Introduction
A promising and recently identified alternative to
classical antibiotic treatment is the use of immunomodulators,
which enhance immune reactions via the stimulation of non-specific
systems, such as granulocytes, macrophages, complement, certain
T-lymphocytes and various effector substances (1,2).
Several studies have focused on identifying compounds that are able
to modulate the biological response of immune cells thereby
enhancing the immunity of the host against various diseases
(3,4). The increasing interest in folk
medicine is due numerous well known plant remedies that are able to
exert their anti-infective influence by directly affecting the
pathogen, in addition to affecting immune cells by improving their
activity. These effects were partially contributed to by the
stimulation of the natural and adaptive defense mechanisms of the
host organism (5). Recent advances
have successfully identified a large number of macromolecules that
regulate the host-defense system and the majority of which result
in enhancement, amplification and/or diversion of immune responses
in positive and therapeutically desirable directions (6).
Potentilla indica (formally known as
Duchesnea indica) is a member of the Rosaceae family, which
is native to eastern and southern Asia and is commonly termed a
mock strawberry. It exhibits a moderate cytotoxic effect against
various cancerous cell lines (7–10)
and limited inhibitory activity against normal cell growth
(11). However, few studies were
conducted to investigate the additional bioactivities of this
plant, such as its immunomodulatory effect. Conversely,
Dendrophthoe pentandra is a type of mistletoe that grows on
the rambutan tree. Although mistletoe is a parasitic plant, it has
been widely administered in traditional medicine for the treatment
of coughs and cancer, and as a diuretic agent. Furthermore, various
antioxidant compounds, such as flavonol glycoside and quercitrin,
have been isolated from the ethanol extract of D. pentandra
and the compounds were shown to contribute to its high antioxidant
activity (12). Moreover, it has
been demonstrated to enhance the phagocytic activity of macrophages
(9). To the best of our knowledge,
there is currently no in vitro study on the
immunoproliferative effect of the ethanol extract of P.
indica and D. pentandra against splenocytes and
thymocytes. Therefore, the aim of the present study was to
determine the immunomodulatory effects of these plant extracts on
mice splenocyte and thymocyte proliferation and viability.
Materials and methods
Plant materials
P. indica and D. pentandra were
collected from George Town, Penang, Malaysia and were identified by
the Forest Research Institute (Kuala Lumpur, Malaysia). The plant
leaves were air-dried at room temperature and 2 g was ground, and
soaked in 100 ml ethanol for three days. The extracts were filtered
using Whatman filter paper grade 1 (Sigma-Aldrich, St. Louis, MO,
USA) and dried via evaporation in a reduced-pressure atmosphere
using an Aspirator A-3S (EYELA, Tohoku, Japan) at <45°C. This
process was repeated three times and the yield was 8.7%, w/w. The
dried residue was suspended in dimethylsulfoxide (DMSO; Fisher
Scientific, Loughborough, UK) as extract stock. Briefly, 0.1 g
dried extract was dissolved in 1 ml DMSO to prepare a 10 mg/ml
stock extract. The sub-stock solution (0.2 mg/ml) was prepared by
diluting 20 μl stock solution in 980 μl serum-free Dulbecco’s
modified Eagle’s medium (DMEM; Sigma-Aldrich). The percentage of
DMSO used was <0.5% and the stock and sub-stock solutions were
stored at 4°C.
Animals
Imprinting control region mice (age, 5–8 weeks) were
used in the present study. The mice were purchased from the Animal
House, Universiti Putra Malaysia (Selangor, Malaysia). The mice
were housed under standard conditions at 25±2°C and fed with
standard pellets and tap water. The mice were protected from
stress. The present study was approved by the Institutional Animal
Care and Use Committee of the Universiti Putra Malaysia (Ref:
UPM/FPV/PS/3.2.1.551/AUP-R2).
Preparation of mouse thymus and spleen
cell suspensions
Following the sacrifice of the mice by cervical
dislocation, the thymus and spleen were removed and washed three
times using Hanks’ balanced salt solution (Sigma-Aldrich). The
thymus and spleen were pulverized separately using a rubber syringe
plunger and pushed through an 80 μm sterile wire mesh screen in
phosphate-buffered saline (PBS), which was supplemented with 0.1%
(w/v) bovine serum albumin (BSA) and 2 mg/ml EDTA (PBS/BSA/EDTA;
Sigma-Aldrich) solution, to obtain a single cell suspension. For
the spleen cell suspension, the red blood cells were removed using
a lysis buffer and the spleen and thymus cell suspensions were
washed twice using the PBS/BSA/EDTA solution and suspended in DMEM,
which was supplemented with 10% heat inactivated fetal bovine serum
(PAA, Pasching, Austria). Cell counting was performed using a
hemocytometer to determine the number of lymphocytes within the
cell suspension.
MTT cell proliferation assay
The lymphocytes were harvested during the
logarithmic growth phase and seeded in 96-well plates at a density
of 5×105 cells/ml with a final volume of 100 μl/well.
Following incubation for 24 h, 100 μl P. indica or D.
pentandra extract (200 μg/ml) was loaded into the well plates
and serially diluted. After 24, 48 and 72 h treatment, 20μl MTT (5
mg/ml) was added to each well for 4 h. Subsequently, the
supernatant was removed and the MTT crystals were solubilized with
100 μl anhydrous DMSO per well. Thereafter, the cell viability was
measured using a μQuant™ ELISA reader (BioTek Instruments Inc.,
Winooski, VT, USA) at 570 nm absorbance and the percentage of cell
proliferation was calculated.
Bromodeoxyuridine (BrdU) incorporation
assay
A 96-well plate was used to allocate the different
concentrations of extracts (100, 50 and 1 μg/ml) from either P.
indica or D. pentandra for incubation with the
splenocytes at 5×105 cells/ml media. A BrdU ELISA kit
(Chemicon, Temecula, CA, USA) was used with splenocytes, according
to the manufacturer’s instructions, to estimate the extent of cell
proliferation. The three incubation periods (24, 48 and 72 h) per
extract were analyzed three times and three independent repeats of
the assay were performed on the cells from the control group. The
plate was read at an absorbance of 450 nm using the μQuant ELISA
reader.
Trypan blue exclusion method
The splenocytes and thymocytes (5×105
cell/ml) were treated with 100 or 50 μg/ml P. indica or
D. pentandra extract in 6-well plates for either 24, 48 or
72 h. The untreated cells served as the negative control. Following
the incubation period, the cells were harvested and pelleted at 200
× g for 10 min. The pellets were suspended in 0.4% Trypan blue dye
(Sigma-Aldrich) and 10 μl mixture was placed in a hemocytometer
(Sigma-Aldrich) and the cells were counted under a phase contrast
light microscope (Eclipse Ti, Nikon, Melville, NY, USA). Each of
the extracts and the control were assayed five times in
triplicate.
Statistical analysis
The results were expressed as the mean ± standard
error of the mean and statistical analyses were performed using
SPSS version 16.0 (SPPS Inc., Chicago, IL, USA). The differences
between the means were evaluated using one way analysis of
variance, followed by Duncan’s test and P≤0.05 was considered to
indicate a statistically significant difference.
Results and Discussion
Effects on splenocyte and thymocyte
proliferation observed by MTT assay
The predominant function of the thymus is to develop
immature T cells into immunocompetent T cells, thus, the thymus
contains 99% of mature T lymphocytes (13). By contrast, the spleen contains a
relatively homogenous fraction of B and T lymphocytes, consisting
of ~60% B cells and 40% T cells (14). Thus, an evaluation of the
immunoproliferative effect on these cells provides an understanding
of the influence of the leaf extract on T and B cells. The
MTT-based lymphocyte proliferation assay was performed on specific
immune cells at different incubation periods and the proliferation
effects of P. indica and D. pentandra were analyzed
using various stock concentrations, between 1.563 and 100 μg/ml.
Two mitogens were used in the present study; lipopolysaccharide
(LPS) as the B cell mitogen and concanavalin A (Con A) as the T
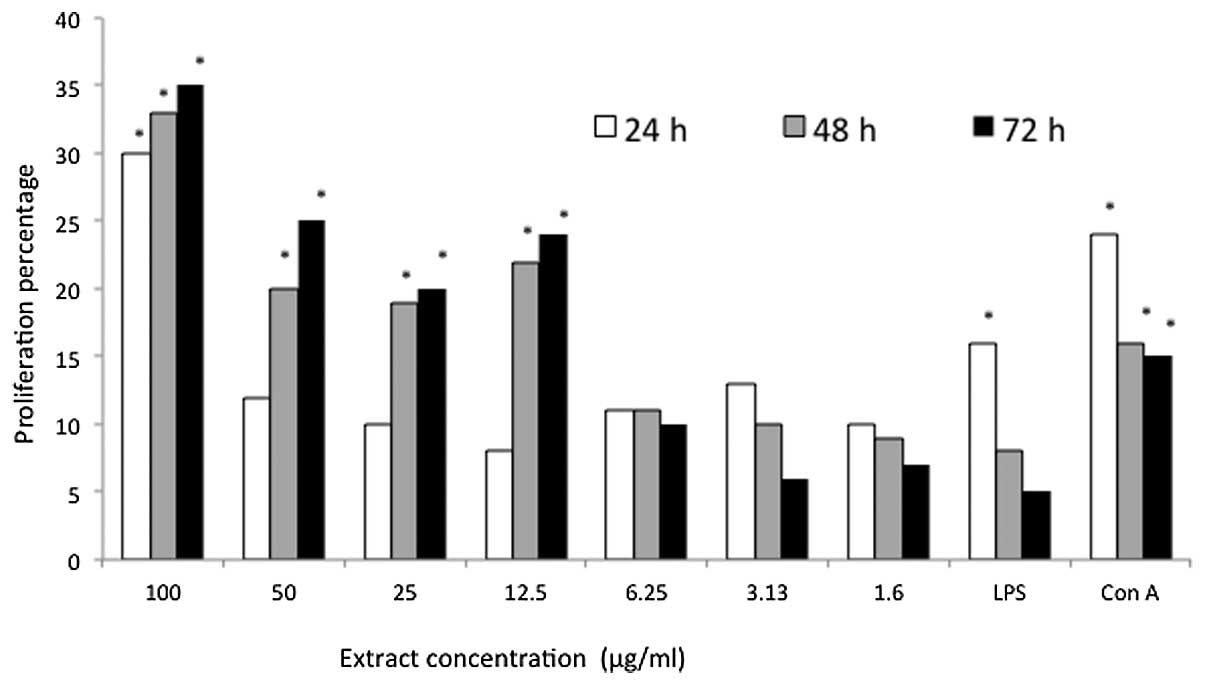
cell mitogen. All of the P. indica extract concentrations
stimulated the proliferation of the mice splenocytes at all of the
incubation times; however, an inhibition of 0.1256% was observed at
the 6.25 μg/ml concentration following 48 h of incubation (Fig. 1). By contrast, the proliferation of
the mice splenocytes induced by 100 μg/ml P. indica extract
increased from 30% at 24 h to 33% after 48 h of treatment. The
greatest proliferation (35%) was obtained following 72 h of
treatment with the same concentration of extract. Furthermore, the
two positive controls were observed to stimulate proliferation of
the mice splenocytes in a time-dependent manner. However, treatment
with P. indica extract indicated an improved stimulatory
effect, exhibiting 30 and 19% more proliferation than the LPS and
Con A groups, respectively, following 72 h of treatment.
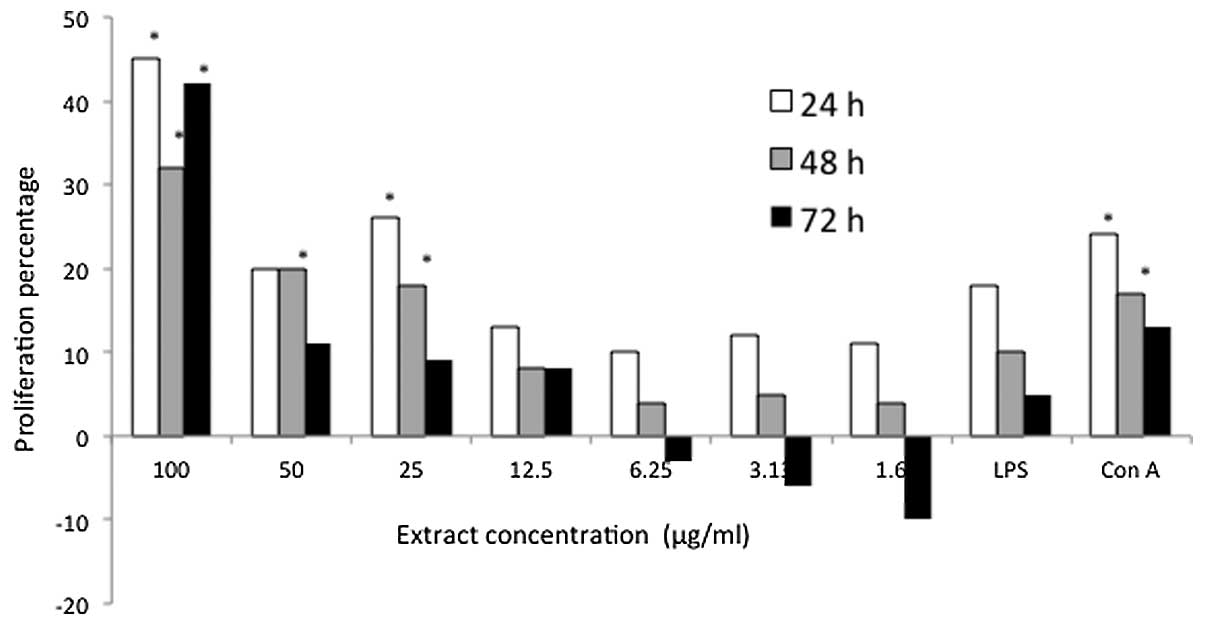
Furthermore, the predominant immunostimulatory effect of D.
pentandra extract on the mice splenocytes was observed at a
concentration of 100 μg/ml (Fig.
2). The highest proliferation was observed in the mice
splenocytes that were treated with 100 μg/ml D. pentandra
extract, although the immunostimulatory effect of D.
pentandra extract on the mice splenocytes was markedly weaker
at low concentrations. Overall, the pattern of mouse splenocyte
proliferation, induced by P. indica and D. pentandra
extracts, were somewhat comparable as the two extracts exhibited
the majority of active proliferation at a concentration of100
μg/ml.
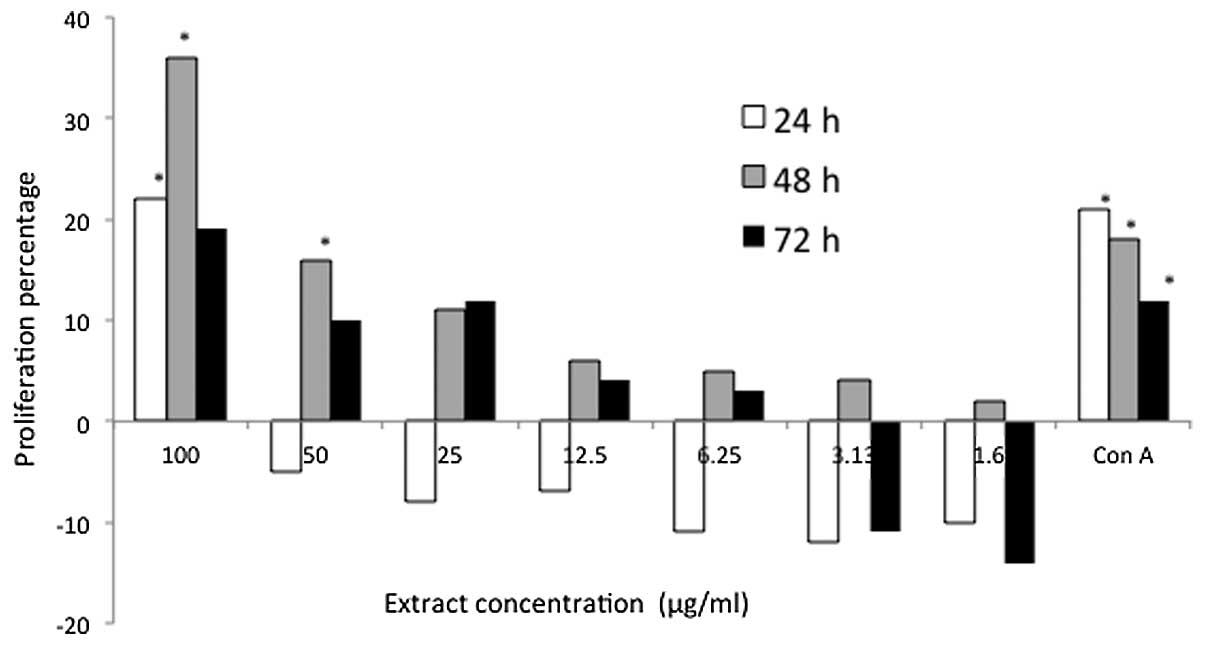
In addition, P. indica exhibited a marked
proliferation effect on T cells, exhibiting induction of 21, 35 and
17% after treatment for 24, 48 and 72 h, respectively, at a
concentration of 100 μg/ml (Fig.
3). The highest stimulatory effect by P. indica extract
on the mouse thymocytes (proliferation, 35%) was observed following
48 h at a concentration of 100 μg/ml. Moreover, it was evident that
the proliferation of mouse thymocytes reduced significantly
throughout the treatment periods when they were treated with low
concentrations (≤50 μg/ml) of P. indica extract. When
compared with the stimulatory effect of 100 μg/ml P. indica
extract, thymocyte proliferation, which was induced by the extract
remained higher than the proliferation that was induced by Con A. A
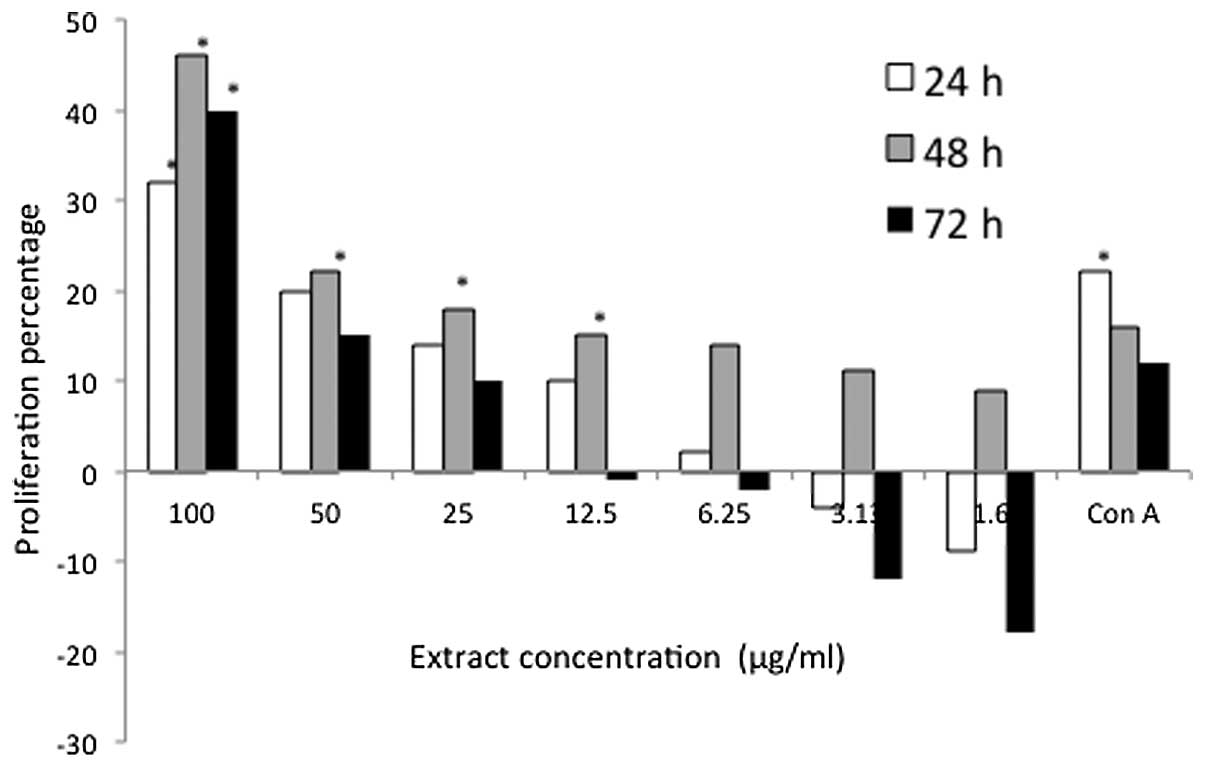
notable proliferation effect was observed at the highest
concentration (100 μg/ml) of D. pentandra extract, with
values of 33, 44 and 41% observed throughout the 24, 48 and 72 h
treatment periods, respectively (Fig.
4). Similarly, the stimulatory effect of 100 μg/ml D.
pentandra extract on mouse thymocyte proliferation was greater
than that of the positive control.
BrdU incorporation assay on
splenocytes
DNA synthesis in cells treated with P. indica
extract for 24 and 48 h was markedly reduced at all of the
concentrations that were employed in the present study (100, 50 and
1 μg/ml), indicating a non-stimulatory effect, which ranged from
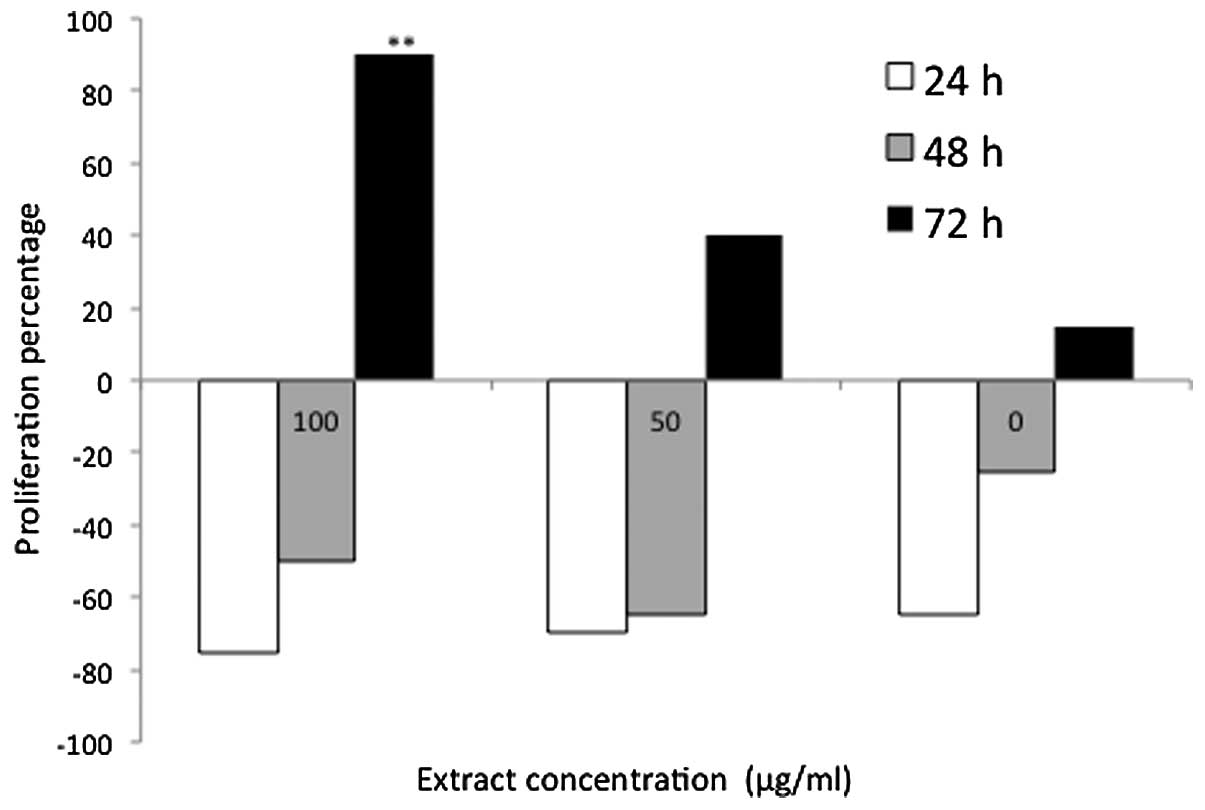
−28 to −78% (Fig. 5). An
incremental increase in DNA synthesis in the P.
indica-treated cells was observed following a prolonged
incubation period of 72 h with 1, 50 and 100 μg/ml P. indica
extract; the DNA synthesis rate was 87, 18 and 42%, respectively.
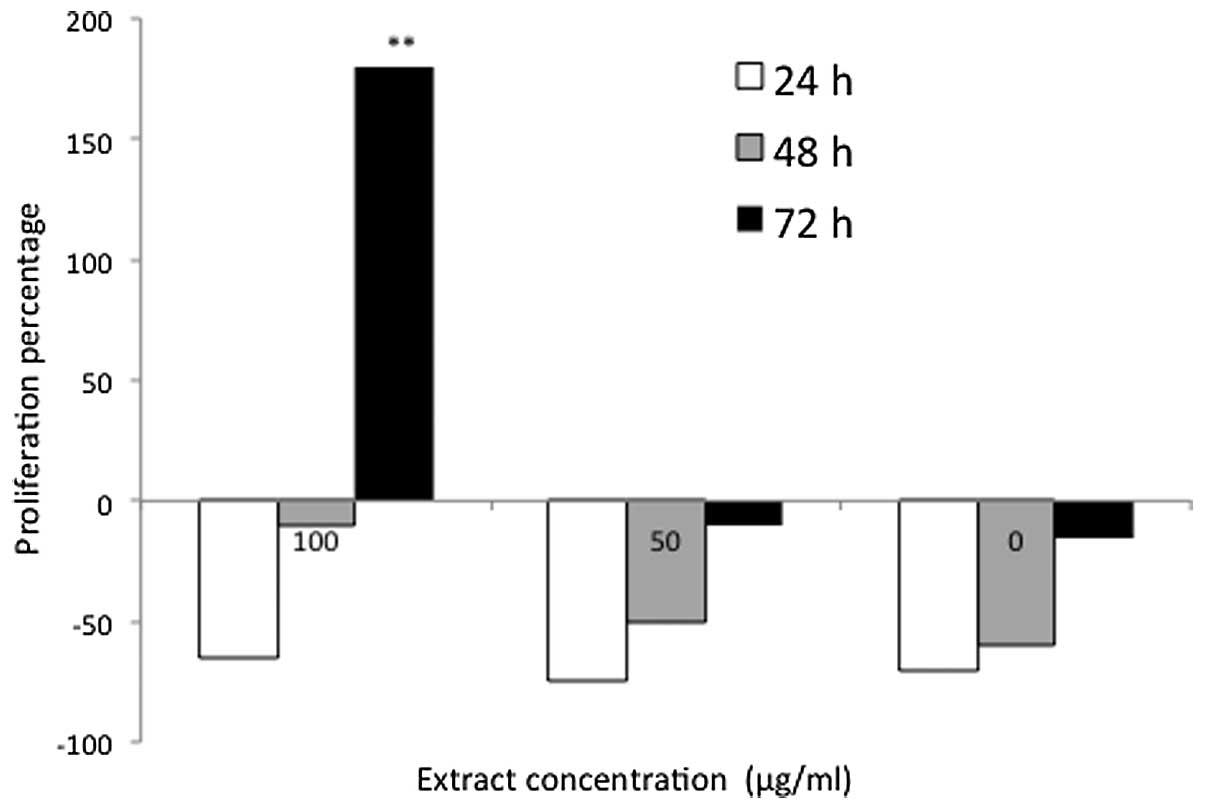
The D. pentandra-treated cells exhibited the greatest rate
of DNA synthesis, which reached 187% following 72 h of treatment
with 100 μg/ml D. pentandra extract (Fig. 6). This demonstrated a 100%
elevation in the proliferation rate of the D.
pentandra-treated cells when compared with the P.
indica-treated cells under matching concentration and
incubation conditions. However, this was the only positive result
that was observed in the D. pentandra-treated cells, whereas
the non-stimulatory effect, ranging from −9 to −81%, was observed
at all of the other concentrations and incubation periods. These
results indicated that P. indica- and D.
pentandra-treated cells exhibited the highest rate of DNA
synthesis following 72 h of treatment, when the greatest
concentration (100 μg/ml) was used. The cells were sustained for
longer in the medium of the two extracts without exhibiting signs
of inhibition or toxicity, however, MTT assays have previously been
reported to overestimate proliferation (15) or underestimate the growth
inhibitory effects of specific cytokines (16). These assays exhibited less
sensitivity when compared with fluorescent labeling techniques
(17) and occasionally failed to
detect the proliferation of lymphocytes (18–20).
In the present study, as the MTT and BrdU assays indicated positive
correlations, it could be deduced that these techniques were
effective in detecting the proliferation of mice splenocytes and
thymocytes.
Trypan blue exclusion assay
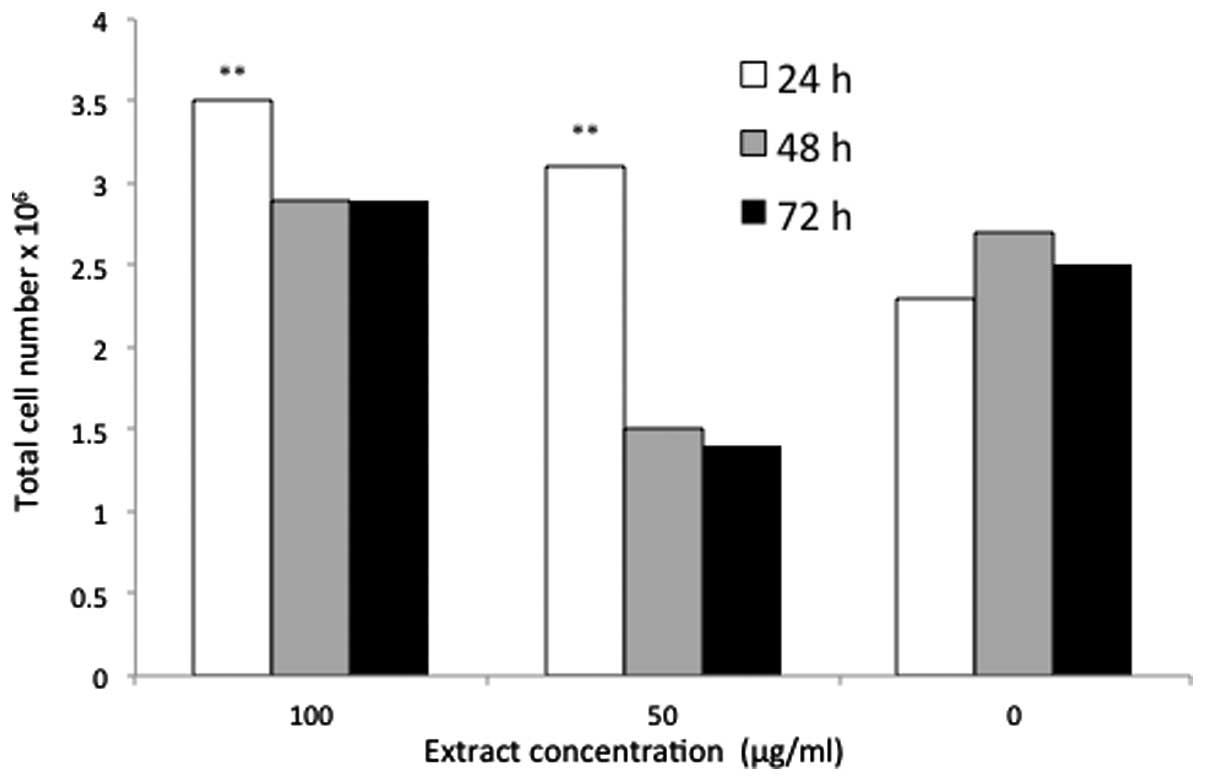
The total cell population was increased to
3.5×106, 2.85×106 and 2.8×106
cells following treatment for 24, 48 and 72 h, respectively, with
100 μg/ml P. indica extract. The total cell number
(3.5×106) was the highest proliferation rate achieved as
a result of treatment with P. indica extract, which was
followed by 3.1×106 cells that was observed following 24
h of treatment with 50 μg/ml P. indica extract (Fig. 7). The greatest cell number
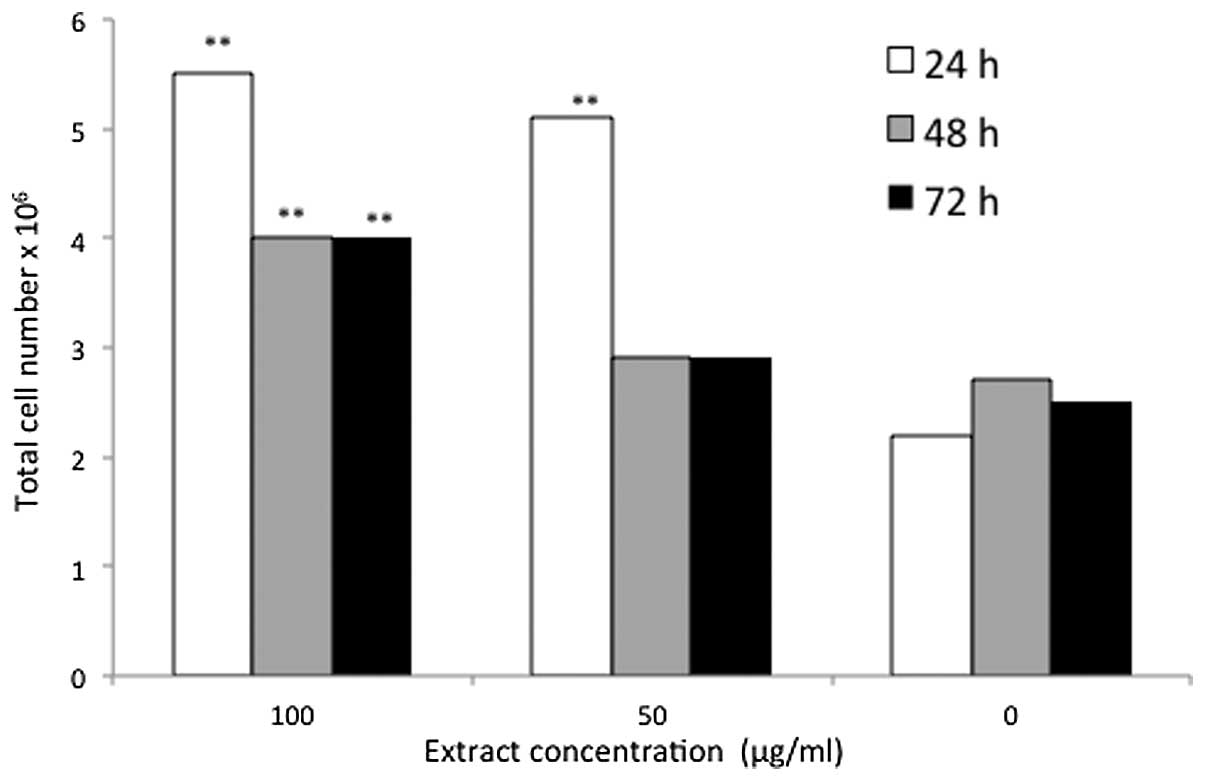
(5.54×106 cells) was achieved following 24 h of
treatment with 100 μg/ml D. pentandra, this was followed by
5.12×106 cells at the concentration of 50 μg/ml.
Following 48 and 72 h, the total cell population resulting from
treatment with 100 μg/ml D. pentandra extract did not
differ; 4.16×106 cells were obtained over the two time
periods. Moreover, a comparable pattern was observed in the D.
pentandra treatment group at a concentration of 50 μg/ml, where
2.88×106 cells were obtained following incubation for 48
and 72 h (Fig. 8). The two
extracts demonstrated that increases in cell number were time- and
dose-dependent and the highest cell population count was observed
following 24 h of incubation with the greatest concentration (100
μg/ml) of P. indica or D. pentandra extract. When
comparing the two extracts, D. pentandra exhibited a greater
stimulatory effect regarding the number of viable cells, the rate
of DNA synthesis and the total cell population. However, the effect
of these extracts requires further investigation to isolate and
evaluate the active metabolites from the extracts, which contribute
to the immunomodulatory effect on thymocytes and splenocytes.
In conclusion, P. indica and D.
pentandra extracts stimulated the proliferation of mice
splenocytes and thymocytes in a time- and dose-dependent manner.
The D. pentandra extracts demonstrated a greater stimulatory
effect on mice splenocytes and thymocytes when compared with the
P. indica extracts. The ability of these plant extracts to
modulate innate immune functions suggests promising further
therapeutic development on wound healing and inhibition of tumor
growth through modulation of lymphocytes.
References
|
1
|
Beutler B: Innate immunity: an overview.
Mol Immunol. 40:845–859. 2004. View Article : Google Scholar
|
|
2
|
Tzianabos AO: Polysaccharide
immunomodulators as therapeutic agents: structural aspects and
biologic function. Clin Microbiol Rev. 13:523–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farnsworth NR, Akerele O, Bingel AS, et
al: Medicinal plants in therapy. Bull World Health Organ.
63:965–981. 1985.PubMed/NCBI
|
|
4
|
Baker JT, Borris RP, Carté B, et al:
Natural product drug discovery and development: new perspectives on
international collaboration. J Nat Prod. 58:1325–1357. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guerra RN, Pereira HA, Silveira LM and
Olea RS: Immunomodulatory properties of Alternanthera tenella
Colla aqueous extracts in mice. Braz J Med Biol Res.
36:1215–1219. 2003.
|
|
6
|
Wagner H: Search for plant derived natural
products with immunostimulatory activity (recent advances). Pure
Appl Chem. 62:1217–1222. 1990. View Article : Google Scholar
|
|
7
|
Peng B, HU Q, Liu X, et al: Duchesnea
phenolic fraction inhibits in vitro and in vivo growth of cervical
cancer through induction of apoptosis and cell cycle arrest. Exp
Biol Med (Maywood). 234:74–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng B, Chang Q, Wang L, et al:
Suppression of human ovarian SKOV-3 cancer cell growth by Duchesnea
phenolic fraction is associated with cell cycle arrest and
apoptosis. Gynecol Oncol. 108:173–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao L, Zhang SL, Tao JY, et al:
Anti-inflammatory mechanism of a folk herbal medicine, Duchesnea
indica (Andr) Focke at RAW264.7 cell line. Immunol Invest.
37:339–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kayser O, Masihi KN and Kiderlen AF:
Natural products and synthetic compounds as immunomodulators. Exp
Rev Anti Infect Ther. 1:319–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shoemaker M, Hamilton B, Dairkee SH, Cohen
I and Campbell MJ: In vitro anticancer activity of twelve Chinese
medicinal herbs. Phytother Res. 19:649–651. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhodes J: Discovery of immunopotentiatory
drugs: current and future strategies. Clin Exp Immunol.
130:363–369. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nowell PC: Phytohemagglutinin: an
initiator of mitosis in cultures of normal human leukocytes. Cancer
Res. 20:462–466. 1960.PubMed/NCBI
|
|
14
|
Andersson J, Sjöberg O and Möller G:
Mitogens as probes for immunocyte activation and cellular
cooperation. Transplant Rev. 11:131–177. 1972.PubMed/NCBI
|
|
15
|
Wong KT and Tan BK: In vitro cytotoxicity
and immunomodulating property of Rhaphidophora korthalsii. J
Ethnopharmacol. 52:53–57. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar
|
|
17
|
Durrieu C, Degraeve P, Carnet-Pantiez A
and Martial A: Assessment of the immunomodulatory activity of
cheese extracts by a complete and easy to handle in vitro screening
methodology. Biotechnol Lett. 27:969–975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jabbar SA, Twentyman PR and Watson JV: The
MTT assay underestimates the growth inhibitory effects of
interferons. Br J Cancer. 60:523–528. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergler W, Petroianu G and Schadel A:
Feasibility of proliferation studies using the BrdU and MTT assays
with a head and neck carcinoma cell line. ORL J Otorhinolaryngol
Relat Spec. 55:230–235. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen CH, Campbell PA and Newman LS: MTT
colorimetric assay detects mitogen responses of spleen but not
blood lymphocytes. Int Arch Allergy Appl Immunol. 93:249–255. 1990.
View Article : Google Scholar : PubMed/NCBI
|