Introduction
The annual incidence of upper urinary tract calculi
has steadily increased in Asia and this trend is likely to continue
in the near future (1,2). The calculi may lead to
hydronephrosis, a type of renal atrophy that may result in renal
insufficiency or a unilateral non-functioning kidney (NFK)
(3). In our previous study, a
gender difference in the development of NFK was observed among
patients with urolithiasis (4).
Sex hormones may contribute to this phenomenon.
Estrogen has been implicated in the pathophysiology
of chronic kidney disease and has been shown to provide a
protective effect against chronic renal damage (5–7). The
protective effects likely act through the renin-angiotensin system
(8–10), nitric oxide pathway (11,12),
extracellular matrix metabolic pathway, inflammatory response
pathway (13–17), lipid metabolic pathway and numerous
other pathways (18–20).
However, few groups have reported whether estrogen
is able to preserve the renal function in unilateral ureteral
obstruction (UUO). This study aimed to test the hypothesis that
estrogen administration is able to preserve the split renal
function in UUO and that estrogen deficit has a harmful effect.
Material and methods
Experimental model
Experiments were performed in 15 female
Sprague-Dawley (SD) rats [supplied by the Department of Laboratory
Animal Science, Fudan University, Shanghai, China; Certificate No.
2009001901383, SCXK (Shanghai) 2009-0019] aged 6–8 weeks and
weighing 200±10 g. The study was carried out in strict accordance
with the Guide for the Care and Use of Laboratory Animals (8th
edition, 2011). The protocol was approved by the Animal Welfare and
Ethics Committee, Fudan University. Female castration, UUO creation
and single-photon emission computed tomography (SPECT/CT) were
performed under anesthesia of sodium pentobarbital (50 mg/kg,
intraperitoneally).
For the low-estrogen model, female castration
(ovariectomy, OVX) was performed with the removal of both ovaries
and a small section of uterus through two small waist incisions. To
create the high-estrogen model, estrogen with a vehicle of tea oil
injection was intraperitoneally injected (10 μg/rat) on alternate
days. To create the normal-estrogen model, the rats were left
untreated. After 3 weeks, the blood was withdrawn from the caudal
vein for the determination of serum estrogen concentrations.
For UUO creation, a low midline abdominal incision
was made under anesthesia. After the ureter was mobilized and
isolated with minimal dissection, it was ligated with two 4-zero
silk sutures (Johnson-Johnson, Shanghai, China) at the
ureterovesical junction. The wound was sutured using 2% lidocaine
solution as an anaesthetic and hydropathically compressed (Shanghai
Zhaohui Pharmaceutical Co., Ltd., Shanghai, China) to relieve
pain.
Study design
The rats were randomly divided into three groups:
high- (5 rats, Estrogen administration + UUO), normal- (5 rats,
only UUO) and low- (5 rats, OVX + UUO) estrogen groups.
Radioimmunoassay of estrogen was performed to confirm the different
estrogen levels. UUO was performed followed by 3 weeks of estrogen
modeling. On days 2, 6 and 16 after surgery, split renal function
[glomerular filtration rate (GFR)] was measured by SPECT/CT with
99mTc-labelled diethylene triamine pentaacetate (DTPA). A
preliminary experiment showed marked nephrosis and cystic changes
of the obstructed kidney on day 16; therefore, pre-surgery and
post-surgery (day 17) serum creatinine levels were also measured.
Routine immunohistochemistry (IHC), pathology and
electron-microscopic (EM) examinations were performed to compare
histological differences. The rats were sacrificed with an overdose
of anesthetic (sodium pentobarbital, 100 mg/Kg,
intraperitoneally).
Evaluation
Evaluation included split renal function (GFR),
serum creatinine, pathology and EM examinations for all three
groups.
For the evaluation of GFR, the rat was fastened to
the scanning table in the supine position under anesthesia. Bolus
injection of 99mTc-DTPA (30 μCi/100 g) was carried out
intravenously and a standard renal scan was performed. In order to
avoid random errors, the results were calculated three times by
three professional doctors of nuclear medicine and then
averaged.
For serum creatinine evaluation, a blood sample was
withdrawn from the caudal vein or inferior vena cava and subjected
to biochemical analysis.
For pathology and EM examinations, specimens of both
kidneys were fixed by glutaraldehyde and formalin. EM examination
was performed on a Philips CM120 transmission electron microscope
(Philips, Amsterdam, The Netherlands). Hematoxylin and eosin
(H&E) staining, and IHC staining of TGF-β and α-SMA were
performed on paraffin-embedded tissue blocks. The H&E-stained
slides were reviewed by the same pathologist. The positive staining
area percentages of TGF-β and α-SMA slides were measured using
Image-Pro Plus (IPP) V 6.0 software (Media Cybernetics, Rockville,
MD, USA) with an average of five high magnification fields for each
slide. The slides were visualized under the same circumstances at
the same time.
Statistical analysis
SPSS software (version 19.0; IBM, Armonk, NY, USA)
was used for statistical analysis. The groups of data were normally
distributed with P>0.05. The Student’s t-test and analysis of
variance test were used to compare the data of three groups for
categorical variables. P<0.05 was considered to indicate a
statistically significant result.
Results
A total of 15 female SD rats were included in the
present study with five rats in each group. Three rats died of
accidental anesthesia overdose. No other complications were
observed
Changes in serum estrogen
Blood samples was obtained after 3 weeks of estrogen
modeling by medication or surgery. The concentration of estrogen
was 89.01±11.19 pg/ml for the low-estrogen group, 135.97±26.23
pg/ml for the normal-estrogen group and 209.68±13.86 pg/ml for the
high-estrogen group (P<0.001). Thus, the various estrogen animal
models were established successfully.
Prior to modeling, the rats appeared identical with
the same weight, body morphology and hair color pattern. After 3
weeks of modeling, the rats in the low-estrogen group appeared
fatter and had significantly less lustrous hair when compared with
the rats in the other groups.
Changes in split glomerular filtration
rate
The GFRs of rats were compared prior to surgery and
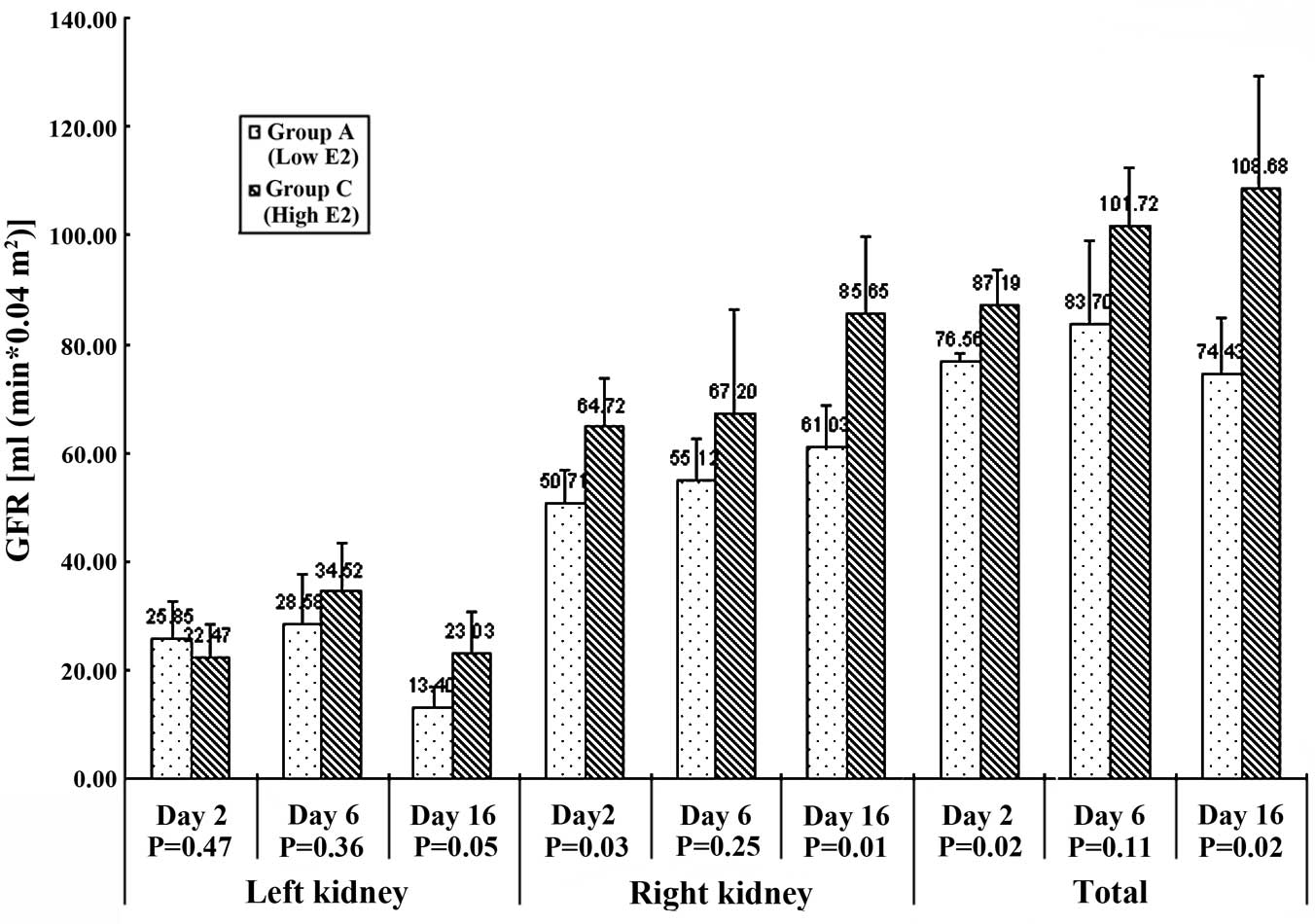
on days 2, 6 and 16 after surgery (Table I, Fig.
1). On day 0, there was no significant difference in bilateral
GFR among the three groups. In the acute (early) stage (day 2), the
GFR of the obstructed kidney was significantly decreased
(P<0.05) in the three groups while the GFR of the contralateral
healthy kidney showed a greater compensatory rise in the normal-
(67.70±1.15 ml/min/0.04 m2) and high-estrogen
(64.72±9.25 ml/min/0.04 m2) groups than in the
low-estrogen (50.71±6.25 ml/min/0.04 m2) group
(P<0.05). The same trend was observed for the total GFR.
 | Table IGFR and serum creatinine of three
estrogen level groups [group A, low estrogen (n=4); group B, normal
estrogen (n=3); group C, high estrogen (n=5)]. |
Table I
GFR and serum creatinine of three
estrogen level groups [group A, low estrogen (n=4); group B, normal
estrogen (n=3); group C, high estrogen (n=5)].
| Group | P-value |
|---|
|
|
|
|---|
| Variable | A | B | C | ANOVA | A vs. B | B vs. C | A vs. C |
|---|
| Estrogen (pg/ml) | 89.01±11.19 | 135.97±26.23 | 209.68±13.86 | 0.000 | 0.022 | 0.002 | 0.000 |
| Serum creatinine
(μmol/m) | | | | | | | |
| Day 0 | 30.75±0.50 | 42.33±15.31 | 36.20±3.83 | 0.196 | 0.178 | 0.405 | 0.027 |
| Day 17 | 52.75±1.89 | 51.00±13.45 | 59.40±13.16 | 0.523 | 0.801 | 0.435 | 0.356 |
| P-value | 0.000 | 0.502 | 0.015 | | | | |
| GFR (ml/min/0.04
m2) (days after surgery) | | | | | | | |
| Left kidney | | | | | | | |
| Day 0 | 51.19±2.23 | 50.37±2.54 | 51.10±2.92 | 0.908 | 0.670 | 0.733 | 0.964 |
| Day 2 | 25.85±6.95 | 33.64±6.24 | 22.47±6.01 | 0.107 | 0.184 | 0.065 | 0.470 |
| Day 6 | 28.58±8.95 | 42.56±17.89 | 34.52±9.05 | 0.332 | 0.309 | 0.528 | 0.360 |
| Day 16 | 13.40±3.64 | 22.69±7.76 | 23.03±7.88 | 0.124 | 0.162 | 0.955 | 0.052 |
| Right kidney | | | | | | | |
| Day 0 | 50.71±2.73 | 50.64±4.52 | 51.66±3.80 | 0.901 | 0.980 | 0.741 | 0.687 |
| Day 2 | 50.71±6.25 | 67.70±1.15 | 64.72±9.25 | 0.021 | 0.006 | 0.514 | 0.031 |
| Day 6 | 55.12±7.79 | 58.71±6.37 | 67.20±19.07 | 0.437 | 0.533 | 0.400 | 0.249 |
| Day 16 | 61.03±7.45 | 55.20±3.37 | 85.65±14.13 | 0.005 | 0.232 | 0.012 | 0.014 |
| Total | | | | | | | |
| Day 0 | 101.90±3.85 | 101.01±6.25 | 102.76±5.77 | 0.903 | 0.824 | 0.699 | 0.804 |
| Day 2 | 76.56±11.68 | 101.34±6.01 | 87.19±6.41 | 0.001 | 0.015 | 0.029 | 0.018 |
| Day 6 | 83.70±15.60 | 101.27±19.87 | 101.72±10.82 | 0.201 | 0.278 | 0.974 | 0.105 |
| Day 16 | 74.43±10.50 | 77.90±11.02 | 108.68±20.86 | 0.021 | 0.694 | 0.034 | 0.021 |
In the medium stage (day 6), the GFR of the
obstructed kidney remained significantly lower than that of the
contralateral side (P<0.05). For the healthy side, the three
groups reached the same compensatory level with no significant
difference (P=0.437).
In the chronic (late) stage (day 16), the GFR of the
obstructed kidney continued to decrease with the high-estrogen
group (23.03±7.88 ml/min/0.04 m2) being significantly
better preserved than the low-estrogen group (13.40±3.64
ml/min/0.04 m2) (P=0.05). The GFR of the contralateral
healthy kidney compensated more in the high-estrogen group
(85.65±14.13 ml/min/0.04 m2) than in the low- and
medium-estrogen groups (61.03±7.45 and 55.20±3.37 ml/min/0.04
m2, respectively) (P=0.01). The same trend was observed
for change of total GFR (P<0.05).
Changes in serum creatinine
Pre- and post-surgery serum creatinine levels showed
no differences among the groups (P>0.05) while significant
increases existed within each group prior to and following surgery
(P<0.01; Table I).
Changes in pathology and EM
visualization
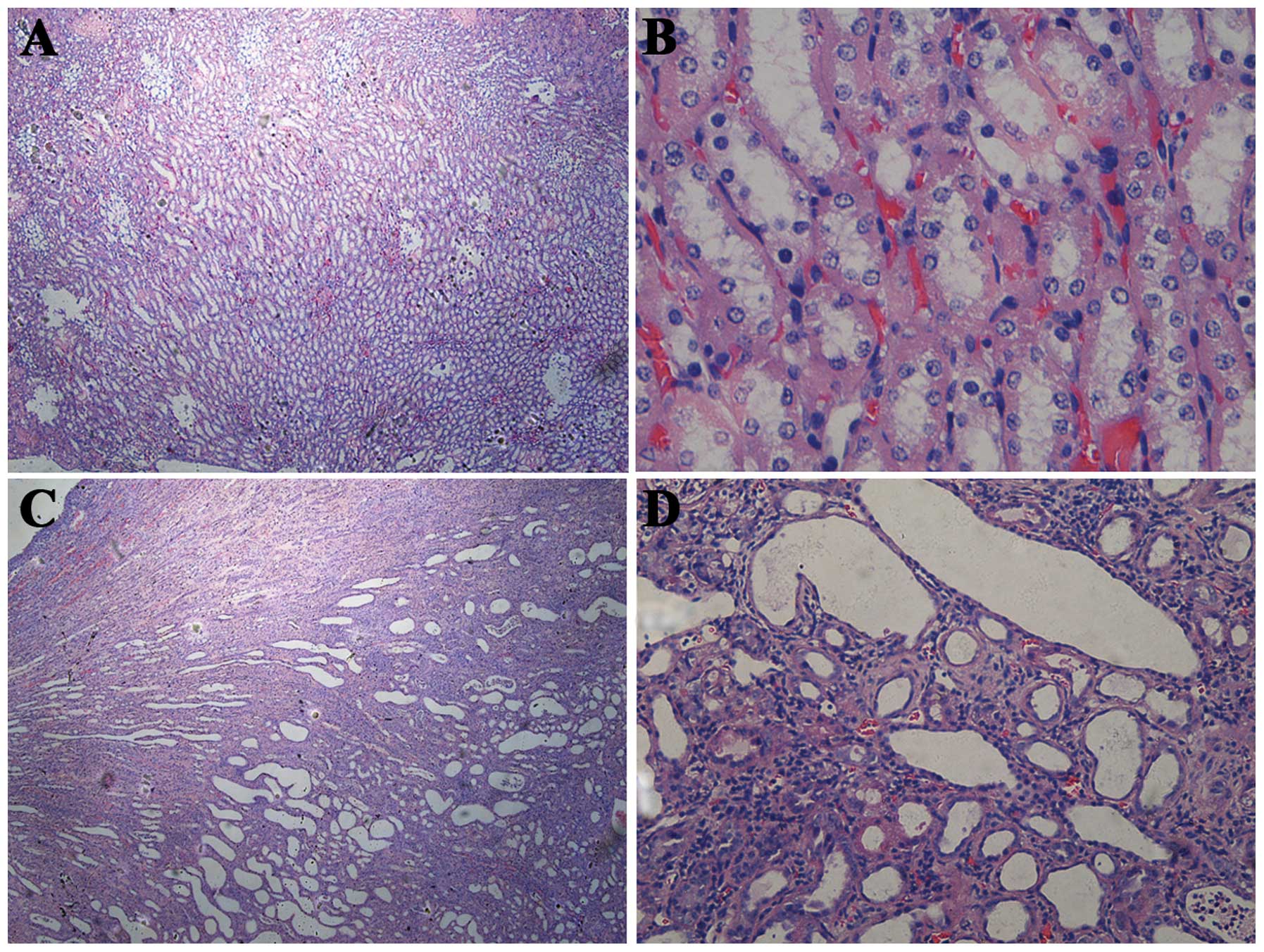
H&E staining revealed no significant difference
in the damage among the three groups. However, on day 17 (chronic
stage), compared with the healthy kidneys, the obstructed kidneys
developed a conspicuous tubulointerstitial injury characterized by
tubular dilatation and atrophy, interstitial inflammation and a
marked interstitial fibrosis (Fig.
2).
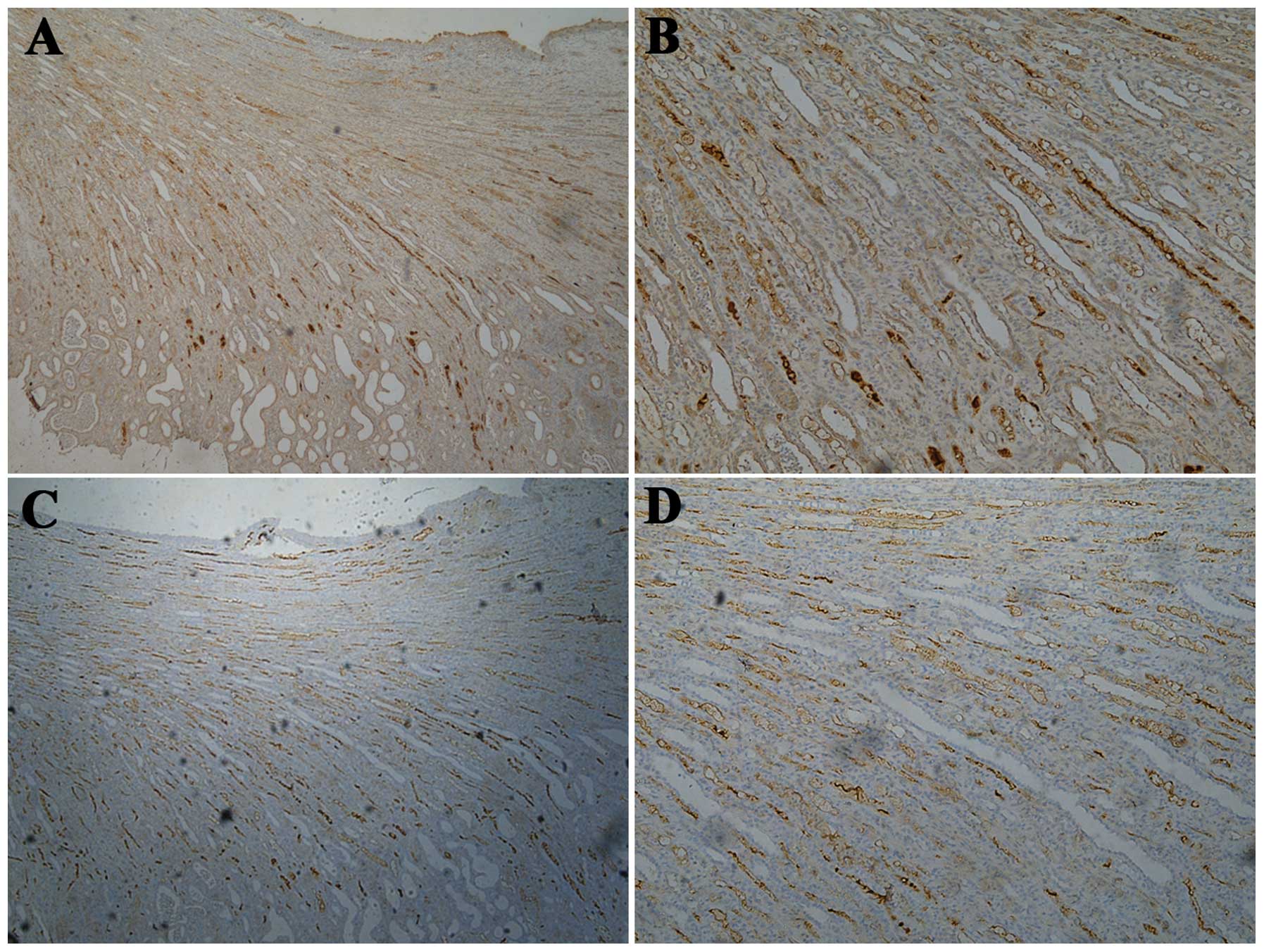
During IHC examination, significant renal fibrosis
was analyzed using IPP V 6.0 software to measure the positive
staining area percentage of TGF-β and α-SMA (Fig. 3, Table II). TGF-β is a protein that
controls proliferation, cellular differentiation and other
functions in the majority of cells, and is an important target for
renal interstitial fibrosis. With the reduction in estrogen
concentration, enhanced TGF-β expression was observed in the
cytoplasm of the tubular epithelial cells in the present study. The
low-estrogen group had a significantly higher expression of TGF-β
than the other two groups (P=0.05 vs. the normal-estrogen group;
P=0.03 vs. the high-estrogen group). α-SMA is a protein that is
involved in cell motility, structure and integrity. α-SMA is a
major constituent of the contractile apparatus and is commonly used
as a marker of myofibroblast formation. With the reduction in
estrogen concentration, enhanced α-SMA expression was also observed
in the renal interstitium in the present study. The low-estrogen
group had a significantly higher expression of α-SMA than the
high-estrogen group (P=0.008).
 | Table IISemi-quantitative analysis (Image-Pro
Plus V6.0) for IHC staining of the three estrogen level groups
[group A, low estrogen (n=4); group B, normal estrogen (n=3); group
C, high estrogen (n=5)]. |
Table II
Semi-quantitative analysis (Image-Pro
Plus V6.0) for IHC staining of the three estrogen level groups
[group A, low estrogen (n=4); group B, normal estrogen (n=3); group
C, high estrogen (n=5)].
| Positive
percentage | P-value |
|---|
|
|
|
|---|
| Protein | Group A | Group B | Group C | ANOVA | A vs. B | B vs. C | A vs. C |
|---|
| TGF-β | 58.83±9.47 | 40.36±13.04 | 42.93±10.81 | 0.05 | 0.05 | 0.76 | 0.03 |
| α-SMA | 38.97±10.19 | 32.24±10.75 | 19.09±9.37 | 0.02 | 0.41 | 0.10 | 0.01 |
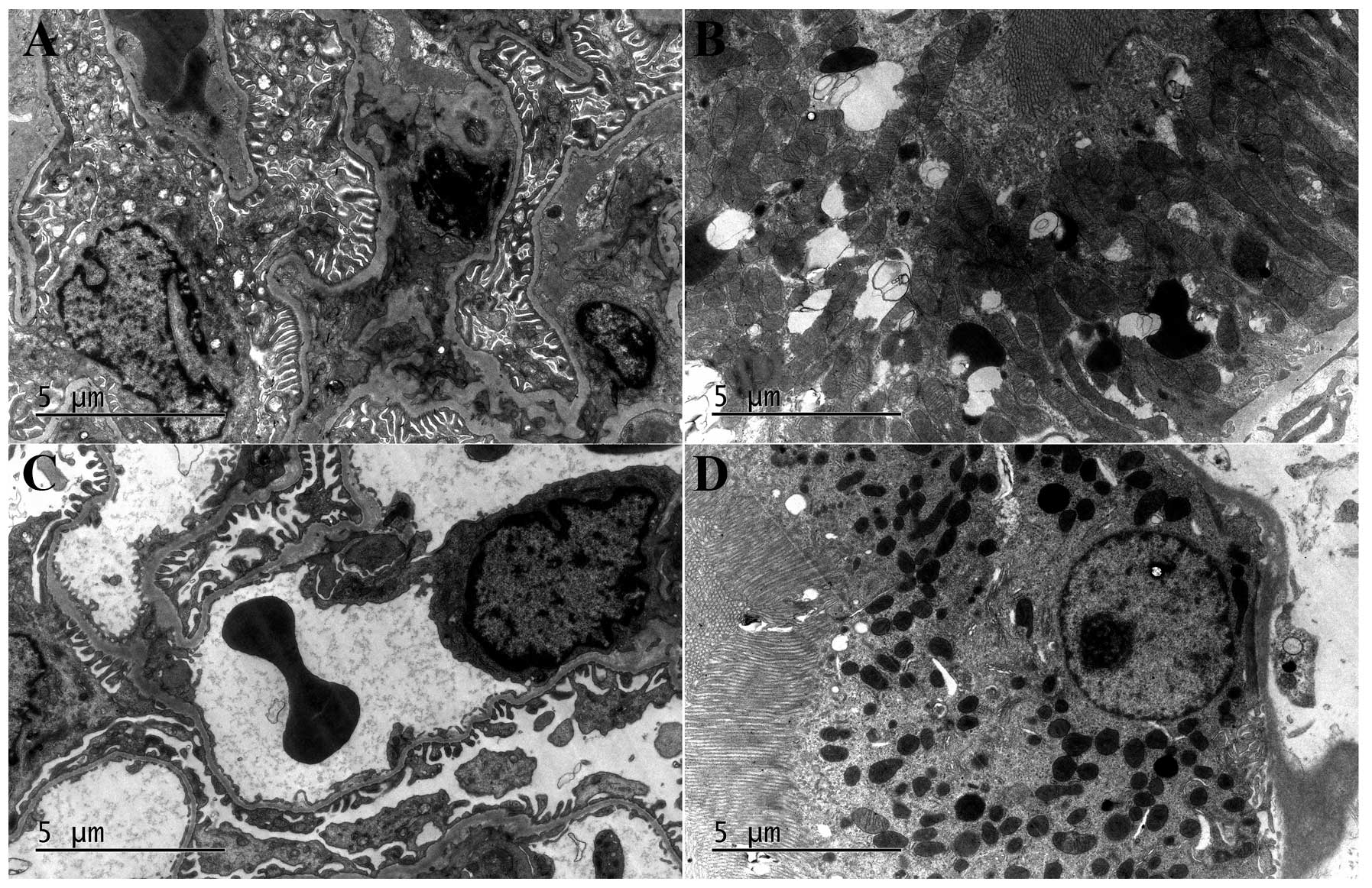
Under EM examination, no significant difference in
damage was identified among the three groups. Normal capillary
endothelial cells, basement membranes, epithelial cells and foot
processes in healthy kidneys were observed. In addition, no obvious
abnormalities in the capillary lumen with erythrocytes within the
renal glomerulus area and normal mitochondria, proximal tubule
epithelial cells and vertically arranged microvilli in the proximal
tubule area were observed in the healthy kidneys. For the
obstructed kidneys, changes typical of hydronephrosis were
observed, including increased numbers of epithelial cells,
swelling, shedding and vacuole formation in the mitochondria of
epithelial cells in the renal glomerulus area, and autophagic
vacuoles, vacuolization and lipofuscin with no evident
abnormalities in the mitochondria and microvilli in the proximal
tubule area (Fig. 4).
Discussion
Urinary tract stones may occasionally be solved
without any treatment, but may also lead to heavy hydronephrosis
and NFK. We previously conducted a survey which found that the
prevalence of NFK remained constant regardless of the increased use
of endoscopic techniques and screening, with females, in particular
elderly women, being more likely to develop NFK (4). Therefore, the gender difference and
the effect of estrogen was investigated using a UUO model in the
present study.
The treatment of obstructive nephropathy is by
relief of obstruction. However, while patients are undergoing
conservative medical treatment of small ureteral stones, waiting
for extracorporeal shock wave lithotripsy (SWL) or ureteroscopy
treatment, or even after SWL or ureteroscopy treatment, the
corresponding ureter remains obstructed during passage. For certain
patients, such obstruction may remain for several weeks. Therefore,
preservation of the renal function under such conditions is
invaluable. In addition, certain studies have observed that the
harmful effect of obstructive nephropathy may continue even after
relief of the obstruction. The recovery of renal function is a
long-term process (21). Thus, the
identification of a medicine that is able to augment the
progression of renal function is likely to be valuable for clinical
application.
The present study is a controlled experiment to
evaluate the role of estrogen in the progression of renal function
loss during a phase of chronic complete ureteral obstruction from
the perspective of morphological and functional study. To the best
of our knowledge, these objectives have not been achieved in
published urological studies.
From the point of functional study, estrogen
contributed to a renoprotective effect during UUO. The
high-estrogen group exhibited a better preserved split renal
function (GFR) for the obstructed kidney and superior compensatory
effect for the contralateral kidney, particularly during the
chronic stage.
The creatinine level does not differentiate between
impaired split renal function and reflects only the total renal
function. The rise in creatinine level did not differ among groups
due to the compensatory effect of the healthy kidney. Thus,
SPECT/CT is better than creatinine level monitoring for evaluating
the split renal function more precisely.
From the point of morphological study, estrogen also
contributes to the renoprotective effect of interstitial fibrosis
during UUO. Significant hydronephrosis changes and inflammatory
infiltration were observed in the obstructed kidney by H&E
staining and EM examination. In addition, IHC staining of TGF-β and
α-SMA was able to differentiate between different estrogen level
groups. TGF-β and α-SMA are the typical markers of renal
interstitial fibrosis. TGF-β controls cell proliferation and is one
of the most important signs of renal fibrosis involved in Smad
signaling pathways (22). α-SMA is
a marker of myofibroblast formation, which plays a crucial role in
the development and progression of renal tubulointerstitial
fibrosis (23). In the present
study, the low-estrogen group demonstrated significantly deeper
staining of these two markers, which indicated that severe renal
interstitial fibrosis had occurred and a high estrogen level was
able to downregulate its progression.
As observed in a previous study (4), the majority of female NFK patients
with urolithiasis were postmenopausal (mean, 53.4±13.3 years old).
This may be due to the sharp decline in estrogen levels following
menopause causing the loss of a renoprotective effect of renal
function for female patients with urolithiasis and leading to NFK.
The present study has demonstrated, for the first time, that
estrogen is able to protect renal functions in acute and chronic
UUO.
Estrogens are a group of compounds named for their
importance in the estrous cycle of humans and other animals. They
are the primary female sex hormones. Estrogens, in females, are
produced primarily by the ovaries and, during pregnancy, the
placenta. Estrogens are also produced in smaller amounts by other
tissues such as the liver, adrenal glands and breasts. For
adolescent girls, estrogen is able to promote formation of female
secondary gender characteristics, produce libido (24), regulate the fluid balance (25) and cause calcium deposition in the
bones.
Previous study has shown that estrogen is able to
alleviate the progression of chronic kidney disease (5). Therefore, the high prevalence of NFK
in older women with urolithiasis in our previous study may be due
to the sharp decline of estrogen levels (4). Ji et al observed that estrogen
influenced the severity of injury in a renal wrap-induced renal
injury animal model; estrogen treatment protected against
glomerular and tubular damage (5).
Fung et al also performed a cross-sectional and 10-year
prospective study of postmenopausal estrogen therapy and observed
improved blood pressure and renal function (GFR) among continuous
estrogen users (6).
In addition, estrogen is involved in multiple
pathways in the progression of obstructive nephropathy. The
arachidonic acid metabolic, renin-angiotensin, nitric oxide,
aquaporin and extracellular matrix pathways, together with
transport disorders of sodium and potassium, the failure of urine
acidification due to hydrogen ion transport disorders, and
secretion disorders of a variety of peptides and proteins are
involved in the development of obstructive nephropathy (26,27).
Estrogen is involved in many of these pathways. For
renin-angiotensin pathways, Baiardi et al observed that
estrogen was able to upregulate the angiotensin II receptor (AT2R)
to protect renal function (9).
Oelkers (28) and Gallagher et
al (29) also found that
estrogen was able to upregulate angiotensinogen and the AT2R, and
downregulate renin, angiotensin-converting enzyme and angiotensin
II, which also protected renal function. For nitric oxide pathways,
Thompson and Khalil found that estrogen activates
endothelium-dependent vascular relaxation pathways, including
NO-cGMP and prostacyclin-cAMP pathways, which has potential
beneficial vascular effects (11).
Sandberg also analyzed nitric oxide synthesis (NOS) disorders in a
renal wrap model of hypertension in rats and observed that estrogen
regulates NOS and NO to preserve renal function (12). For extracellular matrix pathways,
Karl et al found that estrogen in vitro prevented
TGF-β1 stimulation of a Smad-responsive reporter construct and
increased MMP-2 expression and activity that alleviated renal
interstitial fibrosis (30). This
study also demonstrated that estrogen downregulated TGF-β. Guccione
et al found that estrogen was able to upregulate the MAPK
cascade, which in turn stimulated the synthesis of AP-2 protein.
The resultant increased AP-2/DNA binding activity leads to
increased synthesis of MMP-2 and increased metalloproteinase
activity, which may contribute to the protective effect of female
gender on renal disease progression (31). Zdunek et al (32) found that the ability of estrogen to
reverse TGF-β1-stimulated type IV collagen synthesis was mediated
by the downregulation of CK2 activity and ultimately collagen IV
protein synthesis was reduced. Neugarten et al (33) obtained similar results. In
addition, estrogen is able to inhibit podocyte injury (18) and mesangial apoptosis (20), stimulate vascular endothelial
growth factor (VEGF) expression to maintain the healthy intrarenal
vasculature (19) and preserve
renal function. Estrogen is involved in pathways that may mediate
the progression of obstructive nephropathy.
Women <60 years old with low bone density,
flushes, sweats, vaginal dryness, loss of libido and climacteric
depression are treated with estrogen (hormone replacement therapy,
HRT) by gynecologists and the majority of general practitioners.
However, the popular use of estrogen has been reduced by the 2002
Women’s Health Initiative study due to adverse effects, including
increased risk of breast cancer, endometrial cancer, thromboembolic
disease and stroke being reported (34). A previous study suggested that HRT
is more likely to be a tumor promoter than a de novo-inducer
(35). So, whether to use HRT or
not remains unclear. At present, the recommendations state
categorically that the safety of HRT largely depends on age, adding
that healthy women <60 years old should not be unduly concerned
about the safety profile of HRT (36). Therefore, the benefits of HRT given
for a clear indication are many and the risks are few. Further
study is required to investigate the merit and demerit of the use
of estrogen (HRT).
The limitations of the present study include a small
sample size and the use of a complete UUO animal model rather than
a partial UUO model. However, laboratory, functional and
morphological examinations were performed to obtain further
information. Three professional nuclear medicine doctors calculated
the GFR and five high magnification fields were obtained for each
IHC slide to calculate the expression of TGF-β and α-SMA to
minimize bias. A partial UUO model is relatively difficult to
establish with a consistent degree of obstruction that affects the
renal function. Therefore a complete UUO was used to maintain
consistency of the experimental conditions. Future studies to
design a partial UUO model and further investigate the mechanism of
estrogen are required.
In conclusion, estrogen administration preserved the
renal function of obstructive kidney in a unilateral ureteral
obstruction animal model and enhanced the compensatory effect of
the contralateral kidney. SPECT/CT examination (GFR) is an
effective method of measuring split renal function.
Acknowledgements
The study was funded by the National Natural Science
Foundation of China (No. 81272835) and the Shanghai Municipal
Education Commission Foundation(No. 11ZZ08).
References
|
1
|
Yasui T, Iguchi M, Suzuki S and Kohri K:
Prevalence and epidemiological characteristics of urolithiasis in
Japan: national trends between 1965 and 2005. Urology. 71:209–213.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi T, Yamane A, Okasho K, et al:
Incidence of upper urinary tract stone during 15 years in Tajima
area, Japan: a hospital-based study. Urol Res. 37:305–310.
2009.PubMed/NCBI
|
|
3
|
Tanagho EA: Urinary obstruction &
stasis. Smith’s General Urology. Tanagho EA and McAninch JW: 17th
edition. McGraw-Hill; New York: pp. 1662008
|
|
4
|
Mao S, Jiang H, Wu Z, et al: Urolithiasis:
the most risk for nephrectomy in nonrenal tumor patients. J
Endourol. 26:1356–1360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji H, Menini S, Mok K, et al: Gonadal
steroid regulation of renal injury in renal wrap hypertension. Am J
Physiol Renal Physiol. 288:F513–F520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fung MM, Poddar S, Bettencourt R, et al: A
cross-sectional and 10-year prospective study of postmenopausal
estrogen therapy and blood pressure, renal function, and
albuminuria: the Rancho Bernardo Study. Menopause. 18:629–637.
2011.PubMed/NCBI
|
|
7
|
Cherikh WS, Young CJ, Kramer BF, et al:
Ethnic and gender related differences in the risk of end-stage
renal disease after living kidney donation. Am J Transplant.
11:1650–1655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Topcu SO, Pedersen M, Nørregaard R, et al:
Candesartan prevents long-term impairment of renal function in
response to neonatal partial unilateral ureteral obstruction. Am J
Physiol Renal Physiol. 292:F736–F748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baiardi G, Macova M, Armando I, et al:
Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in
the rat. Regul Pept. 124:7–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Armando I, Jezova M, Juorio AV, et al:
Estrogen upregulates renal angiotensin II AT(2) receptors. Am J
Physiol Renal Physiol. 283:F934–F943. 2002.PubMed/NCBI
|
|
11
|
Thompson J and Khalil RA: Gender
differences in the regulation of vascular tone. Clin Exp Pharmacol
Physiol. 30:1–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sandberg K: Mechanisms underlying sex
differences in progressive renal disease. Gend Med. 5:10–23. 2008.
View Article : Google Scholar
|
|
13
|
Li C, Shi Y, Wang W, et al: alpha-MSH
prevents impairment in renal function and dysregulation of AQPs and
Na-K-ATPase in rats with bilateral ureteral obstruction. Am J
Physiol Renal Physiol. 290:F384–F396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Efrati S, Berman S, Chachashvili A, et al:
Rosiglitazone treatment attenuates renal tissue inflammation
generated by urinary tract obstruction. Nephrology (Carlton).
14:189–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alvarez A, Hermenegildo C, Issekutz AC, et
al: Estrogens inhibit angiotensin II-induced leukocyte-endothelial
cell interactions in vivo via rapid endothelial nitric oxide
synthase and cyclooxygenase activation. Circ Res. 91:1142–1150.
2002. View Article : Google Scholar
|
|
16
|
Rodríguez E, López R, Paez A, et al:
17Beta-estradiol inhibits the adhesion of leukocytes in TNF-alpha
stimulated human endothelial cells by blocking IL-8 and MCP-1
secretion, but not its transcription. Life Sci. 71:2181–2193.
2002.PubMed/NCBI
|
|
17
|
Mori M, Tsukahara F, Yoshioka T, et al:
Suppression by 17beta-estradiol of monocyte adhesion to vascular
endothelial cells is mediated by estrogen receptors. Life Sci.
75:599–609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silbiger SR: Raging hormones: gender and
renal disease. Kidney Int. 79:382–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang DH, Yu ES, Yoon KI and Johnson R: The
impact of gender on progression of renal disease: potential role of
estrogen-mediated vascular endothelial growth factor regulation and
vascular protection. Am J Pathol. 164:679–688. 2004. View Article : Google Scholar
|
|
20
|
Negulescu O, Bognar I, Lei J, et al:
Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a
casein kinase 2-dependent mechanism. Kidney Int. 62:1989–1998.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito K, Chen J, El Chaar M, et al: Renal
damage progresses despite improvement of renal function after
relief of unilateral ureteral obstruction in adult rats. Am J
Physiol Renal Physiol. 287:F1283–F1293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng XM, Chung AC and Lan HY: Role of the
TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond).
124:243–254. 2013.
|
|
23
|
Ina K, Kitamura H, Tatsukawa S and
Fujikura Y: Significance of α-SMA in myofibroblasts emerging in
renal tubulointerstitial fibrosis. Histol Histopathol. 26:855–866.
2011.
|
|
24
|
Heiman JR, Rupp H, Janssen E, et al:
Sexual desire, sexual arousal and hormonal differences in
premenopausal US and Dutch women with and without low sexual
desire. Horm Behav. 59:772–779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Curtis KS: Estrogen and the central
control of body fluid balance. Physiol Behav. 97:180–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frøkiaer J1 and Sørensen SS: Eicosanoid
excretion from the contralateral kidney in pigs with complete
unilateral ureteral obstruction. J Urol. 154:1205–1209.
1995.PubMed/NCBI
|
|
27
|
Yarger WE, Schocken DD and Harris RH:
Obstructive nephropathy in the rat: possible roles for the
renin-angiotensin system, prostaglandins, and thromboxanes in
postobstructive renal function. J Clin Invest. 65:400–412. 1980.
View Article : Google Scholar
|
|
28
|
Oelkers WK: Effects of estrogens and
progestogens on the renin-aldosterone system and blood pressure.
Steroids. 61:166–171. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gallagher PE, Li P, Lenhart JR, Chappell
MC and Brosnihan KB: Estrogen regulation of angiotensin-converting
enzyme mRNA. Hypertension. 33:323–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karl M, Berho M, Pignac-Kobinger J, et al:
Differential effects of continuous and intermittent
17beta-estradiol replacement and tamoxifen therapy on the
prevention of glomerulosclerosis: modulation of the mesangial cell
phenotype in vivo. Am J Pathol. 169:351–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guccione M, Silbiger S, Lei J, et al:
Estradiol upregulates mesangial cell MMP-2 activity via the
transcription factor AP-2. Am J Physiol Renal Physiol.
282:F164–F169. 2002.PubMed/NCBI
|
|
32
|
Zdunek M, Silbiger S, Lei J and Neugarten
J: Protein kinase CK2 mediates TGF-beta1-stimulated type IV
collagen gene transcription and its reversal by estradiol. Kidney
Int. 60:2097–2108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neugarten J, Acharya A, Lei J and Silbiger
S: Selective estrogen receptor modulators suppress mesangial cell
collagen synthesis. Am J Physiol Renal Physiol. 279:F309–F318.
2000.PubMed/NCBI
|
|
34
|
Studd J: ‘PROFOX’ - the post HRT
nightmare. Climacteric. 14:217–219. 2011.
|
|
35
|
Dietel M: Hormone replacement therapy
(HRT), breast cancer and tumor pathology. Maturitas. 65:183–189.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brown S: IMS updates its recommendations
on HRT. Menopause Int. 17:752011.PubMed/NCBI
|