be used for tumor
treatment, particularly for cancer treatment (1,2), but
controversial issues remain in its clinical use (1). Unrelieved hyperthermia is a cause of
mortality, particularly in elderly individuals (1,3).
Hyperthermia not only directly induces cell injury of body tissues,
but also causes the body to release large amounts of inflammatory
mediators and cells with extensive biological activities to induce
a systemic inflammatory response and immune dysfunction. Thus,
hyperthermia causes systemic inflammatory response syndrome (SIRS),
aggravating injuries to various organs and ultimately results in
multiple organ dysfunction syndrome (MODS) (3,4). A
study reported that hyperthermia-induced elevation of the levels of
heat shock protein 70 relieved the extent of the pulmonary fibrosis
of rats in response to the induction of acute lung injury by
lipopolysaccharide (LPS) administration (5). Therefore, the effects of hyperthermia
on the lung and its mechanism of action remain unclear at present.
In the clinic, cases of fatal hyperthermia caused by various
intraoperative factors are frequently reported (6–9), and
a comprehensive treatment measure is the key to successful
treatment.
Ulinastatin (UTI) has been used in the process of
successfully treating a case of malignant hyperthermia (MH)
(10). UTI is a typical
Kunitz-type protease inhibitor. A number of studies have suggested
that UTI may be able to protect against acute lung injuries caused
by endotoxins and mechanical damage (11–13).
Additional studies have shown that UTI may be able to protect
against acute lung injuries induced by LPS in rats (14–16).
However, there are a few studies concerning whether UTI has an
intervention effect on hyperthermia-induced lung injury (17–19).
The purpose of the present study was to observe the
cellular morphological changes of the lung tissue in rats with
hyperthermia and the effects of intervention with UTI
administration at different time points on cellular morphology.
These observations were conducted to explore the mechanism of
action by which UTI treats fatal hyperthermia in the clinic and the
importance of the time of application.
Materials and methods
Experimental animals and grouping
A total of 40 specific pathogen-free Sprague Dawley
male rats with body weights ranging from 180 to 220 g were provided
by the Experimental Animal Center of Southern Medical University
(Guangzhou, China) and randomly divided into five groups, with
eight rats in each group. The groups were as follows: The C group
(the rats were maintained at room temperature, without medication),
the H group (the rats were placed at high temperature, without
medication), the HU group (5×104 U/kg UTI was
administered to the rats prior to heating), the HU1 group
(5×104 U/kg UTI was administered after 1 h of heating),
and the HU2 group (5×104 U/kg UTI was administered after
2 h of heating). UTI was provided by Tianpu Biochemical
Pharmaceutical Co. Ltd. (Guangzhou, China). This study was
conducted in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health (ninth edition, 2010). The animal use protocol
was reviewed and approved by the Institutional Animal Care and Use
Committee of Nanfang Hospital, Southern Medical University.
Indicators and methods
The rats were anesthetized with 3% pentobarbital
(Nembutal; 45 mg/kg; SERVA, 921019, Shanghai, China) by
intraperitoneal injection and placed into a heating chamber with a
biological oxygen supply (the Artificial Climate Simulation Chamber
for Animals, developed by the Tropical Medicine Faculty of Southern
Medical University). Also, the previously examined rectal
temperature was used as the basic value. Subsequently, the rats in
all groups other than the C group were heated in the heating
chamber at 35°C and a relative humidity of 60%. For the HU, HU1 and
HU2 groups, 5×104 U/kg UTI (dissolved in 5 ml normal
saline) was administered at the initiation of heating, and after 1
h and 2 h of heating, respectively. For the other two groups, an
equivalent volume of normal saline was administered at the
beginning of the experiment. After 2.5 h of heating, all rats were
removed from the heating chamber and treated as subsequently
described. The time at the start of the experiment was expressed as
T0. During the experiment, the respiratory frequency and rectal
temperature of the rats were measured and recorded once every 30
min. A total of six recordings at different time points during the
experiment were conducted and they were expressed as T0–T150,
respectively.
W/D ratio determination of the lung
tissue
After removal of the rats from the heating chamber,
the rat thoracic cavity of each group (n=8) was opened to remove
the left upper lung tissue before perfusion with paraformaldehyde.
Water and blood staining on the lung tissue surface was absorbed
with filter paper, and the tissue was weighed in a weighing disk
(wet weight), dried in a constant temperature oven at 80°C and
weighed again (dry weight). Subsequently, the W/D ratio was
calculated.
Acquisition and observation of specimens
for light microscopy
Following the opening of the thoracic cavity, buffer
solution containing 4% paraformaldehyde was rapidly perfused via
the right ventricle. The left lower lung tissue was removed, soaked
and fixed in 4% paraformaldehyde for 24 h, conventionally
dehydrated with alcohol, embedded with paraffin wax and sectioned
into ultrathin slices. The slices were stained with hematoxylin and
eosin and were then observed for pathological changes under a Nikon
microscope (Nikon TS100-F, Nikon, Tokyo, Japan) and
photographed.
Acquisition and observation of specimens
for electron microscopy
The thoracic cavities of three rats from each group
were opened to remove the right upper lung tissue, and each tissue
sample was torn into small sections with a size of ~1
mm3. The tissue sections were soaked in 2%
glutaraldehyde (this step was completed within 1 min), fixed with
2% osmic acid, dehydrated with gradient alcohol, embedded with
epoxy resin and sectioned into ultrathin slices. The slices were
stained with uranium acetate and lead citrate, and were observed
and photographed under a H-7500 Transmission Electron Microscope
(Hitachi, Tokyo, Japan).
Statistical analysis
The data were analyzed using SPSS statistical
software, version 13.0 (SPSS, Inc., Chicago, IL, USA), and the
experimental data were expressed as the mean ± standard deviation.
One-way analysis of variance was used for comparisons of the mean
value of various parameter indicators among the groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Comparisons of the general data
No significant difference in the body weight, basic
rectal temperature and rectal temperatures of the rats at various
time points were identified among the groups (all P>0.05;
Table I).
 | Table IComparison of the weight and rectal
temperature of the rats at different time points. |
Table I
Comparison of the weight and rectal
temperature of the rats at different time points.
| | Rectal temperature
(°C) |
|---|
| |
|
|---|
| Group (n=8) | Weight (g) | T0 | T30 | T60 | T90 | T120 | T150 |
|---|
| C | 185.8±30.5 | 37.2±0.2 | 37.1±0.2 | 37.1±0.2 | 37.2±0.1 | 37.1±0.1 | 37.2±0.4 |
| H | 194.5±11.8 | 37.3±0.2 | 38.6±0.2 | 39.0±0.3 | 39.8±0.3 | 40.8±0.5 | 42.3±0.4 |
| HU | 194.9±8.3 | 37.1±0.1 | 38.5±0.4 | 38.9±0.4 | 39.6±0.6 | 40.6±0.3 | 42.1±0.4 |
| HU1 | 192.2±11.7 | 37.2±0.2 | 38.7±0.3 | 39.1±0.3 | 39.8±0.2 | 40.6±0.3 | 42.1±0.4 |
| HU2 | 190.3±6.8 | 37.2±0.2 | 38.6±0.3 | 38.9±0.3 | 39.8±0.5 | 40.7±0.5 | 42.2±0.5 |
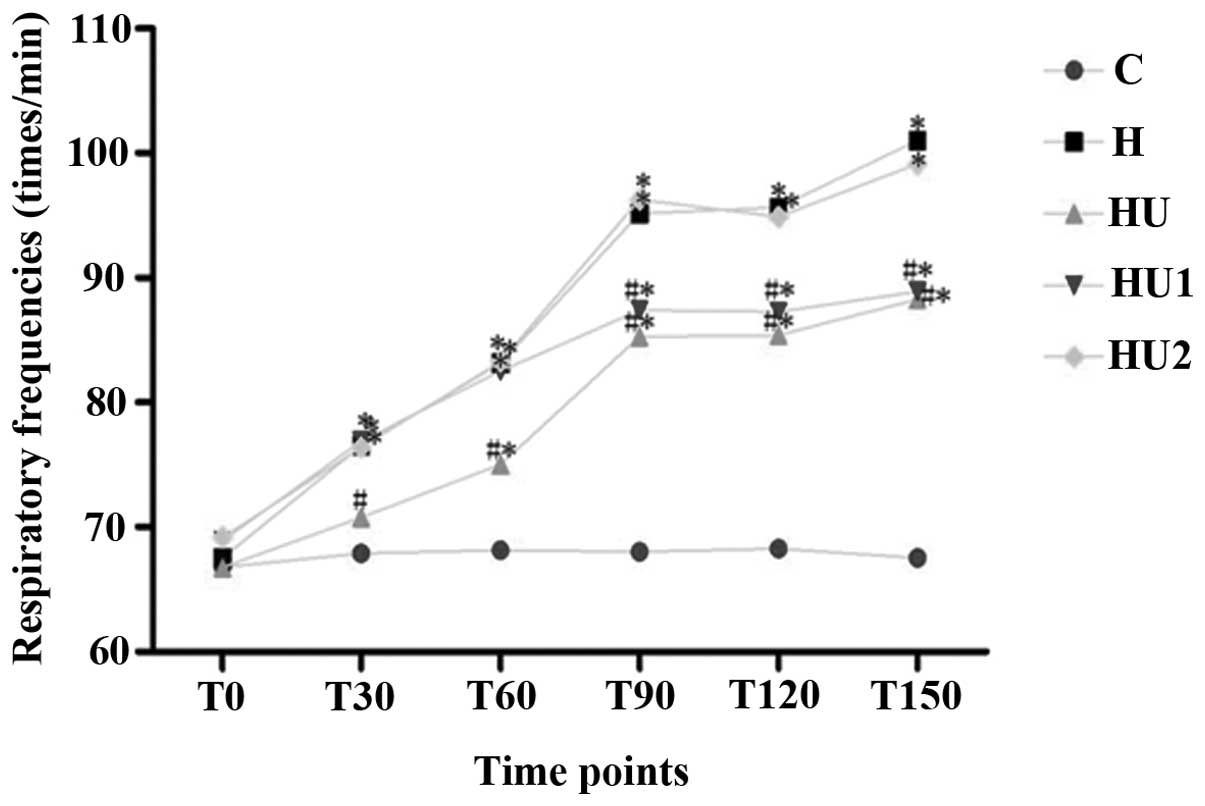
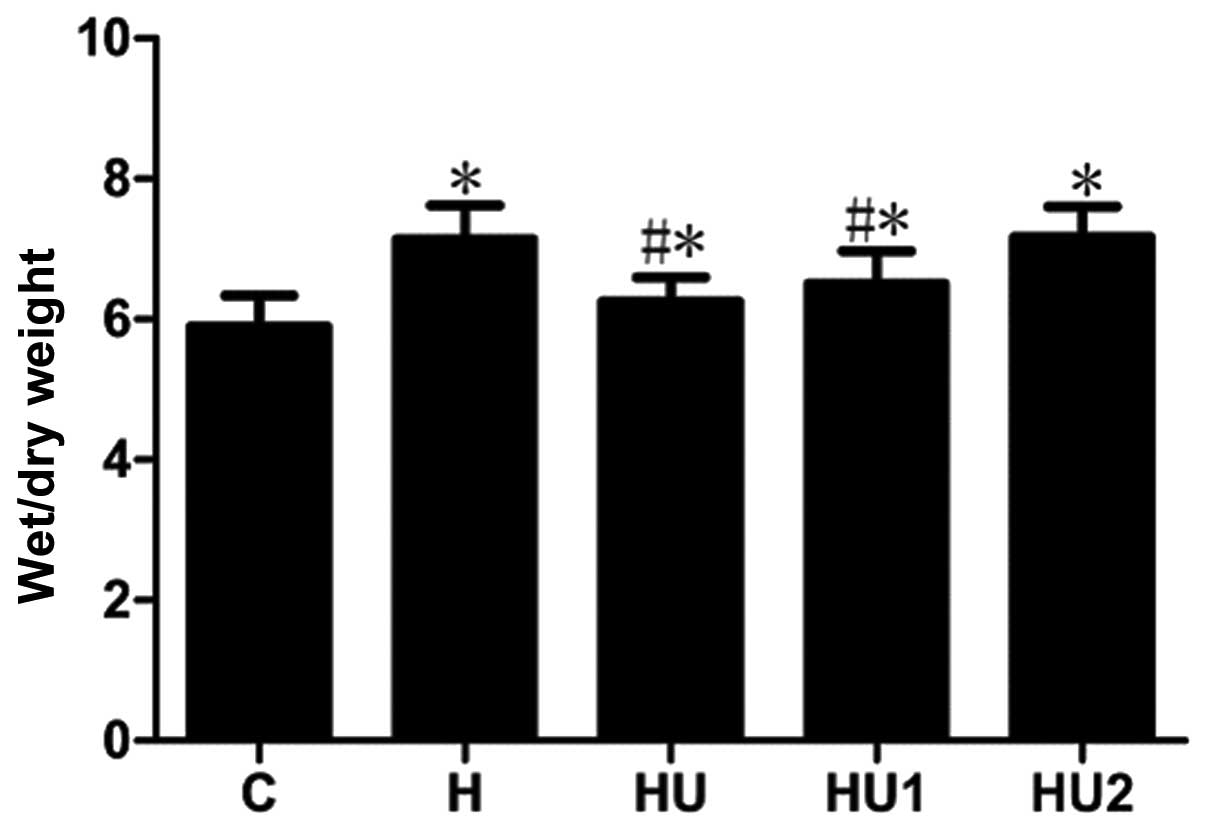
Changes of the pulmonary respiratory
frequencies and lung tissue W/D ratios of the rats in the various
groups
During the experiment, the respiratory frequencies
and lung tissue W/D ratios of the rats in the various hyperthermia
groups were markedly increased. Compared with the those of the C
group, the measurements of the hyperthermia groups were
significantly different (all P<0.05) with the exception of the
respiratory rate of group H1 at 30 and 60 min. In addition, the
respiratory frequencies and lung tissue W/D ratios of the HU and
HU1 groups were significantly lower than those of the H group (all
P<0.05). Between the HU2 and H groups, no significant
differences in the respiratory frequency and lung tissue W/D values
were identified (P>0.05, Figs.
1 and 2).
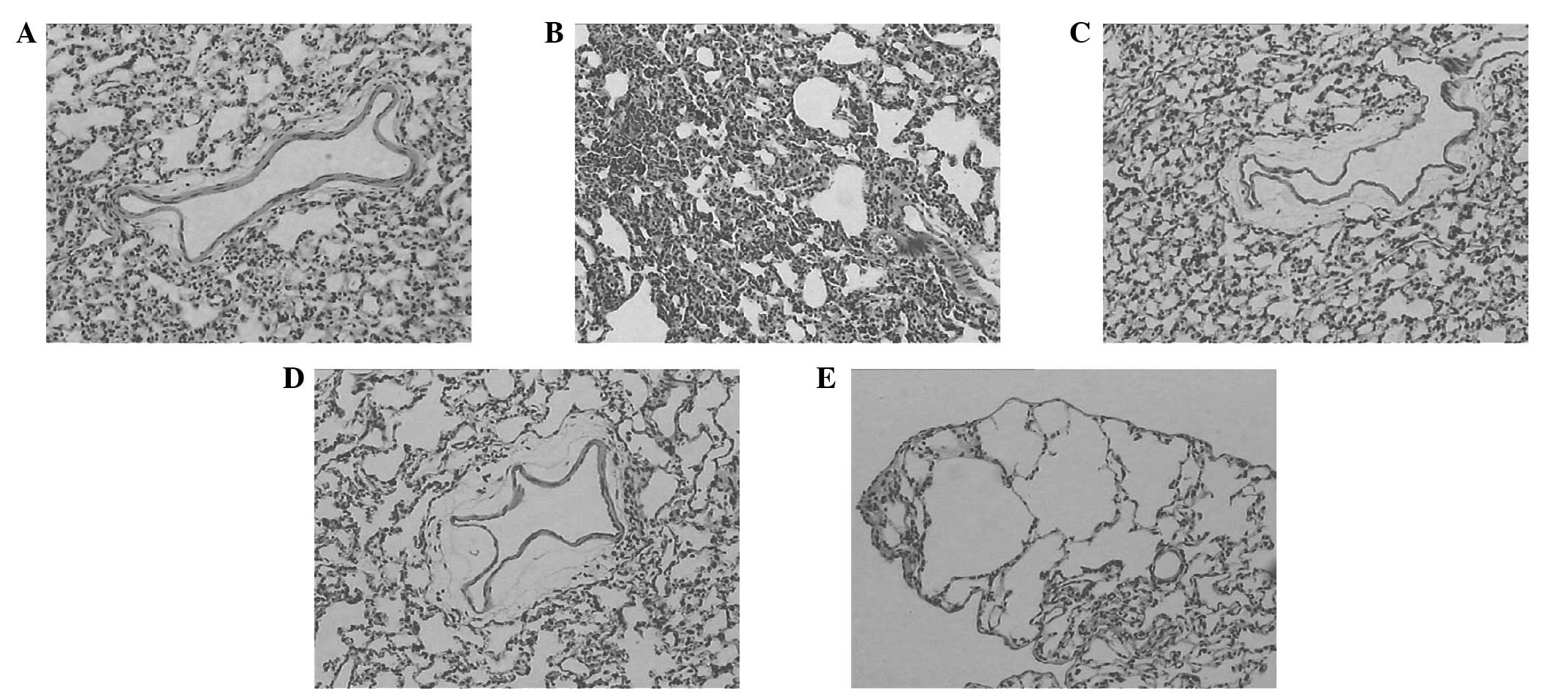
Pulmonary histopathology changes under
light microscopy
In the C group, the bronchial pulmonary alveoli
tissues of the animals were integrated; the bronchial wall did not
present hyperemia and edema; the pulmonary alveoli did not present
shrinkage or dilation; and no clear exudation in the alveolar space
was observed (Fig. 3A). Compared
with those of the rats in the C group, the lung tissues of the rats
in the hyperthermia treatment groups presented pathological change
to various extents. Among them, the pathological changes of the H
group (Fig. 3B) were the most
severe. The alveolar wall was thickened, twisted and deformed, and
pulmonary interstitial hyperemia, and pulmonary alveoli collapsed
and patchy atelectasis were observed. Also, pneumorrhagia and
emphysema were visible. The pathological changes in the HU and HU1
groups were milder than those of the H group. It was observed that
the bronchial surrounding tissues were loose, the alveolar wall
presented mild edema, and no clear exudation was visible in the
alveolar space (Fig. 3C and D). In
the HU2 group, the pulmonary alveolar epithelia were swollen, and
the alveolar wall was thinned or broken to form bullae of the lung
(Fig. 3E).
Electron microscopy examination
results
Under the electron microscope, it was observed in
the C group that the pulmonary alveoli were integrated; the nuclear
membrane was complete; the nuclear chromatin was uniform; and
mitochondria, rough endoplasmic reticula, Golgi apparatus and
lysosomes and lamellated bodies arranged in concentric circles or
in parallel were present in the cytoplasm of the type II epithelial
cells (Fig. 4A). The changes in
the H group were the most evident. In the type II epithelial cells
of the pulmonary alveoli, the mitochondria were swollen, the cell
ridges were shortened, the microvilli were thinned and increased in
number, and the alveolar wall was thickened. An increased number of
infiltrating neutrophils were visible, and a large number of red
blood cell fragments were deposited in the pulmonary alveoli
(Fig. 4B). The changes in the HU
and HU1 groups were milder than those of the H group. The cell
membranes of the type II epithelial cells were complete, and the
cytoplasmic organelles were almost normal in structure, which was
in line with the observations in the C group (Fig. 4C and D). In the HU2 group, the type
II epithelial cells of the pulmonary alveoli had shed and detached
from the basement membrane, the lamellated bodies were reduced, and
the microvilli were thinned and increased in number (Fig. 4E).
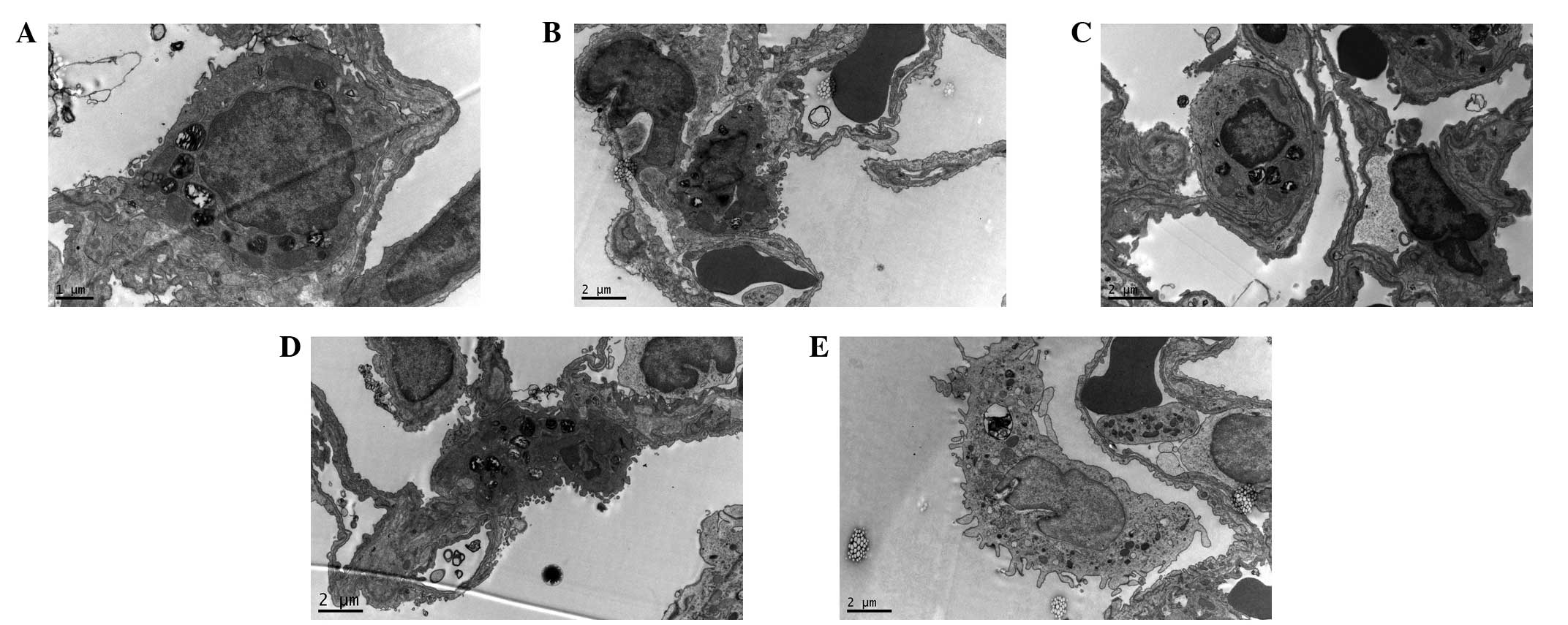 | Figure 4Ultrastructure of the lung tissues of
the rats in the different groups. Uranium acetate and lead citrate
staining. (A) C group (magnification, ×9,700); (B) H group
(magnification, ×5,800); (C) HU group (magnification, ×5,800); (D)
HU1 group (magnification, ×5,800); and (E) HU2 group
(magnification, ×5,800). C, normal control group; H, hyperthermia
without medication; HU, hyperthermia and UTI pretreatment; HU1,
administered with UTI 1 h of heating; HU2, administered with UTI 2
h of heating. UTI, ulinastatin. |
Discussion
As there are numerous methods of causing an
over-high body temperature, there are a number of different damage
mechanisms of the body associated with hyperthermia (6–10).
Previous studies have shown that high temperature and humidity act
as the main factors in the preparation of a hyperthermic animal
model of heat stroke (17,18). It was determined from our
preliminary experiments that a temperature of 35°C and humidity of
60% satisfied the requirements for model establishment. In this
environment, the body temperature of rats rose to 42–43°C in 3 h,
and a high survival rate was maintained. The experimental results
of the present study showed that compared with those of the C
group, the lung tissues of the rats in the various hyperthermia
treatment groups presented different extents of pathological
changes. Among them, the pathological changes of the H group were
the most severe. Under a light microscope, it was observed that the
alveolar wall was thickened, twisted and deformed, and pulmonary
interstitial hyperemia, and pulmonary alveolar and patchy
atelectasis had appeared. Also, pneumorrhagia and emphysema were
visible. Under an electron microscope, it was identified that in
the type II epithelial cells of the pulmonary alveoli, the
mitochondria were swollen, the cell ridges were shortened, the
microvilli were thinned and increased in number, and the alveolar
wall was thickened. Also, an increased number of infiltrating
neutrophils were visible, and a large number of red blood cell
fragments were deposited in the pulmonary alveoli. It may be
inferred from these results that hyperthermia possibly causes lung
tissue damage via the following two mechanisms. One possibility is
associated with the direct damage of the cell membrane and
intracellular structures caused by hyperthermia. A study (19) reported that a high temperature
markedly damaged the close connecting structures of cardiac muscle
cells, epithelial cells of the pulmonary alveoli and capillary
endothelial cells, and the normal barrier function of the cell
membrane was not maintained, as observed by lanthanum nitrate
tracer electron microscopy. In the present study, it was observed
that hyperthermia caused the type II epithelial cells of the
pulmonary alveoli to shed and detach from the basement membrane,
the lamellated bodies were reduced, and the microvilli were thin
and increased in number. The differences between the two studies
are possibly associated with the differences in how the electron
microscopy was performed. Once the cell membrane barrier function
of lung tissue is damaged, cell edema is caused. With aggravation
of cell injury, various cellular organelles, including the
mitochondria, Golgi apparatus and endoplasmic reticula, present
corresponding function disorders. In 1992, Marino et al
(20) observed that when the body
temperature was >42°C, intracellular mitochondrial oxidative
phosphorylation of skeletal muscle becomes dysfunctional. Another
study showed that hyperthermia caused marked reductions in the
respiratory control ratio and the oxidative phosphorylation
efficacy of rat myocardial cell mitochondria, and the reduction in
the Ca2+ ATP enzyme activity and Ca2+ content
of myocardial cell mitochondria caused mitochondrial function
disorders (21). This is in line
with the observations of the ultrastructure of type II epithelial
cells of rats with systemic hyperthermia in the present study. The
other mechanism is hyperthermia causes the body to greatly release
inflammatory mediators and cells to induce a systemic inflammatory
response and immune dysfunction, thus causing SIRS. Zheng et
al (22) investigated the
early inflammatory factor levels of rats with heat stress and
identified that the levels of the major proinflammatory cytokines
[including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8
and IL-10] in rats with heat stress were increased within 24 h of
the establishment of heat stress, and the systemic inflammatory
response was evident. These cytokines connect and coordinate with
each other to form a complex network system, amplify the
inflammatory reaction through a positive feedback mechanism and
aggravate lung tissue damage. Among the mechanisms of
hyperthermia-induced lung damage, it remains unclear which is the
main cause. Naučienė et al (23) hypothesized that mitochondrial
damage was the main mechanism of action of hyperthermia. The
mechanisms of hyperthermia-induced lung damage require
investigation in further studies.
UTI is a trypsin inhibitor isolated and purified
from human urine. UTI inhibits the activities of a variety of types
of proteins, carbohydrates and lipid hydrolases in the body,
scavenges oxygen free radicals, relieves local tissue peroxidation,
inhibits the synthesis of excess superoxide and myocardial
depressant factor, and reduces the excessive release of
inflammatory cytokines, resulting in improved microcirculation and
immune regulation. UTI has been widely used for treating clinical
critical diseases, including severe acute pancreatitis and
disseminated intravascular coagulation (11–13).
A number of studies have shown that UTI is able to effectively
reduce the levels of serum proinflammatory cytokines (TNF-α, IL-1,
IL-6 and IL-8) in patients with pyemia and promote the synthesis
and secretion of the inhibitory proinflammatory cytokine IL-10 to
have a bidirectional regulatory effect on the inflammatory response
and thus relieve an excessive inflammatory response (14,24).
The results in the present study showed that the W/D values of the
HU and HU1 groups, which received early UTI intervention, were
significantly lower than those of the H group, and the pathological
changes were milder. These results suggest that early UTI
application attenuates hyperthermia-induced lung injury and
protects the lungs. This is in line with the results of a previous
study which demonstrated the protective effect of UTI against acute
lung injury in rats caused by endotoxins and LPS (5,15,16).
A study (16) hypothesized that
the mechanism of action of UTI was associated with the ability of
UTI to inhibit TNF-α generation via the p38 mitogen-activated
protein kinase signaling pathway at the transcriptional level to
affect early acute lung injury, thereby protecting the lung. It is
of note that in the present study, the pathological changes of the
lung tissue of the rats in the HU2 group with UTI intervention in
late stage were evident and similar to those of the H group. It may
be speculated that prior to the action of UTI when administered in
the late stage, hyperthermia has caused irreversible damage to the
cells of the lung tissue. UTI intervention was conducted after 2 h
of heat stress in the HU2 group, and the body temperatures of the
majority of the rats rose to >42°C by the end of this study. A
previous study suggested that when the body temperature is
>42°C, intracellular mitochondrial oxidative phosphorylation
dysfunction, cell injury and heart failure and heart failure are
likely to occur (20). The
aforementioned results indicate that UTI should be applied as early
as possible for treating and preventing hyperthermia-induced lung
damage in the clinic.
During surgery, there are numerous factors that may
induce fatal hyperthermia (6–9),
including pyemia, thyroid storm, pheochromocytoma and MH. MH
(25) refers to the
anesthetic-induced abnormally high metabolic status of skeletal
muscle and this causes rhabdomyolysis. It is a rare complication of
anesthesia with an extremely high mortality rate, and its typical
symptoms include masticatory spasm, skeletal myotonia, respiratory
acidosis and rapid elevation of body temperature (>38.8°C), as
well as plasma creatine kinase elevation and myoglobinuria during
anesthesia. Following the exclusion of other potential causes,
fatal hyperthermia is diagnosed as MH. Mitochondrial injury plays
an important role in the pathological mechanism of MH occurrence
(26), and further studies are
required to investigate whether UTI has a protective effect on
mitochondrial injury and whether early intervention with UTI is
effective for treatment of the MH-susceptible population.
To the best of our knowledge, there are no studies
on the applications and trials of UTI in rescuing patients with
fatal hyperthermia. The results of the present study show that
early intervention with UTI relieves the extent of
hyperthermia-induced lung tissue cell damage in rats with
hyperthermia and has a certain protective effect on the lung. This
study provides an experimental basis for the reasonable application
of UTI in cases of intraoperative fatal hyperthermia in the
clinic.
Acknowledgements
This study was supported by the Dean’s Foundation of
Nanfang Hospital, Southern Medical University (2011).
References
|
1
|
Szasz A, Szasz N and Szasz O: Hyperthermia
results and challenges. Oncothermia: Principles and Practices.
Springer; Netherlands: pp. 17–18. 2011
|
|
2
|
Takagi M, Sakata K, Someya M, et al: The
combination of hyperthermia or chemotherapy with gimeracil for
effective radiosensitization. Strahlenther Onkol. 188:255–261.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Varghese GM, John G, Thomas K, Abraham OC
and Mathai D: Predictors of multi-organ dysfunction in heatstroke.
Emerg Med J. 22:185–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leon LR, Blaha MD and DuBose DA: Time
course of cytokine, corticosterone, and tissue injury responses in
mice during heat strain recovery. J Appl Physiol (1985).
100:1400–1409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hagiwara S, Iwasaka H, Matsumoto S,
Noguchi T and Yoshioka H: Association between heat stress protein
70 induction and decreased pulmonary fibrosis in an animal model of
acute lung injury. Lung. 185:287–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noguchi I, Ohno H, Takano K, Shimada R,
Sasao M and Shimonaka H: Fatal hyperthermia due to dental
treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
101:e61–e64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hernandez JF, Secrest JA, Hill L and
McClarty SJ: Scientific advance in the genetic understanding and
diagnosis of malignant hyperthermia. J Perianesth Nurs. 24:19–34.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Firstenberg M, Abel E, Blais D and
Andritsos M: Delayed malignant hyperthermian after routine cononary
artery bypass. Ann Thorac Surg. 89:947–948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pişkin B, Atac MS, Konca E, Yildirim M,
Avsever H and Sevketbeyoğlu H: A Suspected case of malignant
hyperthermia after tooth extraction: case report. J Oral Maxillofac
Surg. 69:1331–1334. 2011.PubMed/NCBI
|
|
10
|
Ouyang MW, Qin ZS, Chen ZQ, Xiao JF, Liu
XJ and Gu MN: A report of fulminant malignant hyperthermia in a
patient during operation. J South Med Univ. 30:2611–2612. 2010.(In
Chinese).
|
|
11
|
Okuhama Y, Shiraishi M, Higa T, et al:
Protective effects of ulinastatin against ischemia-reperfusion
injury. J Surg Res. 82:34–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inoue K, Takano H, Sato H, Yanagisawa R
and Yoshikawa T: Protective role of urinary trypsin inhibitor in
lung expression of proinflammatory cytokines accompanied by lethal
liver injury in mice. Immunopharmacol Immunotoxicol. 31:446–450.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Huang W, Chen S, Li Z, Wang W and
Wang M: Changes of tumor necrosis factor-alpha and the effects of
ulinastatin injection during cardiopulmonary cerebral
resuscitation. J Huazhong Univ Sci Technology Med Sci. 24:269–271.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inoue K, Takano H, Yanagisawa R, et al:
Protective role of urinary trypsin inhibitor in acute lung injury
induced by lipopolysaccharide. Exp Biol Med (Maywood). 230:281–287.
2005.
|
|
15
|
Bae HB, Jeong CW, Li M, Kim HS and Kwak
SH: Effects of urinary trypsin inhibitor on
lipopolysaccharide-induced acute lung injury in rabbits.
Inflammation. 35:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Liu F, Liu H, et al: Urinary
trypsin inhibitor attenuates lipopolysaccharide-induced acute lung
injury by blocking the activation of p38 mitogen-activated protein
kinase. Inflamm Res. 60:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bouchama A, Roberts G, Al Mohanna F, et
al: Inflammatory, hemostatic, and clinical changes in a baboon
experimental model for heatstroke. J Appl Physiol (1985).
98:697–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang HM, Bodenstein M and Markstaller K:
Overview of the pathology of three widely used animal models of
acute lung injury. Eur Surg Res. 40:305–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li JH, Zhu GB, Chen L, Wang TR and Li SH:
The experimental study of the heat stress on the functional injury
and persisting injury. Sichuan Medical Journal. 31:1747–1750.
2010.(In Chinese).
|
|
20
|
Marino A, Pellegrini F, Lucchesi AM,
Roncucci P, Cosimi A and Logi G: Multiorgan damage in exertion
heatstroke. Minerva Anestesiol. 58:393–395. 1992.(In Italian).
|
|
21
|
Qian LJ, Gong JB and Cheng SQ:
Mitochondrial mechanism of heat stress-induced injury in rat
cardiomyocyte. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 16:133–136.
2000.(In Chinese).
|
|
22
|
Zheng CY, Zhang W and Liang YG:
Inflammatory factor level and ulinastatin intervention in the early
stage of heat stress in rats. Journal of Medical Postgraduates.
24:25–28. 2011.(In Chinese).
|
|
23
|
Naučienė Z, Zūkienė R, Degutytė-Fomins L
and Mildažienė V: Mitochondrial membrane barrier function as a
target of hyperthermia. Medicina (Kaunas). 48:249–255.
2012.PubMed/NCBI
|
|
24
|
Aosasa S, Ono S, Mochizuki H, Tsujimoto H,
Ueno C and Matsumoto A: Mechanism of the inhibitory effect of
protease inhibitor on tumor necrosis factor alpha production of
monocytes. Shock. 15:101–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anetseder M, Hager M, Müller CR and Roewer
N: Diagnosis of susceptibility to malignant hyperthermia by use of
a metabolic test. Lancet. 359:1579–1580. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishio H, Sato T, Fukunishi S, et al:
Identification of malignant hyperthermia-susceptible ryanodine
receptor type 1 gene (RYR1) mutations in a child who died in a car
after exposure to a high environmental temperature. Leg Med
(Tokyo). 11:142–143. 2009. View Article : Google Scholar
|