Introduction
Breast cancer is a frequently diagnosed type of
cancer and is a predominant cause of mortality among females
worldwide (1). Multidrug
resistance (MDR) and chemotherapeutic agent toxicity are two
predominant obstacles to the success of chemotherapy (2–4). The
molecular mechanisms that lead to MDR include, the activation of
transport and detoxification systems, enhancement of target repair
activities, alteration of drug targets and dysregulation of cell
death pathways (5,6). MDR can result from the overexpression
of transporter proteins, such as P-glycoprotein (P-gP) and other
breast cancer resistance proteins. P-gP is a 170 kDa plasma
membrane protein that facilitates the efflux of chemotherapeutic
agents from tumor cells. P-gP is coded by the MDR-1 gene and
functions as an energy-dependent efflux pump, which rapidly
extrudes a variety of anticancer drugs from target cancer cells,
thus reducing drug cytotoxicity (7–9). A
number of drugs have been reported to overcome MDR effectively and
are, therefore, considered for use with P-gP inhibitors in
conjunction with other anticancer agents during tumor treatment
(10,11). However, the side effects of these
agents compromise their clinical application. Thus, the
identification of novel agents with low toxicity is necessary to
satisfy the requirement in clinical applications.
Resveratrol (trans-3,4′,5-trihydroxystilbene; RES),
a compound obtained primarily from root extracts of the oriental
plant, Polygonum cuspidatum and from red grapes, has been
identified by previous studies as possessing a strong
chemopreventive effect against the development of breast cancer
(12–15). Our previous studies demonstrated
that RES, quercetin or ferulic acid alone are able to inhibit human
breast cancer doxorubicin (DOX)-resistant (MCF-7/DOX) cell
proliferation; moreover, RES more efficiently inhibited cancer cell
proliferation than quercetin or fumaric acid (16). Although RES was reportedly capable
of enhancing the cytotoxicity of anticancer agents by increasing
the intracellular concentrations and inhibiting MDR-1 expression in
solid tumor cell lines, including the MCF-7 cell line (17), the mechanisms that enable RES to
possess a unique antitumor function remain unidentified. The
present study hypothesized that reversing the MDR of cancer cells
may be an important mechanism.
In the present study, the MCF-7/DOX cell line,
characterized by DOX resistance, was used to identify whether RES
was capable of reversing the MDR of MCF-7/DOX cells in response to
DOX and explore the related mechanism.
Materials and methods
Cell culture
MCF-7 and MCF-7/DOX cells (Nanjing KGI Biological
Technology Development Co. Ltd., Nanjing, China) were cultured in
RPMI-1640 medium (Gibco-BRL, Rockville, MD, USA), which was
supplemented with 10% fetal calf serum (Gibco-BRL) at 37°C in a
humidified 5% CO2 atmosphere. The MCF-7/DOX cells were
maintained in a culture medium with or without supplementation of
1.0 μg/ml DOX (Haizheng Medicine Co. Ltd., Zhengjiang, China) two
weeks prior to the planned experiments.
Reversal index (RI) assay
To determine the MDR of the MCF-7/DOX cells to
chemotherapeutic agents, the MCF-7 and MCF-7/DOX cells were seeded
on 96-well plates (2×104 cells/well) and incubated with
various concentrations of DOX (0, 0.01, 0.1, 1, 10 or 100 μM)
dissolved in dimethylsulfoxide (DMSO) at a final concentration of
0.1% DMSO. RPMI-1640 culture medium served as a negative control
and RPMI-1640 culture medium supplemented with 0.1% DMSO, served as
a vehicle control. The cytotoxicity of DOX was measured via an MTT
assay (18). Following 48 h of
incubation, 200 μl MTT solution (0.5 mg/ml) was added to each well
and incubated for 4 h at 37°C. The supernatants were transferred to
new 96-well plates and the absorbance was recorded at a wavelength
of 570 nm in microplate reader [Multiskan MK3; Thermo Electric
(Shanghai) Technology Instrument Co., Ltd., Shanghai, China].
The half maximal inhibitory concentration
(IC50) was defined as the concentration of the drug that
resulted in 50% inhibition of cell growth and was obtained via
regression analysis between the drug concentration and cell
inhibition rate. The RI value was calculated by dividing the
IC50 value of the MDR (MCF-7/DOX) cells by the value of
the sensitive (MCF-7) cells.
Intrinsic cytotoxicity assay
The in vitro cytotoxicity of RES was measured
via an MTT assay. Briefly, MCF-7 and MCF-7/DOX cells at a
confluence level of 80–90% were digested and re-seeded on 96-well
culture plates (2×104 cells/well). The cells were
incubated at 37°C in a 5% CO2 atmosphere. Following 24
h, the culture medium was refreshed with RPMI-1640 that was
supplemented with various concentrations of RES (4, 8, 12 or 16 μM)
dissolved in DMSO with a final concentration of 0.1%
(Sigma-Aldrich, St. Louis, MO, USA). RPMI-1640 culture medium
served as a negative control and RPMI-1640 culture medium
supplemented with 0.1% DMSO, served as vehicle control. As
described above, following 48 h of incubation, 200 μl MTT solution
(0.5 mg/ml) was added to each well and incubated for 4 h at 37°C.
The supernatants were transferred to new 96-well plates and the
absorbance was recorded at a wavelength of 570 nm, after which the
inhibition ratio (IR) and IC10 values were
calculated.
Reversing drug resistance assay
After seeding 1×104 MCF-7/DOX cells per
well in a 96-well plate for 24 h, the growth medium was refreshed
using a medium that contained RES, DOX or a combination of RES and
DOX. Subsequent to 48 h of exposure, the cytotoxicity of the drugs
was assessed via an MTT assay. The combinational index (Q) was
calculated using the formula: Q=Ea+b/(Ea+Eb-Ea×Eb). Where, Ea+b
represented the combinational inhibition rate of RES and DOX and Ea
and Eb represented the individual inhibition rate of RES and DOX,
respectively. The nature of the drug interaction was defined as: i)
Additive (+) if Q ranged from 0.85 to 1.15; ii)
synergism (++) if Q ranged from 1.15 to 2.0; iii)
subtraction (−) if Q ranged from 0.85 to 0.55; and iv)
antagonism (−−) when the confidence interval was
<0.55. The RI value of RES was calculated by dividing the
IC50 of DOX by the value of the RES and DOX
combination.
Intracellular accumulation of DOX
MCF-7/DOX cells were cultured in the absence or
presence of RES at concentrations of 4, 8, 12 or 16 μM; DOX was
added to the cells to obtain a final concentration of 4, 16 or 64
μM. Following 3 h of incubation, the cells were washed three times
with ice-cold phosphate-buffered saline (PBS) and incubated in
isopropanol overnight at −20°C. The absorbance of the supernatant
was read using a fluorescence spectrofluorometer (Hitachi High-Tech
Companies, Tokyo, Japan) at wavelengths of 470 and 590 nm. The
value of DOX accumulation within the cells was calculated according
to the standard curve (19,20).
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the MCF-7/DOX cells of
the different groups (treated with various concentrations of RES,
DOX or combinations of RES and DOX) using TRIzol Reagent
(Sigma-Aldrich) according to the manufacturer’s instructions.
Thereafter, 2 μg total RNA was used to perform first-strand cDNA
synthesis (Takara Biotechnology, Co. Ltd., Dalian, China) and PCR
was performed using an Applied Biosystems® 7500 RT-PCR
analyzer (Carlsbad, CA, USA). The primer sequences were as follows:
Forward: 5′-CCCATCATTGCAATAGCAGG-3′ and reverse:
5′-GTTCAAACTTCTGCTCCTGA-3′ for the MDR-1 gene, and the length of
the PCR product was 157 bp. The second primer sequence was as
follows: Forward: 5′-CACGTCACACTTCATGATGG-3′ and reverse:
5′-ATGTTTGAGACCTTCAACAC-3′ for β-actin, and the length of the PCR
product was 496 bp. The amplification conditions were 3 min at 94°C
for denaturing, 30 cycles of amplification (94°C for 30 sec, 57°C
for 30 sec and 72°C for 1 min) and a cooling step at 4°C. The PCR
products were subjected to 1% agarose gel electrophoresis and the
spectral density of the bands was visualized and analyzed in a
Bandscan 5.0 image analysis system (Glyko Inc., Hayward, CA, USA).
The relative gene expression of MDR-1 was determined by normalizing
the density of MDR-1 to that of β-actin.
Western blot analysis
The cells were washed with ice-cold PBS and lysed
for 30 min in ice-cold radio-immunoprecipitation assay lysis buffer
(20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM ethylene glycol
tetraacetic acid, 150 mM NaCl, 1% Triton X-100 and protease
inhibitor cocktail; Sigma-Aldrich, St. Louis, MO, USA). The protein
concentration was measured using a bicinchoninic acid protein assay
kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The protein
samples were separated via SDS-PAGE and electroblotted to
polyvinylidene difluoride membranes (Millipore, Billerica,
MA, USA). The membranes were blocked with Tris-buffered saline
(TBS) overnight at 4°C and incubated with primary mouse monoclonal
antibodies (Maixin Biotechnology Co. Ltd. Fuzhou, China) against
P-gP or β-actin for 2 h at room temperature. Following three washes
in TBS with 0.1% Tween-20 (TBST), the membranes were probed with a
secondary horseradish peroxidase-conjugated goat anti-mouse
antibody (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) for 2 h. Following a further three washes with
TBST, the immune complexes were detected by chemiluminescence (KPL
Inc., Gaithersburg, MD, USA). The spectral density of the bands was
visualized and analyzed using a Bandscan 5.0 image analysis system
and the expression of P-gP was obtained by normalizing the density
of P-gP to that of β-actin.
Statistical analysis
Data were expressed as the mean ± standard deviation
(n=4). Statistical significance was assessed using one-way analysis
of variance with SPSS 19.0 software (SPSS Inc., Chicago, IL, USA)
and P<0.05 was considered to indicate a statistically
significant difference.
Results
RES inhibits the proliferation of
MCF-7/DOX and MCF-7 cells
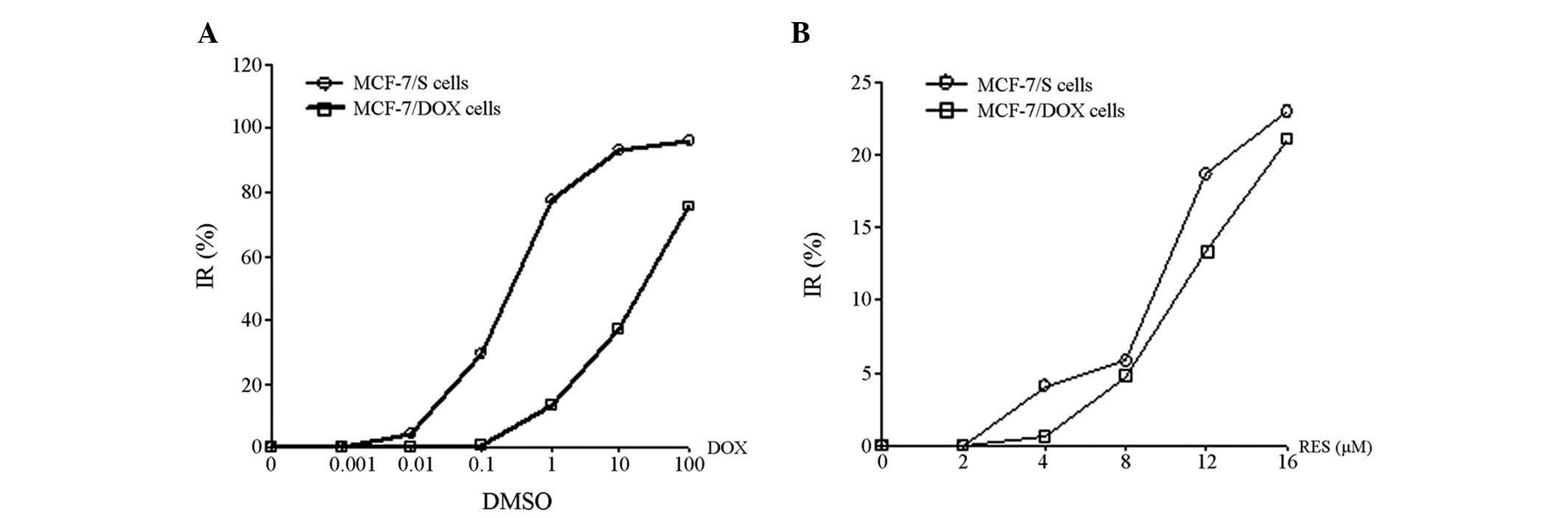
The IC50 values of DOX were 0.39 and
21.38 μM in MCF-7 and MCF-7/DOX cells, respectively (Fig. 1A). The MCF-7/DOX cells were 54.82
times more resistant to DOX, when compared with the MCF-7 cells.
RES was identified to be capable of inhibiting the proliferation of
MCF-7/DOX and MCF-7 cells; however, no significant difference was
demonstrated between the IC10 of RES on MCF-7 cells
(8.46 μM) and that of MCF-7/DOX cells (11.39 μM; P>0.05). In
addition, the proliferation of MCF-7/DOX cells was inhibited by RES
in a dose-dependent manner (Fig.
1B).
RES enhances the cytotoxicity of DOX on
MCF-7/DOX cells
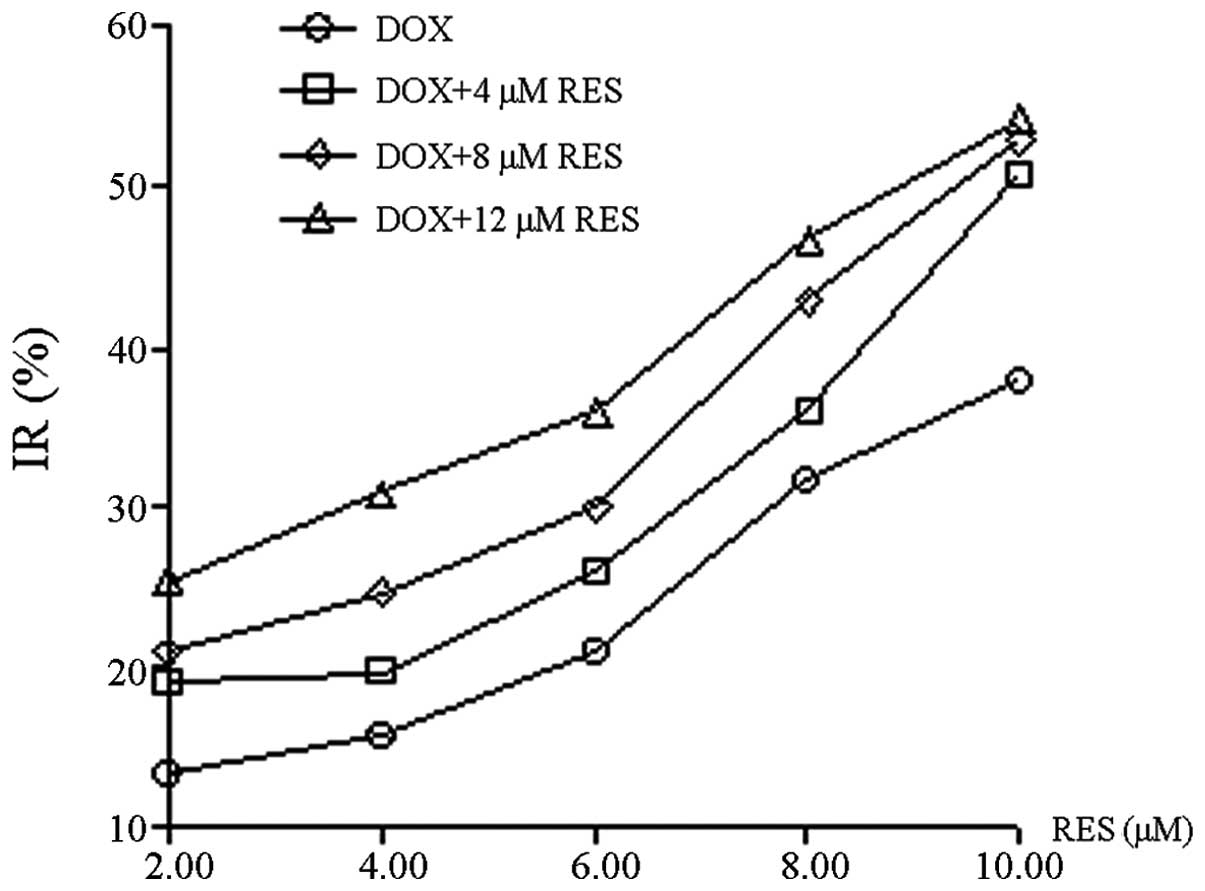
RES was demonstrated to inhibit the growth of
MCF-7/DOX cells in a dose-dependent manner (Fig. 2); with the increase in RES
concentration, the growth of MCF-7/DOX cells gradually decreased.
RES at 12 μM exhibited a comparable inhibitory rate (~10%) on MCF-7
and MCF-7/DOX cells, therefore, the concentration of 12 μM was
considered to be a non-cytotoxic dose. The reversal effect of RES
at a concentration of 12 μM on the MDR of MCF-7/DOX cells was
investigated. Q and RI were calculated based on the IR and 50%
IC50; the values were subsequently used to assess the
combinational inhibitory effect of RES and DOX on the MCF-7/DOX
cells. RES and DOX were identified to synergistically inhibit
MCF-7/DOX cell growth and Q was often >1.15. Moreover, RES
enhanced the inhibitory effect of DOX on cell growth in a
dose-dependent manner and the RI of DOX was increased from 1.950 to
2.355, as the concentration of RES increased from 4 to 12 μM. The
effect of RES on the enhancement of DOX cytotoxicity within
MCF-7/DOX cells is shown in Table
I.
 | Table IAntitumor effects of RES combinined
with DOX on MCF-7/DOX cells (n=4). |
Table I
Antitumor effects of RES combinined
with DOX on MCF-7/DOX cells (n=4).
| 0 μM RES | 4 μM RES | | 8 μM RES | | 12 μM RES | |
|---|
|
|
| |
| |
| |
|---|
| OD | IR (%) | OD | IR (%) | Q | OD | IR (%) | Q | OD | IR (%) | Q |
|---|
| DOX (μM) | | | | | | | | | | | |
| 2 | 0.972±0.094 | 13.37 | 0.910±0.012 | 18.89 | 1.365 | 0.887±0.014 | 20.94 | 1.196 | 0.839±0.029 | 25.22 | 1.010 |
| 4 | 0.947±0.062 | 15.60 | 0.902±0.019 | 19.61 | 1.221 | 0.847±0.034 | 24.51 | 1.249 | 0.775±0.042 | 30.93 | 1.149 |
| 6 | 0.888±0.077 | 20.86 | 0.831±0.077 | 25.94 | 1.219 | 0.786±0.057 | 29.95 | 1.216 | 0.718±0.040 | 36.01 | 1.144 |
| 8 | 0.767±0.088 | 31.52 | 0.718±0.075 | 36.01 | 1.130 | 0.640±0.127 | 42.96 | 1.235 | 0.598±0.036 | 46.70 | 1.147 |
| 10 | 0.695±0.019 | 38.06 | 0.553±0.017 | 50.71 | 1.321 | 0.528±0.039 | 52.94 | 1.291 | 0.513±0.022 | 54.28 | 1.171 |
| IC50 | | | 10.940 | 9.817 | 9.077 |
| RI | | | 1.950 | 2.178 | 2.355 |
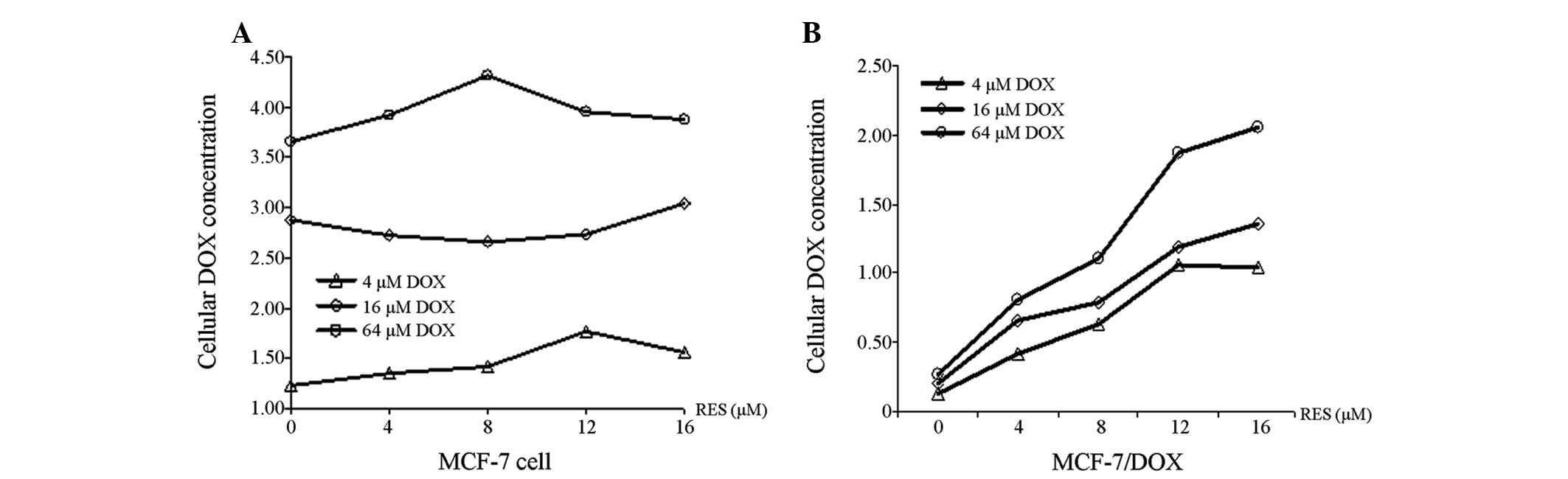
RES increases DOX accumulation within
MCF-7/DOX cells
The capability of RES to promote DOX accumulation
within MCF-7 and MCF-7/DOX cells is shown in Fig. 3. The concentration of DOX in MCF-7
(Fig. 3A) and MCF-7/DOX cells
(Fig. 3B) increased with
increasing DOX treatment, regardless of the RES dose, however, the
concentration of DOX in MCF-7/DOX cells was significantly lower
than that observed in the MCF-7 cells (P<0.01). In addition, RES
was demonstrated to be capable of elevating the concentration of
DOX in MCF-7/DOX cells in a dose-dependent manner, however, this
did not occur in the MCF-7 cells.
RES decreases MDR-1 gene and protein
expression levels within MCF-7/DOX cells
To determine the mechanism by which RES functionally
elevates drug accumulation within MCF-7/DOX cells, MDR-1 mRNA
expression was quantitatively measured using RT-PCR. In addition,
P-gP, a protein encoded by MDR-1, was quantitatively measured using
western blot analysis. The levels of MDRl gene expression (Fig. 4A) and P-gP expression (Fig. 4B) in MCF-7/DOX cells significantly
decreased when the cells were treated with a combination of RES and
DOX (P<0.05).
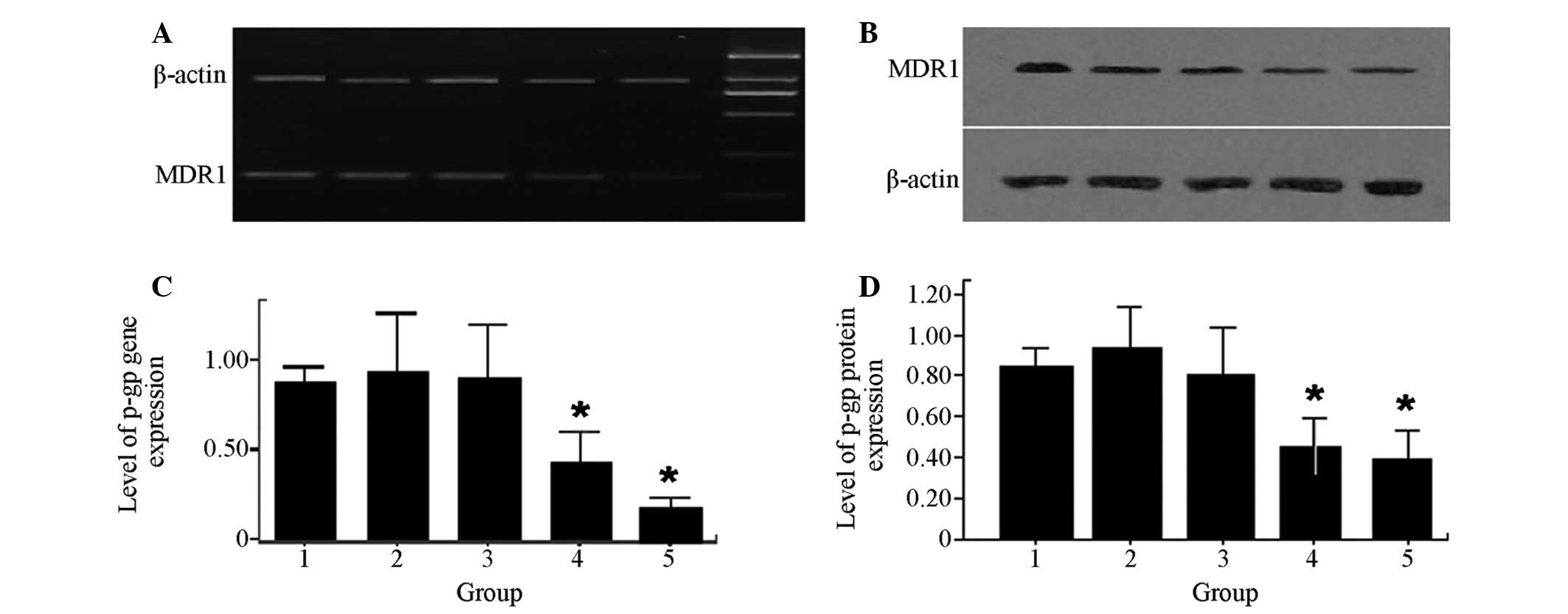 | Figure 4Effects of RES and DOX on MDR-1 gene
and protein expression in MCF-7/DOX cells. (A and C) Lane 1, MDR-1
gene expression levels in MCF-7/DOX cells; lanes 2, 3, 4 and 5,
treatment with 8 μM DOX, 10 μM RES, 6 μM DOX combined with 8 μM
RES, 10 μM DOX combined with 12 μM RES for 48 h, respectively;
β-actin, control; lane M, DNA ladder. (B and D) Lane 1, protein
expression of P-gP in MCF-7/DOX cells; lanes 2, 3, 4 and 5,
treatment with 8 μM DOX, 10 μM RES, 6 μM DOX combined with 8 μM
RES, 10 μM DOX combined with 12 μM RES for 48 h, respectively.
*P<0.05 compared with lane 1. MDR, multidrug
resistance; RES, resveratrol; DOX, doxorubicin. |
Discussion
MDR is a prevalent issue in cancer chemotherapy,
thus, reversing MDR in cancer cells may provide a basis for
overcoming drug resistance, and improving chemotherapy and the
outcome for cancer patients. RES is hypothesized to possess unique
health benefits, including prolonging life, providing
cardiovascular protection and exhibiting anti-inflammatory effects
(21).
In the present study, the inhibitory effect of RES
on human breast cancer cell proliferation was investigated. The
results indicated that RES inhibited the proliferation of MCF-7/DOX
and MCF-7 cells, which was consistent with previous studies that
demonstrated a strong chemopreventive effect of RES against the
development of breast cancer (14,15).
RES inhibited the growth of human cancer cells in vitro,
when administered alone or in combination with other anticancer
drugs (22,23). Furthermore, the effects of RES on
the cytotoxicity of DOX in MCF-7/DOX cells, which exhibited
DOX-resistance was investigated in the present study. The RI of
MCF-7/DOX cells, relative to DOX and RES treatment, was observed to
be significantly higher than that of the group without RES
treatment. These results demonstrated that RES enhanced the DOX
cytotoxicity effect within MCF-7/DOX cells, which indicated a
synergistic effect of RES and DOX.
In addition, the mechanism by which RES enhanced DOX
cytotoxicity was investigated. It was identified that, when
combined with DOX, RES elevated the concentration of DOX in
MCF-7/DOX cells in a dose-dependent manner, while promoting DOX
accumulation in the MCF-7/DOX cells. This result provides a partial
explanation for why RES may enhance DOX cytotoxicity within
MCF-7/DOX cells. The results further revealed that the mRNA and
protein expression of the MDR-1 gene were significantly inhibited
by RES, indicating that RES enhanced DOX cytotoxicity via
downregulating MDR-1 expression. In addition, one of the membrane
transport proteins, P-gP was identified in a previous study to
promote the expulsion of anticancer drugs, which is considered to
be a typical MDR mechanism (24).
In conclusion, the mechanism by which RES exerts its
antitumor efficacy remains to be determined. The present study
demonstrated that RES inhibited the proliferation of MCF-7/DOX and
MCF-7 cells in a dose-dependent manner and significantly enhanced
the cytotoxicity of DOX within MCF-7/DOX cells. Moreover, RI was
observed to be significantly higher with RES treatment when
compared with cells without treatment. In addition, RES reversed
MDR in MCF-7/DOX cells, elevated the concentration of DOX within
MCF-7/DOX cells and significantly downregulated the expression of
the MDR-1 gene and P-gP protein. Therefore, it was concluded that
reversing DOX resistance by downregulating MDR-1 expression, is one
of the mechanisms that provides RES with a unique antitumor
function. Thus, these findings indicate that RES may potentially
act as a novel MDR reversal agent for breast cancer therapy.
Acknowledgements
The present study was supported by the Foundation of
Fujian Province Key Laboratory of Environment and Health (GW15;
Fujian, China).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global Cancer Statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Clarke R, Currier S, Kaplan O, Lovelace E,
Boulay V, Gottesman MM and Dickson RB: Effect of P-glycoprotein
expression on sensitivity to hormones in MCF-7 human breast cancer
cells. J Natl Cancer Inst. 84:1506–1512. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robert J: Resistance to cytotoxic agents.
Curr Opin Pharmacol. 1:353–357. 2001. View Article : Google Scholar
|
|
4
|
Avendaño C and Menéndez JC: Inhibitors of
multidrug resistance to antitumor agents (MDR). Curr Med Chem.
9:159–193. 2002.PubMed/NCBI
|
|
5
|
Ross DD: Novel mechanisms of drug
resistance in leukemia. Leukemia. 14:467–473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM and Pastan I: Biochemistry of
multidrug resistance mediated by the multidrug transporter. Annu
Rev Biochem. 62:385–427. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan B, Piwnica-Worms D and Ratner L:
Multidrug resistance transporters and modulation. Curr Opin Oncol.
12:450–458. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mayer LD and Shabbits JA: The role for
liposomal drug delivery in molecular and pharmacological strategies
to overcome multidrug resistance. Cancer Metastasis Rev. 20:87–93.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coley HM: Mechanisms and strategies to
overcome chemotherapy resistance in metastatic breast cancer.
Cancer Treat Rev. 34:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu HJ, Wang JS, Guo QL, Jiang Y and Liu
GQ: Reversal of P-glycoprotein mediated multidrug resistance in
K562 cell line by a novel synthetic calmodulin inhibitor, E6. Biol
Pharm Bull. 28:1974–1978. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, Liu M, Aneja R, Chandra R, Lage H
and Joshi HC: Reversal of P-glycoprotein-mediated multidrug
resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer
Res. 66:445–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, et al: Cancer chemopreventive activity of
resveratrol, a natural product derived from grapes. Science.
275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol:
mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goswami SK and Das DK: Resveratrol and
chemoprevention. Cancer Lett. 284:1–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bishayee A: Cancer prevention and
treatment with resveratrol: from rodent studies to clinical trials.
Cancer Prev Res (Phila). 2:409–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang F, Huang ZJ, Chen J and Wang JL:
Studies of several polyphenolses on multidrug resistance to human
breast carcinoma MCF-7/ADM Cell. J Pract Oncol. 24:121–123.
2009.(In Chinese).
|
|
17
|
Al-Abd AM, Mahmoud AM, El-Sherbiny GA,
El-Moselhy MA, Nofal SM, El-Latif HA, et al: Resveratrol enhances
the cytotoxic profile of docetaxel and doxorubicin in solid tumour
cell lines in vitro. Cell Prolif. 44:591–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hansen MB, Nielsen SE and Berg K:
Re-examination and further development of a precise and rapid dye
method for measuring cell growth/cell kill. J Immunol Methods.
119:203–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chambers SK, Hait WN, Kacinski BM, Keyes
SR and Handschumacher RE: Enhancement of anthracycline growth
inhibition in parent and multidrug resistant Chinese hamster ovary
cells by cyclosporin A and its analogues. Cancer Res. 49:6275–6279.
1989.PubMed/NCBI
|
|
20
|
Ganapathi R, Grabowski D, Rouse W and
Riegler F: Differential effect of the calmodulin inhibitor
trifluoperazine on cellular accumulation, retention, and
cytotoxicity of anthracyclines in doxorubicin
(adriamycin)-resistant P388 mouse leukemia cells. Cancer Res.
44:5056–5061. 1984.
|
|
21
|
Marques FZ, Markus MA and Morris BJ:
Resveratrol: cellular actions of a potent natural chemical that
confers a diversity of health benefits. Int J Biochem Cell Biol.
41:2125–2128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pozo-Guisado E, Merino JM, Mulero-Navarro
S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A and
Fernandez-Salguero PM: Resveratrol-induced apoptosis in MCF-7 human
breast cancer cells involves a caspase-independent mechanism with
downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 115:74–84.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benitez DA, Pozo-Guisado E,
Alvarez-Barrientos A, Fernandez-Salguero PM and Castellón EA:
Mechanisms involved in resveratrol-induced apoptosis and cell cycle
arrest in prostate cancer-derived cell lines. J Androl. 28:282–293.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loo TW and Clarke DM: Location of the
rhodamine-binding site in the human multidrug resistance
P-glycoprotein. J Biol Chem. 277:44332–44338. 2002. View Article : Google Scholar : PubMed/NCBI
|