Introduction
Obstructive jaundice (OJ) is a common
pathophysiological process that occurs in numerous clinical
conditions, including gallstones, stricture of the bile duct and
pancreatic cancer. Surgical or interventional decompression is the
main treatment strategy for OJ patients (1). In the treatment of OJ patients,
severe complications may occur in surgical or interventional
decompression. The procedure of decompression itself is not enough
to prevent the complications. One of the major consequences of OJ
is the development of severe liver injury (2). The mechanisms responsible for the
pathogenesis of OJ-induced liver injury remain largely unknown,
although inflammatory cell infiltration, microvascular perfusion
failure and Toll-like receptor (TLR) activation are reported to be
involved (3,4). At present, there are no effective
treatments to protect against OJ-induced liver injury, thus, novel
therapeutic strategies are urgently required.
Preconditioning is a process where the body is
subjected to mild stress in order to increase its resistance to
further stresses. It has been associated with increased resistance
and protection against numerous types of tissue injuries, including
infected thermal injury, ischemia/reperfusion (I/R) injury and
hemorrhagic shock (5–8). Notably, hyperthermia preconditioning
has been demonstrated to be an effective method to protect against
OJ-induced liver injury in rats (9). The results from this study were in
accordance with previous studies, which demonstrated that
hyperthermic preconditioning enhances the immune response of rats
with OJ (10,11).
Previous studies have demonstrated that numerous
damage-associated molecular pattern (DAMP) molecules, including
high mobility group box 1 (HMGB1), lipoteichoic acid,
lipopolysaccharide (LPS) and heat shock protein, have been
successfully developed as preconditioning agents (12–15).
Furthermore, the protective effect of HMGB1 preconditioning on
hepatic I/R injury was found to involve the downregulation of it’s
receptor TLR4 (16). It is
noteworthy that pretreatment with LPS, lipoteichoic acid and HMGB1
were all demonstrated to have a protective effect against
myocardial I/R injury, suggesting that cross-tolerization may occur
between different preconditioning agents (12–14).
Histones have been previously identified as alarmins or DAMP
molecules, which serve as danger signals in the context of the
‘danger model’ to promote activation of the innate immune system in
response to several types of tissue injury, including OJ-induced
liver injury (17).
Therefore, in the present study it was hypothesized
that preconditioning with histones, which are recently identified
DAMP molecules, may protect against OJ-induced liver injury and the
downregulation of TLR may be involved in this process.
Materials and methods
Reagents
Histones obtained from calf thymus (H9250) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). TRIzol reagent
was purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA). The RevertAid First Strand cDNA Synthesis kit was obtained
from Fermantas (Beverly, MA, USA). The SYBR-Green kit was purchased
from Bio-Rad (Hercules, CA, USA).
Animals
Adult male Sprague-Dawley (SD) rats were purchased
from the Medical Experimental Animal Center of Guangdong Province
(Guangzhou, Guangdong, China). Rats were provided with standard
rodent chow and water ad libitum under a natural day/night
cycle. All the experimental protocols were approved by the Animal
Ethics Committee of the First Affiliated Hospital of Shenzhen
University (Shenzhen, Guangdong, China).
Experimental protocol
In total, 18 SD rats were randomly divided into
three groups, with each group containing six animals. Animals in
group 1 underwent sham surgery (sham group). Animals in group 2
underwent bile duct ligation (BDL) 24 h subsequent to physiological
saline pretreatment (control group), whilst animals in group 3
underwent BDL 24 h subsequent to histone pretreatment (HPC
group).
Prior to surgery, rats were fasted for 12 h with
water ad libitum. Each rat was weighed and anesthetized with
10% chloral hydrate (300 mg/kg) intraperitoneally. Following a
midline incision, the common bile duct was exposed by careful
separation from its surrounding soft tissue and a double-ligature
with 5–0 silk suture was performed, and the bile duct was sectioned
between the ligatures. A two-layer running suture was then used for
abdominal closure with 4–0 dexon and 2–0 nylon. The sham animals
underwent the same surgical procedure with the exception of
ligation and section of the common bile duct. Animals in the
control and HPC groups were intraperitoneally administered 1 ml
physiological saline and 200 μg/kg histones from calf thymus,
respectively, 24 h prior to BDL. All animals were euthanized 14
days subsequent to BDL with an overdose of chloral hydrate.
Sample collection
A second laparotomy was performed once the animals
were anesthetized, 14 days after BDL. Following collection of the
blood samples from the inferior vena cava, the liver was carefully
dissected from its attachment and totally excised. The blood
samples were stored at 4°C for biochemical analysis of total
bilirubin (TB), direct bilirubin (DB) and alanine aminotransferase
(ALT) levels in the serum. The left lobe of the liver was excised
and flushed with physiological saline and then cut into two
sections. One section was immediately frozen in liquid nitrogen and
stored at −80°C for the measurement of mRNA levels of TLR-4, TLR-9
and interleukin-6 (IL-6), whilst the other section was fixed in 40
g/l paraformaldehyde for histopathological analysis.
Histopathological observations
Liver tissues from all the experimental animals were
fixed in 40 g/l formaldehyde and embedded in paraffin. For
histopathological evaluation, 4-mm slides were stained with
hematoxylin and eosin. The sections were scored by an experienced
hepatopathologist in a blinded manner. The histological activity
index (HAI) scoring system has been previously used to evaluate
histopathology in BDL rats (18,19).
In the present study, a modified HAI scoring system was used, which
included the following lesions: piecemeal necrosis, confluent
necrosis, focal (spotty) lytic necrosis, apoptosis, focal
inflammation and portal inflammation.
The levels of bile duct proliferation were also
scored by an experienced hepatopathologist in a blinded manner on a
scale between 0 and 2, with 0 denoting absent or mild; 1 moderate
and 2 severe proliferation (20).
Neutrophils that accumulated in the liver were counted in a blinded
manner in 20 randomly selected fields using a microscope (Olympus
BX50-32H01; Olympus, Tokyo, Japan; magnification, ×400). The data
are expressed as the number of polymorphonuclear neutrophils per
high-power field (PMNs/HPF).
Blood biochemistry
The results from the histopathological analysis were
verified biochemically by measuring the serum levels of ALT, TB and
DB in each experimental group using an autoanalyzer (Hitachi
7600–020; Hitachi, Tokyo, Japan).
Quantitative polymerase chain reaction
(qPCR)
Rat liver samples (0.1 g/per sample) stored at −80°C
were homogenized in 1 ml TRIzol reagent and the total RNA was
isolated in accordance with the manufacturer’s instructions. The
synthesis of cDNA was performed using the RevertAid First Strand
cDNA Synthesis kit. The house-keeping gene β-actin was used as an
internal control to analyze the mRNA expression levels of TLR-4,
TLR-9 and IL-6. The sequences of the PCR primers were designed
based on cDNA sequences from GenBank (http://www.ncbi.nlm.nih.gov/genbank/), and were as
follows: TLR-4, forward 5′-CGCTCTGGCATCATCTTCAT-3′ and reverse
5′-CTCCTCAGGTCAAAGTTGTTGC3′; TLR-9, forward
5′-TGAGCTACAACAGCCAGCCA-3′ and reverse 5′-AATGTCATTGTGTGCCAGGC-3′;
IL-6, forward 5′-GTCAACTCCATCTGCCCTTCAG-3′ and reverse
5′-GGTCTGTTGTGGGTGGTATCCT-3′.
qPCR was performed using SYBR-Green PCR master mix
according to the manufacturer’s instructions and each sample was
analyzed in duplicate. The mRNA levels of each of the genes being
investigated were quantified using the ABI 7700 Sequence Detection
System (Applied Biosystems, Warrington, UK) using the comparative
methods. The quantity of mRNA was calculated using the ΔΔCt method.
Ct values for each gene were normalized to the Ct value of β-actin
(ΔCt = Ct-β-actin - Ct-target). The results
are presented as mRNA fold change: 2−ΔΔCt (ΔΔCt =
ΔCt-sham - ΔCt-HPC, in the HPC group or ΔΔCt
= ΔCt-sham - ΔCt-control, in the control
group).
Statistical analysis
One-way analysis of variance, with subsequent
post-hoc least significant difference tests and Bonferroni tests,
was used for comparison between the experimental groups with
continuous variables. Differences in the distribution of
histopathological scores (modified HAI scores, bile duct
proliferation scores and PMNs/HPF) between the groups were assessed
using the Mann-Whitney U test. P<0.05 was considered to indicate
a statistically significant difference. All analyses were performed
using SPSS statistical software version 10.0 (SPSS, Inc., Chicago,
IL, USA).
Results
Macroscopic observations
Animals that underwent sham surgery (sham group)
showed no alterations in the clinical conditions, specifically in
normal activity, no irritability, no vertical hair, normal body
weight, no yellowed tails, no darkened urine and no pale feces. In
the control and HPC groups however, 24 h after surgery the clinical
conditions of the animals deteriorated, as shown by decreased
activity, irritability, vertical hair, body weight loss, yellowed
tails, darkened urine and pale feces. All the animals survived
until the end of the experiment. Jaundice was observed in the
visceral and parietal peritoneum of all animals with the exception
of animals in the sham group. Varying degrees of ascites, enlarged
livers and dilated bile ducts above the obstruction point were also
observed in all animals with the exception of those in the sham
group.
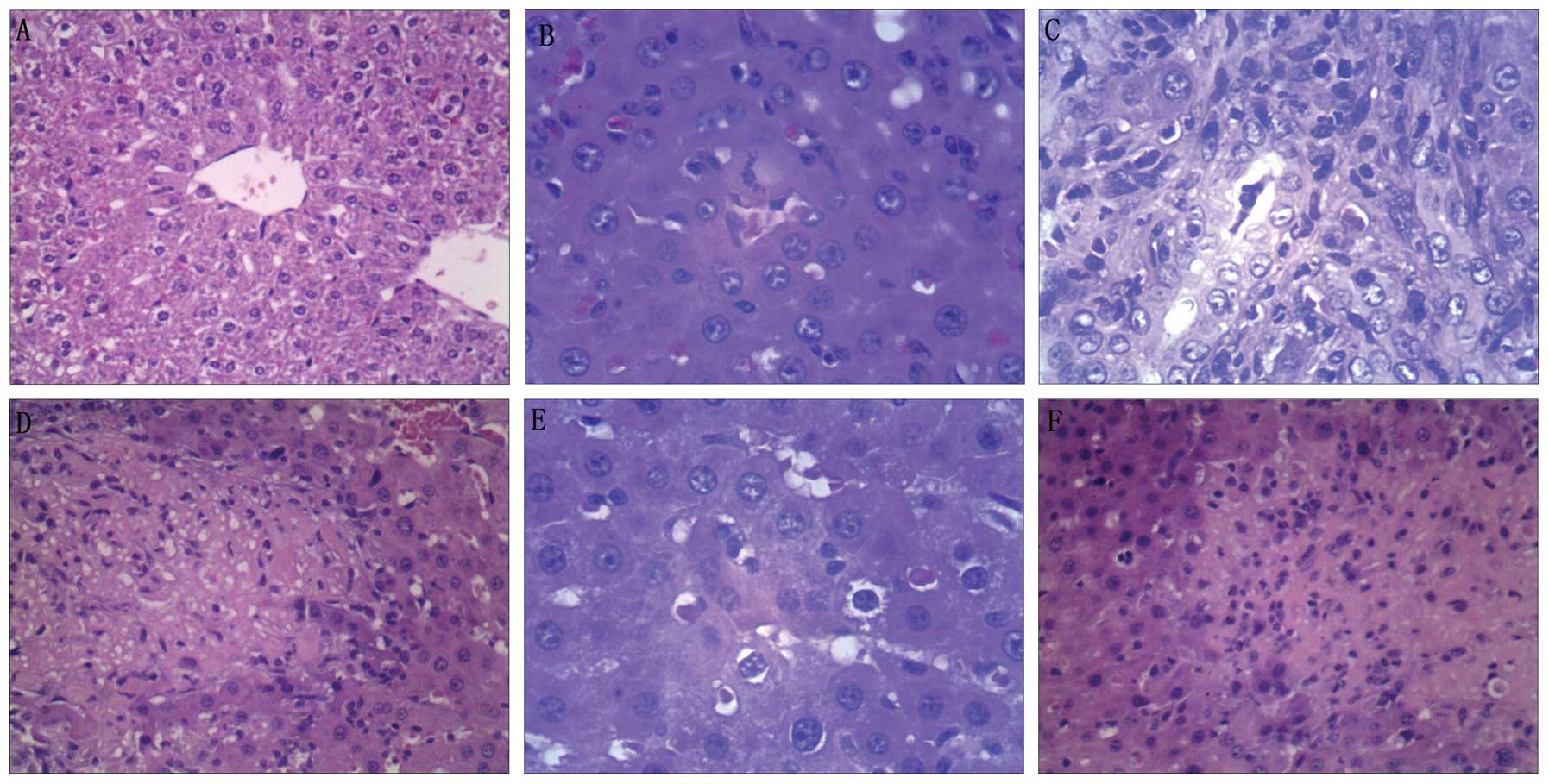
Microscopic observations
No histological alterations were observed in animals
in the sham group. Following euthanasia on day 14 after BDL, severe
liver injury was observed in the control group, as indicated by an
increase in neutrophil infiltration into the liver tissue, as well
as an increase in ductal proliferation and significantly higher
modified HAI scores (P<0.05) compared with the sham group.
(Fig. 1; Table I).
 | Table IHistopathological score of ductal
proliferation, modified HAI and the number of PMNs/HPF in the three
groups. |
Table I
Histopathological score of ductal
proliferation, modified HAI and the number of PMNs/HPF in the three
groups.
| Sham | HPC | Control |
|---|
| Ductal
proliferation | 0 | 1 | 2.0a |
| PMNs/HPF | 0 | 2 | 7.5a,b |
| HAI | 1.5 | 5 | 9.5a,b |
Histone preconditioning significantly ameliorated
the OJ liver injury induced by BDL in the control group, as
indicated by a significant reduction in neutrophil infiltration
into the liver tissue and decreased modified HAI scores of animals
in the HPC group compared with the control group (P<0.05;
Fig. 1; Table I). The results of the
histopathological analysis, including the PMNs/HPF, the bile duct
proliferation scores and the modified HAI scores, of the three
groups are summarized in Table 1.
No significant difference was identified in the ductal
proliferation scores between the HPC group and the control group
(P>0.05).
Blood biochemistry results
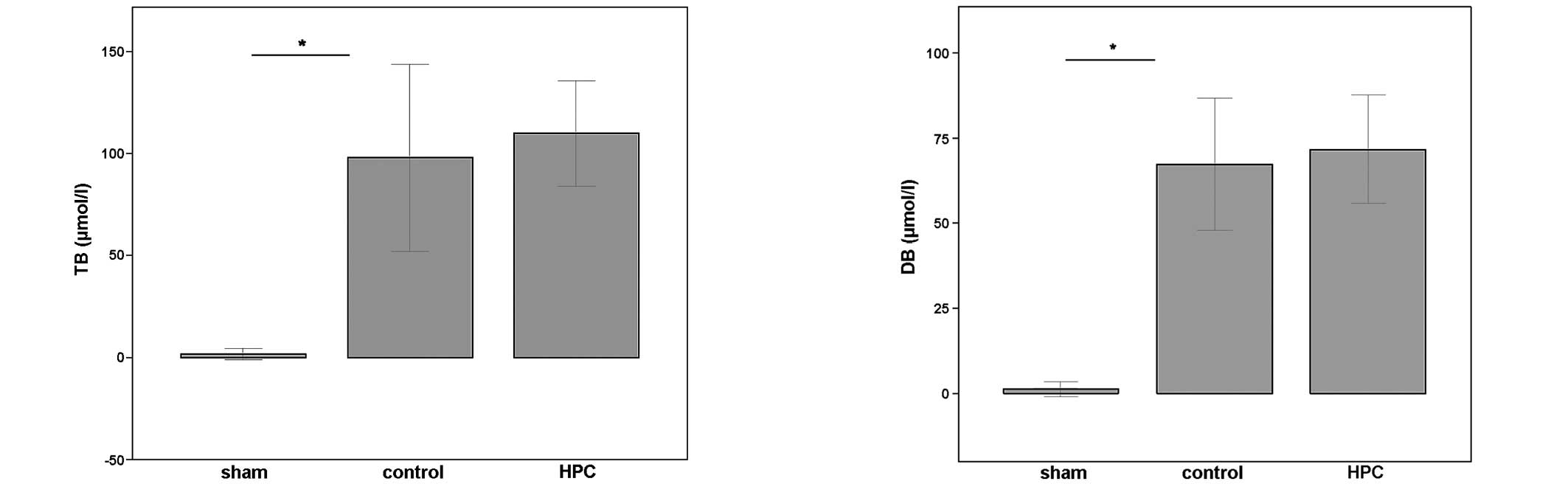
BDL in the control group resulted in significantly
elevated serum levels of TB and DB (Fig. 2) compared with the sham group,
which suggests that the experimental OJ model was successfully
induced. The serum levels of TB and DB in rats preconditioned with
histone proteins prior to being subjected to BDL were not
significantly different from those in the control animals
(P>0.05), indicating that the degree of cholestasis was similar
in the two experimental groups (Fig.
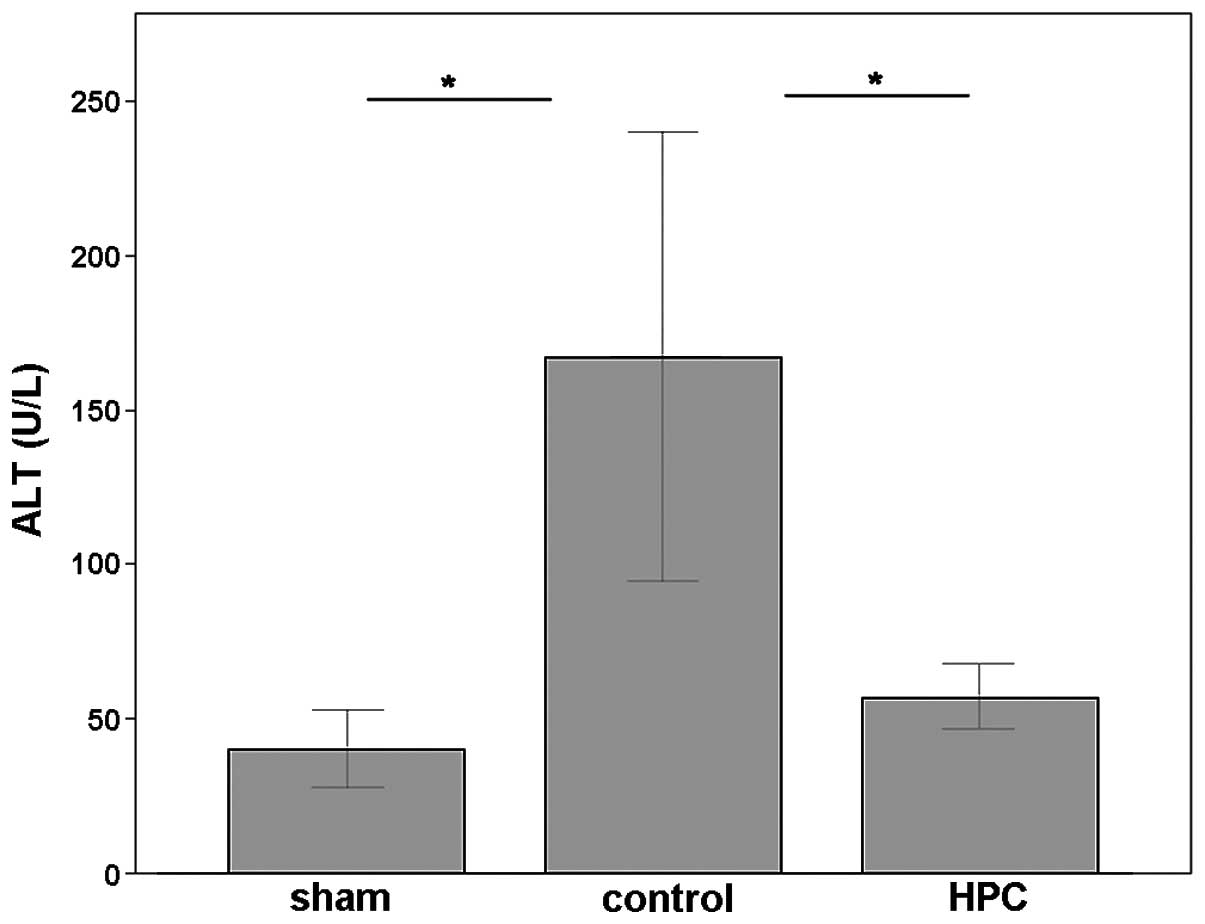
2). OJ liver injury induced by BDL was prominent in the control
group, as shown by the significantly increased serum levels of ALT
(Fig. 3) compared with the sham
group (P<0.05). In accordance with the results of
histopathological studies, histone preconditioning significantly
ameliorated OJ-induced liver injury induced by BDL. The serum
levels of ALT were significantly lower than that of the control
group (P<0.05; Fig. 3).
mRNA expression of IL-6, TLR-4 and
TLR-9
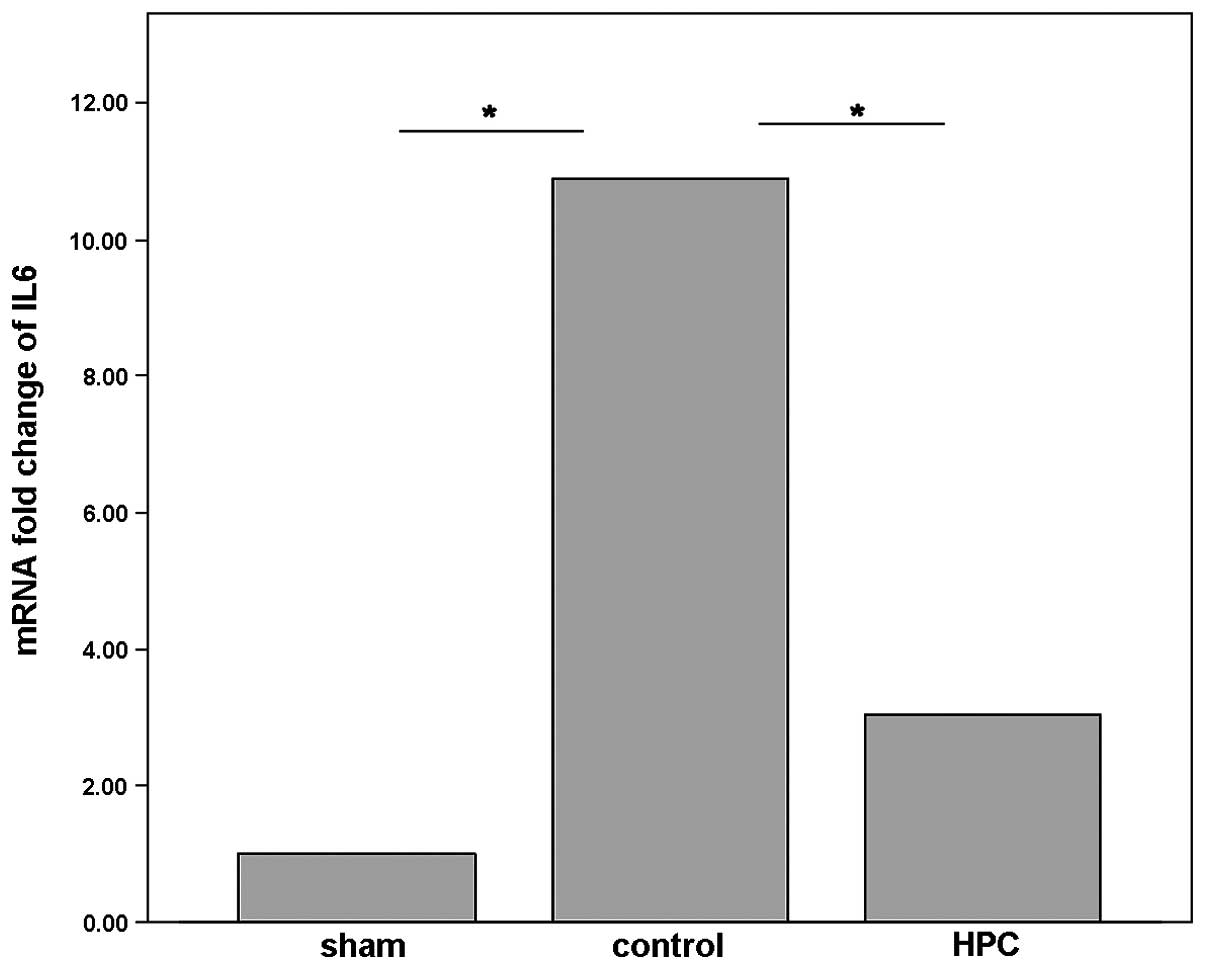
Using qPCR, it was demonstrated that the mRNA
expression levels of IL-6 were significantly upregulated by BDL in
the control group compared with the sham group (P<0.05; Fig. 4), which is consistent with previous
studies (21). However, compared
with the control group, histone preconditioning (HPC group)
significantly downregulated the mRNA expression levels of IL-6
(P<0.05; Fig. 4). In addition,
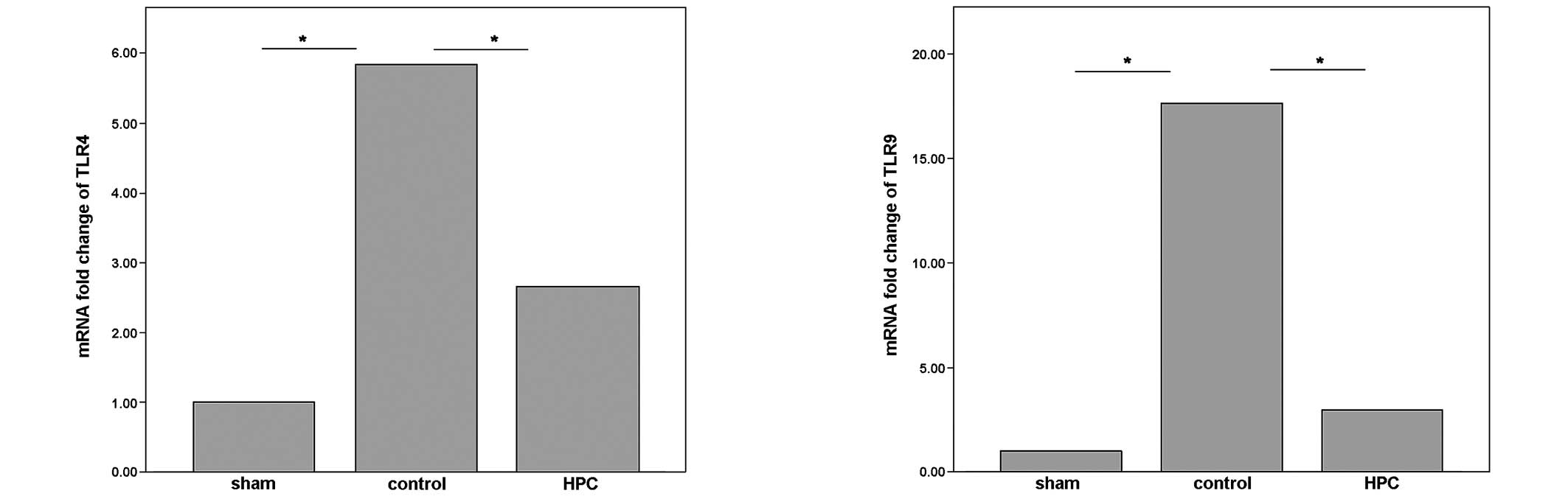
BDL in the control group significantly upregulated the mRNA
expression levels of TLR-4 and TLR-9 (P<0.05; Fig. 5) compared with the sham group,
which is consistent with previous studies (4). In the present study it was
demonstrated that histone preconditioning significantly ameliorated
the upregulation of the mRNA expression levels of TLR-4 and TLR-9.
Animals preconditioned with histones expressed significantly lower
mRNA levels of TLR-4 and TLR-9 (P<0.05; Fig. 5) compared with animals in the
control group.
Discussion
OJ is a common clinical condition, which has been
extensively studied, and is capable of inducing severe liver injury
(2). Surgical, endoscopic and
interventional decompressions are the primary treatment strategies
for patients with OJ. However, biliary intervention has been
demonstrated to augment inflammatory cell infiltration and
aggravate OJ-induced liver injury (22,23).
Preconditioning with DAMP molecules has been demonstrated to be
effective in protecting organs from injury in stressed situations
(12–15). Preconditioning with hyperthermia
(<42°C for 20 min) 12 h prior to being subjected to BDL was
found to significantly ameliorate OJ-induced liver injury (9,10).
However, hyperthermia is difficult to achieve in the clinic, thus,
an effective pharmaceutical drug would be the preferred option.
Histones are a newly identified DAMP molecule,
however, the preconditioning effect of histones on OJ-induced liver
injury remains to be elucidated. In the present study, the effect
of histone preconditioning on OJ-induced liver injury in rats was
investigated. Preconditioning with HMGB1 (20 μg/mouse) has been
demonstrated to significantly protect against hepatic I/R injury,
and preconditioning with LPS (100 μg/kg) has been demonstrated to
significantly protect against hepatic I/R injury, so in our study
we preconditioned experimental animals with 200 μg/kg histone
proteins (16,24). The present study demonstrated that
liver injury in animals in the HPC group was significantly
ameliorated by histone preconditioning compared with the control
group, as indicated by significant differences in the degree of
necroinflammation and a decrease in the number of neutrophils
infiltrating into the liver tissue. The serum levels of ALT were
significantly lower in the HPC group compared with the control
group, indicating that necrosis of hepatocytes was prevented, which
is consistent with the results from the histopathological
analysis.
Inflammation has been revealed to be important in
the development of OJ-induced liver injury (2). In addition, the proinflammatory
cytokine IL-6 has been demonstrated to be important in the
pathogenesis of OJ-induced liver injury (21). Endotoxemia has been found to occur
in patients with jaundice and experimental OJ animals, and LPS was
demonstrated to be capable of inducing the release of
proinflammatory cytokines, including TNF-α and IL-6 (25,26).
Therefore, in the present study, the expression levels of IL-6 in
each group were investigated. Using qPCR, it was demonstrated that
the mRNA expression levels of IL-6 in the control group were
significantly higher compared with the sham group, whilst histone
preconditioning significantly downregulated the mRNA expression of
IL-6. The present study demonstrated that histone preconditioning
protects against OJ-induced liver injury in rats by inhibiting the
release of inflammatory mediators.
The preconditioning effects of HMGB1 and LPS on
hepatic I/R injury were previously reported to be dependent on TLR4
expression (the receptor for HMGB1 and LPS). The receptors for
histones have been demonstrated to be TLR-4 and TLR-9, therefore,
the present study investigated the role of TLR-4 and TLR-9 in the
process of histone preconditioning on OJ-induced liver injury
(16,17,24,27).
The results from the qPCR analysis revealed that the mRNA
expression levels of TLR4 and TLR9 in the liver tissue were
significantly lower compared with the control group, which
indicated that TLR4 and TLR9 may be involved in the process of
histone preconditioning in OJ-induced liver injury.
Histone proteins are assembled with DNA to form
nucleosomes in the nucleus and histone modifications have been
demonstrated to be important in gene transcription (28,29).
Recently, histone proteins have been identified as DAMP molecules
(17,27). Furthermore, the present study
demonstrated that histone proteins may be used as a preconditioning
agent to protect against OJ-induced liver injury. Preconditioning
may be achieved with a variety of stress responses and the most
widely studied method is ischemia (30). The adaptive responses to
preconditioning may enhance the body’s tolerance to further
stresses. Although the exact mechanism of preconditioning has not
yet been fully elucidated, the activation of potassium channels,
metabolic alterations and the generation of nitric oxide have been
suggested to be involved (30–33).
Investigations into the mechanisms underlying the protective
effects of preconditioning have led to the application of DAMP
molecules as preconditioning agents to protect against organ
injury. However, for inflammatory diseases, for example OJ-induced
liver injury, the interactions between different DAMP molecules,
the network of proinflammatory cytokines and downstream signaling
molecules are complex. Therefore, further studies are required to
elucidate the mechanisms and the safety of the preconditioning
molecules, particularly as DAMP molecules are known to be
toxic.
In conclusion, histone preconditioning protects
against OJ-induced liver injury in rats, and TLR4 and TLR9 may be
involved in this process. Histone preconditioning may be a novel
and promising therapeutic strategy for the treatment of OJ-induced
liver injury and possibly other diseases. However, its safety and
underlying mechanisms have not been fully elucidated. Thus, further
investigations are necessary to ascertain the safety and
therapeutic mechanisms underlying the protective effects of histone
preconditioning.
Acknowledgements
The authors would like to thank the Department of
Laboratory of The First Affiliated Hospital of Shenzhen University
for assisting in the analysis of the biochemical data. The present
study was supported by the Science and Technology Project Grant of
Guangdong, China (no. 2012B031800349).
References
|
1
|
Tsuyuguchi T, Takada T, Miyazaki M, et al:
Stenting and interventional radiology for obstructive jaundice in
patients with unresectable biliary tract carcinomas. J
Hepatobiliary Pancreat Surg. 15:69–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gujral JS, Farhood A, Bajt ML and Jaeschke
H: Neutrophils aggravate acute liver injury during obstructive
cholestasis in bile duct-ligated mice. Hepatology. 38:355–363.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koeppel TA, Trauner M, Baas JC, et al:
Extrahepatic biliary obstruction impairs microvascular perfusion
and increases leukocyte adhesion in rat liver. Hepatology.
26:1085–1091. 1997.PubMed/NCBI
|
|
4
|
Huang YH, Wang PW, Tiao MM, et al:
Glucocorticoid modulates high-mobility group box 1 expression and
Toll-like receptor activation in obstructive jaundice. J Surg Res.
170:e47–e55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klune JR, Billiar TR and Tsung A: HMGB1
preconditioning: therapeutic application for a danger signal? J
Leukoc Biol. 83:558–563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He W, Fong Y, Marano MA, Gershenwald JE,
Yurt RW, Moldawer LL and Lowry SF: Tolerance to endotoxin prevents
mortality in infected thermal injury: association with attenuated
cytokine responses. J Infect Dis. 165:859–864. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colletti LM, Remick DG and Campbell DA Jr:
LPS pretreatment protects from hepatic ischemia/reperfusion. J Surg
Res. 57:337–343. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ackerman M, Reuter M, Flohé S, Bahrami S,
Redl H and Schade FU: Cytokine synthesis in the liver of
endotoxin-tolerant and normal rats during hemorrhagic shock. J
Endotoxin Res. 7:105–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao SM, Zhang BM, Guan XD, Li J, Jia YB
and Pan HY: The effect of hyperthermia preconditioning on the liver
function of obstructive jaundice rats. J Pract Med. 23:3037–3838.
2007.
|
|
10
|
Mao SM, Zhang BM, Guan XD, Li J, Pan HY
and Jia YB: The effect of hyperthermia pretreatment on the
subgroups of T lymphocytes in obstructive jaundiced rats. Shijie
Huaren Xiaohua Zazhi. 15:3035–3037. 2007.
|
|
11
|
Güllüoğlu BM, Bekraki A, Cerikçioğlu N,
Söyletir G and Aktan AO: Immunologic influences of hyperthermia in
a rat model of obstructive jaundice. Dig Dis Sci. 46:2378–2384.
2001.PubMed/NCBI
|
|
12
|
Hu X, Jiang H, Cui B, et al:
Preconditioning with high mobility group box 1 protein protects
against myocardial ischemia-reperfusion injury. Int J Cardiol.
145:111–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha T, Hua F, Liu X, et al:
Lipopolysccharide-induced myocardial protection against
ischeamia/reperfusion injury is mediated through a
PI3K/Akt-dependent mechanism. Cardiovasc Res. 78:546–553. 2008.
View Article : Google Scholar
|
|
14
|
Zacharowski K, Frank S and Otto M:
Lipoteichoic acid induces delayed protection in the rat heart, a
comparison with endotoxin. Arterioscler Thromb Vasc Biol.
20:1521–1528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aneja R, Odoms K, Dunsmore K, Shanley TP
and Wong HR: Extracellular heat shock protein-70 induces endotoxin
tolerance in THP-1 cells. J Immunol. 177:7184–7192. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Izuishi K, Tsung A, Jeyabalan G, et al:
Cutting edge: high-mobility group box 1 preconditioning protects
against liver ischemia-reperfusion injury. J Immunol.
176:7154–7158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang H, Evankovich J, Yan W, et al:
Endogenous histones function as alarmins in sterile inflammatory
liver injury through toll-like receptor 9. Hepatology. 54:999–1008.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raetsch C, Jia JD, Boigk G, Bauer M, Hahn
EG, Riecken EO and Schuppan D: Pentoxifylline downregulates
profibrogenic cytokines and procollagen I expression in rat
secondary biliary fibrosis. Gut. 50:241–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishak K, Babtista A, Bianchi L, Callea F,
De Groote J, Gudot F, Denk H, Desmet V, Korb G, MacSween RN,
Phillips MJ, Portmann BG, Poulsen H, Scheuer PJ, Schmid M and
Thaler H: Histological grading and staging of chronic hepatitis. J
Hepatol. 22:696–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hammel P, Couvelard A, O’Toole D, et al:
Regression of liver fibrosis after biliary drainage in patients
with chronic pancreatitis and stenosis of the common bile duct. N
Engl J Med. 344:418–423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimmings AN, van Deventer SJ, Obertop H,
Rauws EA, Huibregtse K and Gouma DJ: Endotoxin, cytokines, and
endotoxin binding proteins in obstructive jaundice and after
preoperative biliary drainage. Gut. 46:725–731. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chuang JH, Chang NK, Huang CC, et al:
Biliary intervention augments chemotactic reaction and aggravates
cholestatic liver injury in rats. J Surg Res. 120:210–218. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martignoni ME, Wagner M, Krähenbühl L, et
al: Effect of preoperative biliary drainage on surgical outcome
after pancreaticoduodenectomy. Am J Surg. 181:52–59. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sano T, Izuishi K, Hossain MA, Kakinoki K,
Okano K, Masaki T and Suzuki Y: Protective effect of
lipopolysaccharide preconditioning in hepatic ischaemia reperfusion
injury. HPB (Oxford). 12:538–545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bemelmans MH, Gouma DJ, Greve JW and
Buurman WA: Cytokines tumor necrosis factor and interleukin-6 in
experimental biliary obstruction in mice. Hepatology. 15:1132–1136.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Bossuyt H, Desmaretz C, Gaeta GB and
Wisse E: The role of bile acids in the development of endotoxemia
during obstructive jaundice in the rat. J Hepatol. 10:274–279.
1990.PubMed/NCBI
|
|
27
|
Xu J, Zhang X, Monestier M, Esmon NL and
Esmon CT: Extracellular histones are mediators of death through
TLR2 and TLR4 in mouse fatal liver injury. J Immunol.
187:2626–2631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Holde KE: Histone modifications.
Chromatin. 4. 1st edition. Springer; New York, NY: pp. 111–148.
1988
|
|
29
|
Strahl BD and Allis CD: The language of
covalent histone modifications. Nature. 403:41–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Opie LH and Sack MN: Metabolic plasticity
and the promotion of cardiac protection in ischemia and ischemic
preconditioning. J Mol Cell Cardiol. 34:1077–1089. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen W, Glasgow W, Murphy E and
Steenbergen C: Lipooxygenase metabolism of arachidonic acid in
ischemic preconditioning and PKC-induced protection in heart. Am J
Physiol. 276:H2094–H2101. 1999.PubMed/NCBI
|
|
32
|
Schulz R, Rose J and Heusch G: Involvement
of activation of AT dependent potassium channels in ischemic
preconditioning in swine. Am J Physiol. 267:H1341–H1352.
1994.PubMed/NCBI
|
|
33
|
Marber MS, Latchman DS, Walker JM and
Yellon DM: Cardiac stress protein elevation 24 hours after brief
ischemia or heat stress is associated with resistance to myocardial
infarction. Circulation. 88:1264–1272. 1993. View Article : Google Scholar : PubMed/NCBI
|