Introduction
Lupus nephritis (LN) is a common and severe
manifestation of systemic lupus erythematosus (SLE). Patients with
a rapidly progressive destruction of renal parenchyma often have a
worse LN prognosis (1,2). In the past 20 years, much evidence
has supported that patients with active LN (International Society
of Nephrology/Renal Pathology Society classes III, IV or V) may be
effectively treated with corticosteroids combined with
immunosuppressive drugs, for example, cyclophosphamide (CYC) or
mycophenolate (MMF) (3–5). Additionally, pulsed intravenous
therapy with high doses of CYC followed by quarterly dosing,
combined with steroids, has long been considered the ideal strategy
for inducing renal remission and preventing renal flares in
patients with severe LN (6).
However, a significant number of patients have refractory disease
or are not able to tolerate these drugs due to significant
drug-related toxicity (7).
Calcineurin inhibitors (CNIs), particularly
cyclosporine A (CsA) and tacrolimus (TAC), are widely used as
immunosuppressive drugs. The principle action of CNIs within T
lymphocytes is the inhibition of phosphatase calcineurin (8). A number of studies have demonstrated
that TAC and CsA may provide equivalent potency and safety as an
induction therapy in the treatment of active LN (9–14).
Given the increasing popularity of CNIs in the treatment of LN, a
meta-analysis was performed in the current study to compare the
efficacy and safety of CNIs with those of CYC in the treatment of
active LN. This was carried out by analyzing the most recently
published controlled trials, including large randomized controlled
trials (RCTs), prospect cohort studies, and case-control
studies.
Materials and methods
Search strategy
A literature search was performed of the PubMed,
Cochrane Library, and Ovid databases up to April 2013. The
literature search was performed using the relevant keywords of
‘cyclosporine A’, ‘CsA’, ‘tacrolimus’, ‘FK506’, ‘lupus nephritis’,
‘nephritis’ and ‘glomerulonephritis’. The search was limited to
articles written in English.
Criteria for selecting articles included
in this meta-analysis
Two authors extracted information from the trials
independently and disagreement was resolved by consensus. In the
primary stage, the titles and abstracts were scanned to exclude any
studies that were clearly irrelevant. In the secondary stage, the
full texts of the remaining articles were read in order to
determine whether they contained information on the topic of
interest. Inclusion criteria consisted of: i) the study design was
an RCT, prospect cohort study or case-control study; ii) the study
was of patients with biopsy-proven LN classes III, IV or V; iii)
the study compared TAC or CsA with CYC in the induction therapy of
LN; and iv) at least one of the following three outcomes was
reported: achievement of complete renal remission (CR), partial
renal remission (PR), both at least six months after therapy, and
common adverse effects including infection, gastrointestinal
symptoms, transient increase in serum creatinine (SCr), glucose
disorders, irregular menstruation, leucopenia and liver function
disorders. Exclusion criteria were: i) abstract not in English; ii)
did not compare TAC or CsA with CYC in the treatment of LN; iii)
did not concern the induction treatment of LN; iv) studies
including children.
Data extraction
The same reviewers independently extracted data from
all primary studies that fulfilled the inclusion criteria, with
disagreement resolved by consensus. Data extracted included study
design, details of treatment protocol, baseline demographics and
clinical, laboratory and renal biopsy information. Data on the
following outcomes were extracted when reported: CR, PR, total
renal remission (TR) and adverse effects.
Outcome measures of this
meta-analysis
The primary outcomes were the proportion of patients
who achieved CR, PR and TR at six months or later after induction
therapy with CNIs or CYC. The secondary outcomes were the relative
risks (RRs) of the adverse effects including infection,
gastrointestinal symptoms, transient increase in SCr, glucose
disorders, irregular menstruation, leucopenia and liver dysfunction
at the end of the respective studies. Definitions of the primary
outcomes used in the original papers were extracted as described in
Table I.
 | Table IDefinitions of complete remission,
partial remission and total remission. |
Table I
Definitions of complete remission,
partial remission and total remission.
| Study (ref.) | Complete
remission | Partial
remission | Total remission |
|---|
| Chen et al
(9) | Proteinuria <0.3
g/24 h with normal urine sediment, Alb≥35 g/l, normal SCr range or
not >15% more than baseline | Proteinuria range
of 0.3–2.9 g/24 h and decrease ≥50% of baseline, Alb≥30 g/l, normal
SCr range or not >15% more than baseline | CR or PR |
| Wang et al
(10) | Proteinuria <0.5
g/24 h with normal urine sediment, Alb≥35 g/l, stable or improved
eGFR≥10% for baseline SCr≥133 umol/l | Stable or improved
eGFR; reduction of proteinuria≥50% of the basal level but still
>0.5 g/24 h; Alb≥30 g/l (≥2 determinations one month apart) | CR or PR |
| Li et al
(11) | Proteinuria <0.3
g/24 h with normal urine sediment, Alb≥35 g/l and stabilization
(±15%) or improvement in SCr at 24 weeks. | Proteinuria
(0.3–2.9 g/24 h) and decrease ≥50% of baseline; Alb≥30 g/l;
stabilization (±30%) in SCr. | CR or PR |
| Szeto et al
(12) | Proteinuria <0.5
g/24 h with normal urine sediment, normal Alb, eGFR≤15% above
baseline | Proteinuria
(0.5–2.9 g/24 h), Alb≥30 g/l, stable renal function | NR |
| Zavada et al
(13) | proteinuria <0.3
g/24 h with normal urine sediment, SCr within the normal range with
stable or not >15% more than baseline | SCr within the
normal range with stable or not >15% more than baseline,
proteinuria decrease ≥50% of baseline and proteinuria <3 g/24 h
if nephritic at baseline or ≤0.5 g/24 h if baseline non-nephritic,
normal urine sediment or C3 improvement ≥25% | CR or PR |
| Austin et al
(14) | Proteinuria <0.3
g/24 h | Proteinuria <2.0
g/d and decrease ≥50% of baseline | NR |
Assessment of trial quality
The quality of each RCT was assessed using a
standard scoring system proposed in the Jadad scale criteria
(15). These included: i) whether
the randomization method was appropriate; ii) whether
double-blindness was mentioned in the trial and whether it was
appropriately performed; iii) whether the description (the patient
number and reasons) of withdrawal and drop-outs was clearly stated.
The studies were classified as high quality if they scored >2.
Otherwise, they were classified as low quality (16,17).
The quality of the cohort and case-control studies was assessed
using the Newcastle-Ottawa Scale (NOS) with certain modifications
to match the requirements of the current study (18). The quality of the studies was
evaluated by examining three items: patient selection,
comparability of CNI and CYC groups, and assessment of outcomes.
For the comparability between the CNI and CYC groups, the focus was
on the following variables: age, gender, proteinuria, serum
albumin, SCr, estimated glomerular filtration rate (eGFR) or
creatinine clearance rate, serum complement component 3, anti
double-stranded DNA antibodies, systemic lupus erythematosus
disease activity index (SLEDAI), pathological type, pathological
activity index and pathological chronicity index. Studies were
graded on an ordinal star scoring scale with higher scores
representing studies of a higher quality. The quality of each study
was graded as either level one (0–5 stars) or level two (6–9
stars).
Statistical analyses
All statistical analysis was performed using Stata
software, version 11.0 (StataCorp LP, College Station, TX, USA).
The fixed-effects model of Mantel-Haenszel was used to estimate the
pooled RR with 95% confidence intervals (CIs) for study outcomes,
using data from all eligible papers. The possibility of
heterogeneity in results across the studies was examined using the
H statistic and I2 index (19).
Heterogeneity was considered statistically significant if P<0.1
in the χ2 test. If no statistical heterogeneity existed
among the trials, a fixed-effects model was selected to analyze the
data. When statistical heterogeneity was detected, the sources of
heterogeneity were explored and subgroup analyses were performed.
The robustness of the pooled effect sizes was evaluated based on
the different types of CNIs (i.e., TAC vs. CsA) or study types
(i.e., RCT vs. Non-RCT). P<0.05 was considered to indicate a
statistically significant difference.
Results
Study selection
Initially, 5,815 articles were identified through
database searches. All abstracts were scanned and nine studies
(20–22) were retrieved for detailed scrutiny.
Thus, 5,806 abstracts were rejected on initial screening (Fig. 1). Three publications were further
excluded as one compared TAC with a placebo (20), one compared TAC with mycophenolate
mofetil (MMF) (21) and one
compared TAC plus MMF with CYC (22). The definitive analyses in the
present meta-analysis included four RCTs (9,11,13,14),
one cohort study (10) and one
case-control study (12) that were
published between 2008 and 2013.
Trial characteristics and qualities
Table II shows the
characteristics of the papers that were included in the
meta-analysis. A total of 265 patients had been assessed in the six
studies. Of the six studies, four provided data for comparing the
efficacy of TAC plus a steroid with that of CYC plus a steroid
(9–11), and two compared the efficacy of CsA
plus a steroid with that of CYC plus a steroid in the induction
therapy of active LN (13,14). For the quality of the RCTs, as
assessed by the Jadad method (15), the Jadad score ranged from 2 to 4
and only one trial was of high quality (Jadad score = 4). For the
quality of the cohort and case-control studies, as assessed by the
NOS (18), the NOS score ranged
from 5 to 8 stars.
 | Table IITrial characteristics and
qualities. |
Table II
Trial characteristics and
qualities.
| Study (ref.) | Country or
area | Study type | Number
enrolled | Age (years) | Comparison | Renal pathology
type | Follow-up duration
(months) | Jadad score
/Newcastle-Ottawa Scale |
|---|
| Chen et al
(9) | China | RCT | TAC 42 | 32±10.8 | TAC+Pred vs. IV
CYC+Pred | Class III, IV,
V | 6 | 4 |
| CYC 39 | 31.9±10.1 | | | |
| Wang et al
(10) | China | CS | TAC 20 | 32±7.7 | TAC+Pred vs. IV
CYC+Pred | Class III, IV,
V | 21.25±15.25 | ******** |
| CYC 20 | 35.7±11.4 | | 16.83±15.85 | |
| Li et al
(11) | China | RCT | TAC 20 | 29 (17–50) | TAC+Pred vs. IV
CYC+Pred | Class III, IV,
V | 6 | 2 |
| CYC 20 | 33 (17–64) | | | |
| Szeto et al
(12) | Hong Kong | CC | TAC 18 | 38.2±10.4 | TAC+Pred vs. PO
CYC+Pred | Class V | 6 | ***** |
| CYC 19 | 36.5±12.2 | | | |
| Zavada et al
(13) | Czech Republic and
Slovakia | RCT | CsA 19 | 30±9 | CsA +Pred vs. IV
CYC+Pred | Class III, IV | 9 | 2 |
| CYC 21 | 28±5 | | | |
| Austin et al
(14) | USA | RCT | CsA 12 | 34 (13–56) | CsA +Pred vs. IV
CYC+Pred | Class V | 12 | 2 |
| CYC 15 | 41 (17–60) | | | |
Trial outcomes
Comparison of the CNI regimen with the
CYC regimen
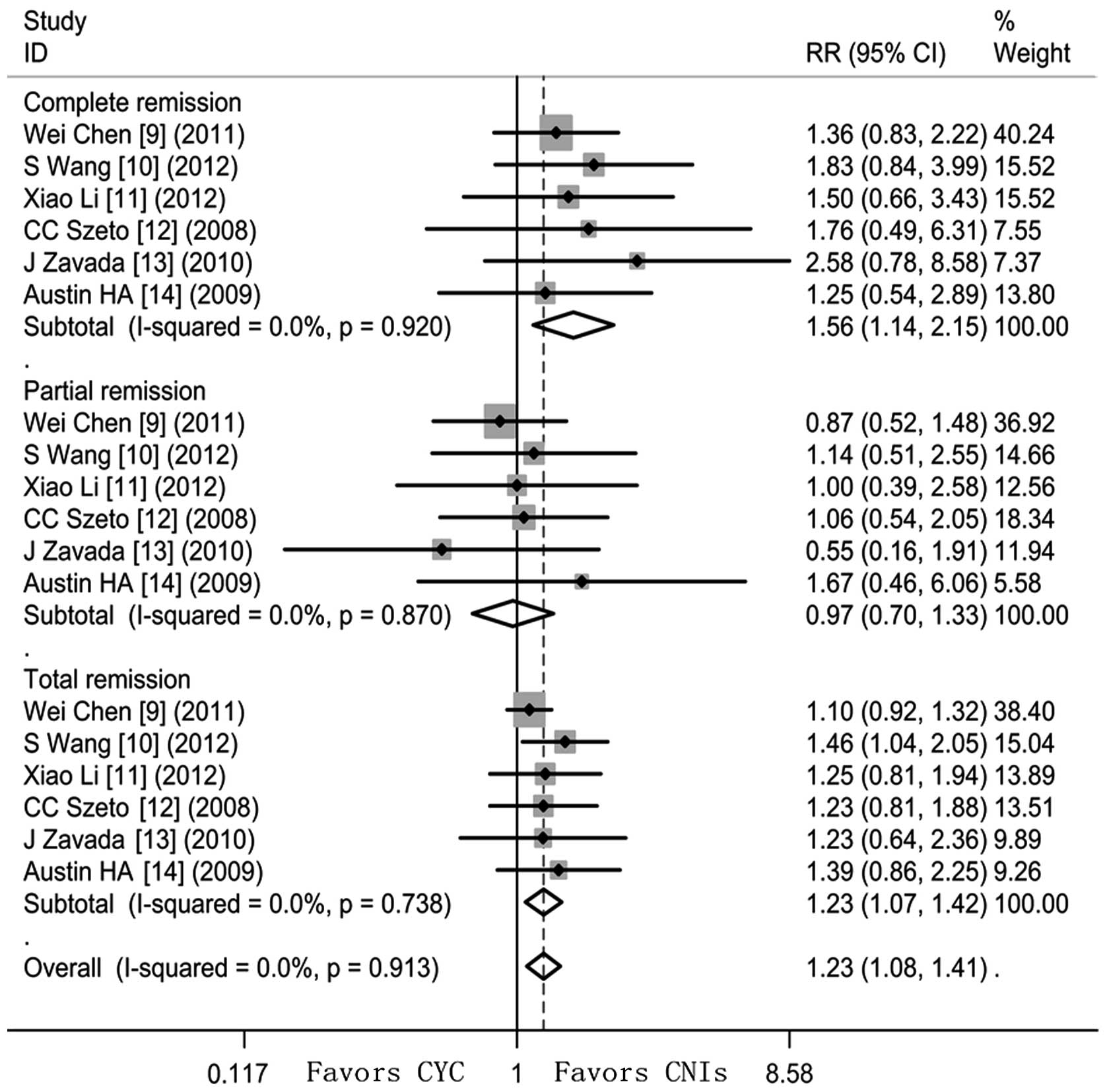
CNIs increased the rates of the following compared
with CYC (Fig. 2): CR (RR=1.56,
95% CI 1.14–2.15, Z=2.74, P=0.006; heterogeneity
χ2=1.44, P=0.920, I2=0%) and TR (RR=1.23, 95%
CI 1.07–1.42, Z=2.87, P=0.004; heterogeneity χ2=2.76,
P=0.783, I2=0%). However, PR did not reach a significant
difference between CYC and CNIs (RR=0.97, 95% CI 0.70–1.33, Z=0.20,
P=0.844; heterogeneity χ2=1.85, P=0.870,
I2=0%).
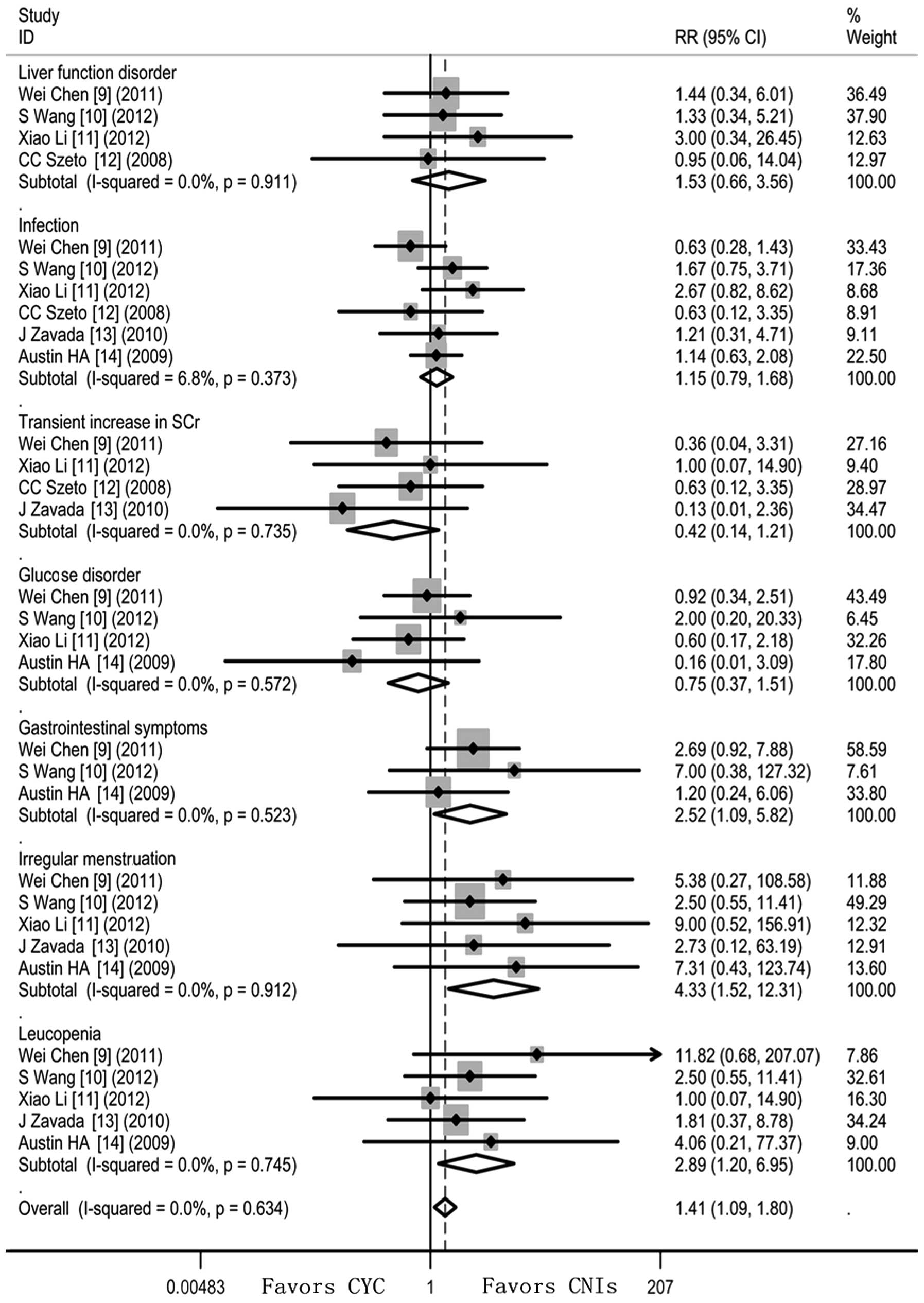
Adverse events
Significantly fewer patients who received CNIs
developed irregular menstruation (RR=4.33; 95% CI 1.52–12.31),
gastrointestinal disorder (RR=2.52; 95%CI 1.09–5.82) and leucopenia
(RR=2.89; 95% CI 1.20–6.95). However, patients receiving CNIs
appeared to have a higher risk of experiencing a transient increase
in SCr (RR=0.42; 95% CI 0.14–1.21) and glucose disorder (RR=0.75;
95%CI 0.37–1.51) though this did not reach statistical
significance. Heterogeneity was undetectable when the effect sizes
of side-effects were evaluated (I2=0). The fixed-effects
model was thus used (Fig. 3).
Sensitivity analyses
The results of the primary and secondary outcomes,
including the RRs of CR, PR, TR and side-effects, remained
generally consistent upon sensitivity analysis based on the type of
CNI (Table III). When the trials
that compared CsA with CYC in the induction therapy of LN were
excluded, TAC showed a better response, higher rate of inducing
remission and lower risk of adverse events than CYC. This was
consistent with the results of the analysis containing all the
trials, although the RR values were slightly lower. CsA did not
reveal a superiority when compared with CYC in the induction
therapy of active LN. When only RCT trials were pooled for
analysis, the incidence of gastrointestinal symptoms became
insignificant. Nevertheless, these results must be interpreted with
caution since the effect sizes were generated from a small number
of RCTs (n=4) and trials that involved TAC (n=4) (9–12)
and CsA (n=2) (13,14) (Table
III).
 | Table IIIResults of sensitivity analyses
(trials exclusion). |
Table III
Results of sensitivity analyses
(trials exclusion).
|
Combined RR
(95% CI) |
|---|
|
|
|---|
| Comparison | CR | PR | TR | Infections | Liver function
disorder | Gastrointestinal
symptoms | Transient SCr↑ | Glucose
disorder | Leucopenia | Irregular
menstruation |
|---|
| TAC vs. CsA |
| All trials | 1.56
(1.14,2.15)a | 0.97
(0.70,1.33) | 1.23
(1.07,1.42)a | 0.87
(0.60,1.26) | 0.65
(0.28,1.52) | 0.40 (0.17,
0.92)a | 2.39
(0.82,6.90) | 1.33
(0.66,2.67) | 0.35
(0.14,0.83)a | 0.23
(0.08,0.66)a |
| TAC vs. CYC | 1.52
(1.06,2.17)a | 0.98
(0.70,1.38) | 1.22
(1.05,1.41)a | 0.87
(0.54,1.40) | 0.62
(0.25,1.51) | 0.31 (0.11,
0.86)a | 1.75
(0.54,5.71) | 6.15
(0.32,117.21) | 0.30
(0.10,0.93)a | 0.25
(0.07,0.83)a |
| CsA vs. CYC | 1.71
(0.86,3.43) | 0.91
(0.38,2.15) | 1.31
(0.87,1.96) | 0.86
(0.48,1.54) | 1.06
(0.07,15.64) | 0.83 (0.46,
4.21) | 7.70
(0.42,140.03) | 0.44
(0.11,1.75) | 0.44
(0.11,1.75) | 0.20
(0.02,1.56) |
| RCT vs.
non-RCT |
| All trials | 1.56
(1.14,2.15)a | 0.97
(0.70,1.33) | 1.23
(1.07,1.42)a | 0.87
(0.60,1.26) | 0.65
(0.28,1.52) | 0.40 (0.17,
0.92)a | 2.39
(0.82,6.90) | 1.33
(0.66,2.67) | 0.35
(0.14,0.83)a | 0.23
(0.08,0.66)a |
| RCT | 1.49
(1.04,2.13)a | 0.91
(0.60,1.36) | 1.19
(1.00,1.41) | 0.91
(0.59,1.41) | 0.61
(0.21,1.77) | 0.47 (0.19,
1.13) | 3.01
(0.75,12.09) | 1.50
(0.71,3.16) | 0.32
(0.11,0.95)a | 0.16
(0.04,0.70)a |
| Non-RCT | 1.81
(0.93,3.53) | 1.09
(0.66,1.83) | 1.35
(1.04,1.76)a | 0.76
(0.37,1.55) | 0.75
(0.19,2,93) | 0.14 (0.01,
2.60) | 1.58
(0.30,8.40) | 0.50
(0.05,5.08) | 0.40
(0.09,1.83) | 0.40
(0.09,1.83) |
Discussion
LN is a major cause of mortality in patients with
systemic lupus erythematosus (SLE). Despite the relatively high
renal remission rate following treatment with CYC, as many as 15%
of patients with LN are refractory to treatment and up to 50% of
patients develop end-stage renal disease (ESRD) during the
treatment (23–26). In addition, CYC is associated with
significant toxicity, particularly infections, malignancy and
infertility (27). Thus, novel
therapeutic strategies are necessary for the improved clinical
management of patients with LN. CsA and TAC have been found to be
safer than, or at least as efficacious as, CYC in inducing renal
remission in a number of published controlled trials (9–14).
The current meta-analysis of six trials involving
265 patients with active LN revealed that, in terms of inducing
renal remission, LN patients demonstrated a better treatment
response to CNIs compared with CYC. In addition, the risks of
developing irregular menstruation, gastrointestinal disorder and
leucopenia were significantly lower in patients undergoing
induction therapy with CNIs compared with those receiving CYC.
However, CNIs were associated with a higher incidence of
experiencing a transient increase in SCr and glucose disorder than
CYC. In the analysis of the two different CNIs, TAC was indicated
to be superior, revealing a better response rate and fewer adverse
side-effects than CYC. Partially due to the small sizes of the
trials, CsA did not demonstrate any superiority when compared with
CYC. Previously reviewed in vivo and in vitro studies
have revealed that TAC is more potent than CsA in its action
(28). Therefore, the results of
the present study, that TAC was superior to CsA in the induction
therapy of LN, were anticipated.
Any systematic review must assess the suitability of
identified trials for pooling in a meta-analysis. In the present
study, all trials that were included in the analyses were
comparable in several respects: duration of follow-up, pathological
activity and chronicity indices, and the age, gender and renal
function of the patients.
The meta-analysis in the current study generally
agrees with previously published RCTs and systematic reviews
(29,30). Although the conclusions are based
on a small number of randomized trials, they are strengthened by
the homogeneity of the study results and lack of any clear
publication bias. The results favoring CNIs (CR, TR and the adverse
effects) strengthen the evidence for the efficacy and safety of
CNIs as alternative induction agents.
Meta-analyses are applied with increasing frequency
compared with RCTs; the latter are considered to provide the
strongest evidence regarding an intervention (31). The use of observational data for
meta-analysis is often dismissed as being inferior in quality to
data from RCTs. However, in many situations, RCTs are not feasible
and only data from observational studies are available. In
addition, even though RCTs mainly produce convincing evidence,
flaws in the design, such as performance bias and detection bias
(32), may compromise the quality
of the evidence that they produce. Thus, at certain times
observational studies may produce more reliable evidence than RCTs.
To our knowledge, >1,000 papers relating to the meta-analysis of
observational studies currently exist. Furthermore, in order to
eliminate the bias from observational studies, a sensitivity
analysis containing RCTs only was performed in the present study
and the results of the meta-analysis did not change
significantly.
Despite the added precautions, there are limitations
to the current study. Firstly, although all the trials included
were similar in terms of the baseline characteristics of the
patients, duration of follow-up, pathological activity and
chronicity indices and renal function, there were also several
differences, including the definitions of complete and partial
remission, the dosages of CNIs and CYC used, and the route of CYC
administration. These differences may have introduced bias into the
analyses. This highlights the need for adequately powered RCTs and
observational studies. Secondly, although statistically significant
results with regards to CR, TR and several side-effects were
obtained, all results were based on a relatively small number of
events and may therefore have been susceptible to random error.
Small meta-analyses should be regarded with caution even in the
presence of statistically significant results (33). However, meta-analyses are able to
combine data from trials with small sample sizes in order to obtain
useful information. Thirdly, the poor quality of the trials, mainly
resulting from the lack of double-blindness and double-dummy
procedures, may have further compromised the validity of the
results. Fourthly, the trials also differed in the ethnicity of the
participants, with four studies including only Asian patients
(10–13). It is known that there are
significant differences in the response of different ethnic groups
to treatment of LN. Previous reports have implied that
African-Americans have a three-fold higher incidence rate of SLE
compared with Caucasians and often develop nephritis (34,35).
Hispanics and Asians also have a greater frequency and severity of
nephritis compared with Caucasians (34). However, there are inadequate data
in the current study to examine the treatment allocation to
ethnicity interactions directly and to make any final conclusions
on this topic. Finally, both proliferative (III and IV) and
membranous (V) lupus nephritis were included in the current study,
although the latter is felt to exhibit a relatively benign course
and a better prognosis. This may have biased the final conclusions
that CNIs are more effective than CYC in all patients with active
LN. In the present meta-analysis, the majority of studies included
LN in classes III, IV or V (9–11),
whereas a smaller number of trials included only LN in class V
(12,14). Among these studies, only Chen et
al (9) performed a subgroup
analysis that was restricted to patients with proliferative lesions
(severe class III and class IV). The study drew the conclusion that
the CR rates were similar between the TAC and CYC groups after
induction treatment in patients with LN classes III or IV. However,
the CR rates were higher in the TAC group than in the CYC group in
patients with LN classes V, V+IV, or V+III, although this
difference was not statistically significant. Owing to the lack of
individual patient data in trials that included small numbers of
class V LN patients, it was not possible to perform a subgroup
analysis. This may have caused bias in the meta-analysis of the
present study. Due to the different pathogenesis between
proliferative (class III and IV) LN and membranous (class V) LN,
the response to CINs may vary. The review performed by Moroni et
al (36) revealed an important
antiproteinuric effect of CsA with a cumulative rate of CR or PR
approaching 90% both in patients with proliferative LN and in
patients with membranous nephropathy. Similarly, Favre et al
(37) concluded that CsA had a
notable effect on proteinuria accompanying proliferative and
membranous glomerulonephritis. With regards to TAC, several studies
(9,12,38)
reported the efficacy and absence of serious adverse effects when
TAC was administered as an induction and maintenance therapy for
patients with proliferative and membranous LN. Therefore, it may be
hypothesized that CNIs are likely to be effective in the treatment
of patients with proliferative and membranous LN. However, the
question of whether CNIs were more efficacious and safer than CYC
respectively for patients with proliferative and membranous LN
remains uncertain. Therefore, the results of the current
meta-analysis, despite being comprehensive, may not fully reflect
the relative efficacy and safety profile of CNIs and CYC and the
results should be interpreted with caution. Nevertheless, every
effort has been implemented to eliminate potential bias and the
sensitivity analyses have improved the robustness of the pooled
estimates.
In conclusion, CNIs, especially TAC, may be
reasonable alternative treatments for patients with active LN who
are insensitive or intolerant to CYC in induction therapy.
Acknowledgements
The authors thank Dr Zemu Wang, Nanjing Medical
University, who provided analysis tools for the present study.
References
|
1
|
Cervera R, Khamashta MA, Font J, et al;
The European Working Party on Systemic Lupus Erythematosus.
Systemic lupus erythematosus: clinical and immunologic patterns of
disease expression in a cohort of 1,000 patients. Medicine
(Baltimore). 72:113–124. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mok CC and Tang SS: Incidence and
predictors of renal disease in Chinese patients with systemic lupus
erythematosus. Am J Med. 117:791–795. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Austin HA III, Klippel JH, Balow JE, et
al: Therapy of lupus nephritis. Controlled trial of prednisone and
cytotoxic drugs. N Engl J Med. 314:614–619. 1986.PubMed/NCBI
|
|
4
|
Carette S, Klippel JH, Decker JL, et al:
Controlled studies of oral immunosuppressive drugs in lupus
nephritis. A long-term follow-up. Ann Intern Med. 99:1–8. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boumpas DT, Austin HA III, Vaughn EM, et
al: Controlled trial of pulse methylprednisolone versus two
regimens of pulse cyclophosphamide in severe lupus nephritis.
Lancet. 340:741–745. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok CC, Wong RW and Lai KN: Treatment of
severe proliferative lupus nephritis: the current state. Ann Rheum
Dis. 62:799–804. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhat P and Radhakrishnan J: B lymphocytes
and lupus nephritis: new insights into pathogenesis and targeted
therapies. Kidney Int. 73:261–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sommerer C, Giese T, Meuer S and Zeier M:
New concepts to individualize calcineurin inhibitor therapy in
renal allograft recipients. Saudi J Kidney Dis Transpl.
21:1030–1037. 2010.PubMed/NCBI
|
|
9
|
Chen W, Tang X, Liu Q, et al: Short-term
outcomes of induction therapy with tacrolimus versus
cyclophosphamide for active lupus nephritis: A multicenter
randomized clinical trial. Am J Kidney Dis. 57:235–244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Li X, Qu L, et al: Tacrolimus
versus cyclophosphamide as treatment for diffuse proliferative or
membranous lupus nephritis: a non-randomized prospective cohort
study. Lupus. 21:1025–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Ren H, Zhang Q, et al: Mycophenolate
mofetil or tacrolimus compared with intravenous cyclophosphamide in
the induction treatment for active lupus nephritis. Nephrol Dial
Transplant. 27:1467–1472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szeto CC, Kwan BC, Lai FM, et al:
Tacrolimus for the treatment of systemic lupus erythematosus with
pure class V nephritis. Rheumatology (Oxford). 47:1678–1681. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zavada J, Pesickova S, Rysava R, et al:
Cyclosporine A or intravenous cyclophosphamide for lupus nephritis:
the Cyclofa-Lune study. Lupus. 19:1281–1289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Austin HA III, Illei GG, Braun MJ and
Balow JE: Randomized, controlled trial of prednisone,
cyclophosphamide, and cyclosporine in lupus membranous nephropathy.
J Am Soc Nephrol. 20:901–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
16
|
Moher D, Pham B, Jones A, et al: Does
quality of reports of randomised trials affect estimates of
intervention efficacy reported in meta-analyses? Lancet.
352:609–613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kjaergard LL, Villumsen J and Gluud C:
Reported methodologic quality and discrepancies between large and
small randomized trials in meta-analyses. Ann Intern Med.
135:982–989. 2001. View Article : Google Scholar
|
|
18
|
Oremus M, Oremus C, Hall GB and McKinnon
MC: Inter-rater and test-retest reliability of quality assessments
by novice student raters using the Jadad and Newcastle-Ottawa
Scales. BMJ Open. 2:e0013682012. View Article : Google Scholar
|
|
19
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyasaka N, Kawai S and Hashimoto H:
Efficacy and safety of tacrolimus for lupus nephritis: a
placebo-controlled double-blind multicenter study. Mod Rheumatol.
19:606–615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yap DY, Yu X, Chen XM, et al: Pilot 24
month study to compare mycophenolate mofetil and tacrolimus in the
treatment of membranous lupus nephritis with nephrotic syndrome.
Nephrology (Carlton). 17:352–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao H, Liu ZH, Xie HL, Hu WX, Zhang HT and
Li LS: Successful treatment of class V+IV lupus nephritis with
multitarget therapy. J Am Soc Nephrol. 19:2001–2010. 2008.
|
|
23
|
Valeri A, Radhakrishnan J, Estes D, et al:
Intravenous pulse cyclophosphamide treatment of severe lupus
nephritis: a prospective five-year study. Clin Nephrol. 42:71–78.
1994.PubMed/NCBI
|
|
24
|
Mok CC, Ho CT, Siu YP, et al: Treatment of
diffuse proliferative lupus glomerulonephritis: a comparison of two
cyclophosphamide-containing regimens. Am J Kidney Dis. 38:256–264.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sesso R, Monteiro M, Sato E, Kirsztajn G,
Silva L and Ajzen H: A controlled trial of pulse cyclophosphamide
versus pulse methylprednisolone in severe lupus nephritis. Lupus.
3:107–112. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Belmont HM, Storch M, Buyon J and Abramson
S: New York University/Hospital for Joint Diseases experience with
intravenous cyclophosphamide treatment: efficacy in steroid
unresponsive lupus nephritis. Lupus. 4:104–108. 1995. View Article : Google Scholar
|
|
27
|
Dooley MA and Ginzler EM: Newer
therapeutic approaches for systemic lupus erythematosus:
immunosuppressive agents. Rheum Dis Clin North Am. 32:91–102. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scott LJ, McKeage K, Keam SJ and Plosker
GL: Tacrolimus: a further update of its use in the management of
organ transplantation. Drugs. 63:1247–1297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YH, Lee HS, Choi SJ, Dai Ji J and Song
GG: Efficacy and safety of tacrolimus therapy for lupus nephritis:
a systematic review of clinical trials. Lupus. 20:636–640. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng J, Huo D, Wu Q, Yang Z and Liao Y: A
meta-analysis of randomized controlled trials comparing tacrolimus
with intravenous cyclophosphamide in the induction treatment for
lupus nephritis. Tohoku J Exp Med. 227:281–288. 2012. View Article : Google Scholar
|
|
31
|
Midgette AS, Wong JB, Beshansky JR, Porath
A, Fleming C and Pauker SG: Cost-effectiveness of streptokinase for
acute myocardial infarction: A combined meta-analysis and decision
analysis of the effects of infarct location and of likelihood of
infarction. Med Decis Making. 14:108–117. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jones T and Evans D: Conducting a
systematic review. Aust Crit Care. 13:66–71. 2000. View Article : Google Scholar
|
|
33
|
Flather MD, Farkouh ME, Pogue JM and Yusuf
S: Strengths and limitations of meta-analysis: larger studies may
be more reliable. Control Clin Trials. 18:568–579; discussion
661–666. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alarcón GS, Friedman AW, Straaton KV, et
al: Systemic lupus erythematosus in three ethnic groups: III. A
comparison of characteristics early in the natural history of the
LUMINA cohort LUpus in MInority populations: NAture vs Nurture.
Lupus. 8:197–209. 1999.
|
|
35
|
Cooper GS, Parks CG, Treadwell EL, et al:
Differences by race, sex and age in the clinical and immunologic
features of recently diagnosed systemic lupus erythematosus
patients in the southeastern United States. Lupus. 11:161–167.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moroni G, Doria A and Ponticelli C:
Cyclosporine (CsA) in lupus nephritis: assessing the evidence.
Nephrol Dial Transplant. 24:15–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Favre H, Miescher PA, Huang YP, Chatelanat
F and Mihatsch MJ: Ciclosporin in the treatment of lupus nephritis.
Am J Nephrol. 9(Suppl 1): 57–60. 1989. View Article : Google Scholar
|
|
38
|
Chen W, Liu Q, Chen W, et al: Outcomes of
maintenance therapy with tacrolimus versus azathioprine for active
lupus nephritis: a multicenter randomized clinical trial. Lupus.
21:944–952. 2012. View Article : Google Scholar : PubMed/NCBI
|