Introduction
Graves’ disease (GD) is a common autoimmune thyroid
disease accounting for ~85% of all clinical hyperthyroidism cases.
Patients produce autoantibodies that activate the thyroid
stimulating hormone receptor (TSHR) on thyroid follicular cells,
leading to thyroid enlargement and excessive production of thyroid
hormones (1–3). The etiology of GD is considered to be
multifactorial, including complex interactions between
environmental, genetic, endogenous and local factors (4,5).
Previous studies have focused on identifying a number of putative
susceptibility genes in GD and several gene loci have been reported
to be associated with a risk of developing GD, including TSHR,
interleukin-21 (IL-21), human leukocyte antigen class I and II,
protein tyrosine phosphatase non-receptor 22 and cytotoxic
T-lymphocyte associated 4 (6–13).
IL-21 is a pleiotropic cytokine that is produced
mainly by activated CD4+ T cells and natural killer (NK)
T cells (14). The activity of
IL-21 is mediated by binding to a composite receptor consisting of
a private receptor (IL-21R) and the common cytokine receptor γ
chain (γc) (15,16).
The expression of IL-21R has been detected in a variety of
lymphohematopoietic cells, as well as fibroblasts, keratinocytes
and intestinal epithelial cells (17–19),
indicating that IL-21 is involved in a wide range of biological
functions. Previously, IL-21 has been shown to play a critical role
in the interactions among B cells, T cells, NK cells and dendritic
cells, and may serve as a pivotal cytokine linking innate and
adaptive immune responses (20).
According to the cellular context of costimulation and the ambient
cytokine environment, IL-21 may promote the proliferation and
differentiation of T cells, alter the production of cytokines and
chemokines, induce the maturation and activation of NK cells and
enhance antibody-dependent cellular cytotoxicity by NK cells
(21–23). In addition, IL-21 has essential
non-redundant regulatory functions on B cell responses and can
promote B cell differentiation and antibody production (24–26),
indicating that IL-21 may also be a causative factor in autoimmune
diseases. IL-21 gene polymorphisms have been reported to be
associated with an increasing number of autoimmune or immunological
diseases, including systemic lupus erythematosus (SLE) (27), rheumatoid arthritis (28,29),
GD (8) and type 1 diabetes
(30).
A previous study demonstrated that GD patients from
Southern China had increased serum expression levels of IL-21
(31). However, whether single
nucleotide polymorphisms (SNPs) of the IL-21 gene are associated
with GD susceptibility remains unclear. In the current study,
rs907715 within the IL-21 gene intronic region was selected as the
tag-SNP and distributions of IL-21/rs907715 gene polymorphisms
among GD patients and healthy controls were analyzed. In addition,
correlations among genotypes and clinical manifestations of GD were
investigated. The aim of the study was to identify the association
between different genotypes at the IL-21 rs907715 gene locus with
GD in a Southern Chinese population.
Materials and methods
Subjects
For the case-control cohort, 211 GD patients (male,
70; female, 141; age, 26–46 years; mean age, 37 years) from
Southern China were recruited from the Department of Endocrinology
at Sun Yat-sen Memorial Hospital (Guangzhou, China). The GD
patients were defined by clinical manifestations and biochemical
criteria of thyrotoxicosis, including thyroid stimulating hormone
levels of <0.05 mIU/l, free triiodothyronine levels of >6.5
pmol/l (normal range, 3.5–6.5 pmol/l), free thyroxine levels of
>22.7 pmol/l (normal range, 11.5–22.7 pmol/l; and had positive
circulating TSHR antibodies or antibodies against thyroglobulin or
thyroid peroxidase (32). The
levels of the hormones mentioned above were measured by ADVIA
Centaur Immunoassay System (Siemens Healthcare, Erlangen,
Germany).
In total, 212 control subjects (male, 78; female,
134; age, 33–50 years; mean age, 41 years) without family history
of thyroid diseases or other autoimmune diseases were recruited
from the Health Care Center at Sun Yat-sen Memorial Hospital. All
the healthy controls were age- and gender-matched with the GD
patients. Informed consent was provided by all the participants and
the experimental protocol was approved by the Ethics Committee of
Sun Yat-sen Memorial Hospital.
Genotyping
Genomic DNA was prepared from peripheral blood
samples using a DNA extraction kit (Omega Bio-Tek, Inc., Norcross,
GA, USA), according to the manufacturer’s instructions. The
concentration and quality of DNA were detected by a nucleic
acid/protein analyzer (Beckman Coulter, Miami, FL, USA) and agarose
gel electrophoresis, respectively. SNPs of IL-21/rs907715 were
determined by direct DNA sequencing following polymerase chain
reaction (PCR). The forward and reverse primers for IL-21/rs907715
were 5′-CCCCAAGTTCCATAAATAGT-3′ and 5′-TTTTTGTATTTTTAGTAGAGACCA-3′,
respectively. PCR was conducted using a genomic DNA template from
each subject at a total volume of 50 μl, which contained 0.25 μl Ex
Taq polymerase (5.0 U/ml; Takara Bio, Inc., Shiga, Japan),
5.0 μl 10X PCR buffer, 4.0 μl dNTP mixture, 3.0 μl DNA template (50
ng/μl), 35.75 μl PCR-grade water and 1.0 μl of each 20 μM primer.
PCR conditions were as follows: 94°C for 4 min; 35 cycles of 94°C
for 45 sec, 58°C for 45 sec and 72°C for 45 sec; and a final
extension at 72°C for 4 min. Following amplification, the PCR
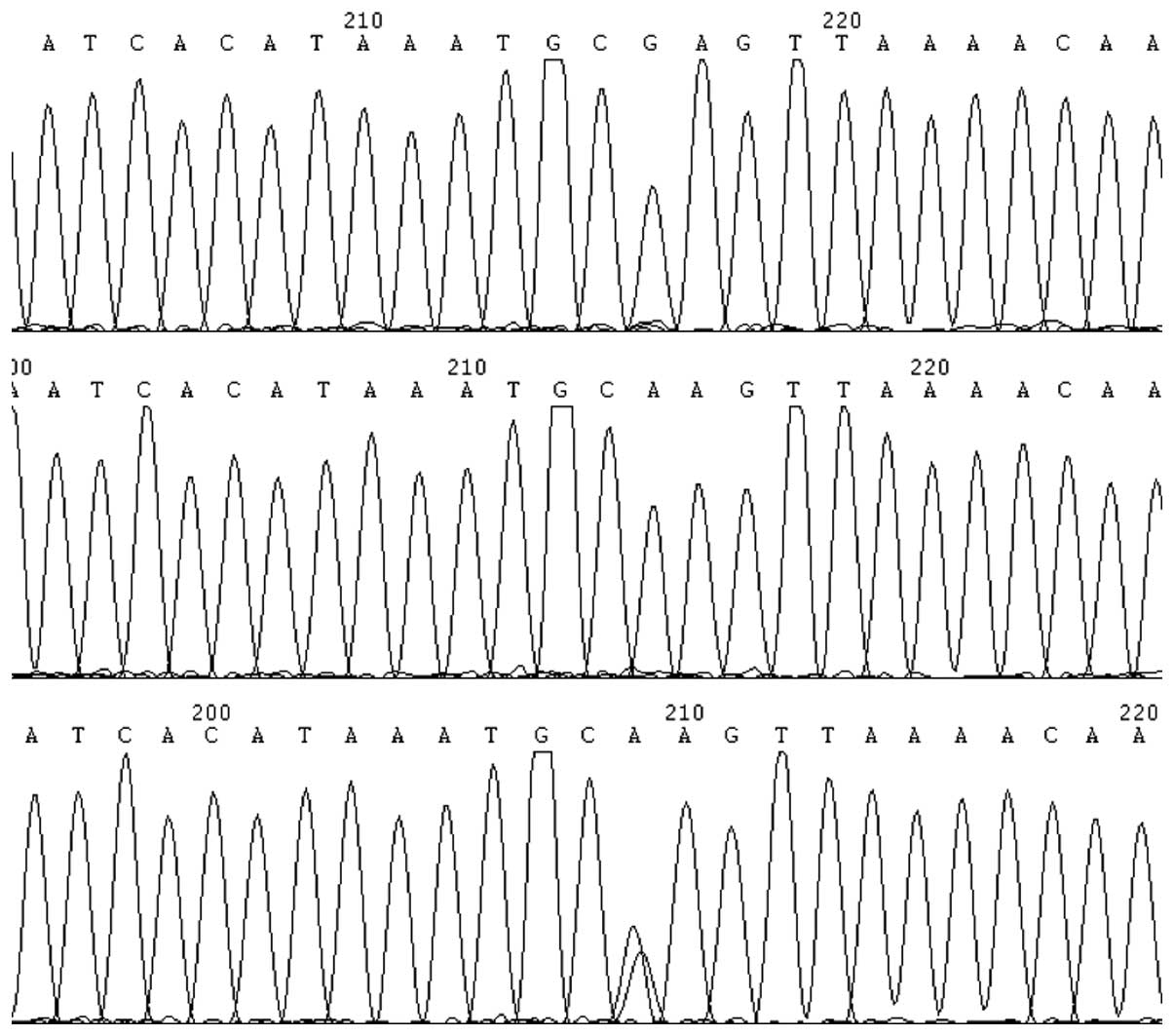
products were submitted for DNA sequencing. The sequences are shown
in Fig. 1.
Clinical phenotype correlations
Correlation analyses between the genotypes/alleles
of IL-21/rs907715 and the clinical characteristics of GD were
performed. The detailed contents were as follows: i) Age of onset
(≤30 vs. ≥31 years), the former represented early onset of GD and
the latter represented the normal age of onset; ii) thyroid size
(≤I vs. ≥II°), goiter size was divided into three degrees by
physical examination (33); iii)
presence or absence of family history of autoimmune thyroid
diseases, including first-degree relatives (parents, children and
siblings) and second-degree relatives (grandparents, uncles and
aunts); iv) presence or absence of ophthalmopathy, which was
defined as a distinctive disorder featured by inflammation and
swelling of the extraocular muscles and eyelid retraction,
periorbital edema, episcleral vascular injection, conjunctive
swelling and proptosis (33,34);
and v) presence or absence of relapse history of GD patients.
Statistical analysis
All genotyping results were analyzed by the
Hardy-Weinberg equilibrium using Excel (Microsoft Office;
Microsoft, Redmond, WA). Allele and genotype frequencies between
the case and control groups were compared using the χ2
test or Fisher’s exact test. The odds ratio (OR) and 95% confidence
interval (CI) were calculated to estimate the disease
susceptibility of specific genotypes and alleles. Statistical
analysis was performed using SPSS software version 20.0 (IBM,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Genotype distributions
A Hardy-Weinberg equilibrium of the genotype
distributions of IL-21/rs907715 polymorphisms was exhibited in the
control group (P>0.05). The allele and genotype frequencies of
IL-21/rs907715 in the case and control groups are listed in
Table I. The distributions of
rs907715 genotypes in GD patients (GG, 35.1%; AG, 48.3%; AA, 16.6%)
differed significantly from those in the healthy controls (GG,
23.0%; AG, 43.0%; AA, 34.0%; χ2=18.500;
P=9.6×10−5). As shown in Table I, the frequency of the GG genotype
was significantly higher in the patients as compared with the
controls (P=6.7×10−3; OR, 1.797; 95% CI, 1.173–2.753).
Conversely, the frequency of the AA genotype was markedly lower in
the patient group compared with the control group
(P=4.0×10−5; OR, 0.387; 95% CI, 0.244–0.613). No
significant difference was observed in the AG genotype distribution
between the patients and matched controls (P=0.263). In addition,
the G allele of IL-21/rs907715 was significantly more frequent in
the GD patients than in the healthy controls. The OR for carrying
the G allele was 1.807 greater in the patients as compared with the
controls (P=2.0×10−5; 95% CI, 1.376–2.374).
 | Table IAllele and genotype frequencies of
IL-21/rs907715 in GD patients and controls. |
Table I
Allele and genotype frequencies of
IL-21/rs907715 in GD patients and controls.
|
Genotype/allele | GD, n (%) | Control, n (%) | χ2 | P-value | OR | 95% CI |
|---|
| Genotype |
| GG | 74 (35.1) | 49 (23.0) | 7.332a |
6.7×10−3 | 1.797 | 1.173–2.753 |
| AG | 102 (48.3) | 91 (43.0) | 1.251b | 0.263 | 1.244 | 0.848–1.825 |
| AA | 35 (16.6) | 72 (34.0) | 16.893c |
4.0×10−5 | 0.387 | 0.244–0.613 |
| Allele |
| G | 250 (59.2) | 189 (44.6) | 18.223 |
2.0×10−5 | 1.807 | 1.376–2.374 |
| A | 172 (40.8) | 235 (55.4) | | | | |
Correlation analyses
Significant differences were observed when the
patients with and without relapse history were compared. The
frequencies of the GG genotype in the relapse and non-relapse
groups were 64.7 and 32.2%, respectively (χ2=12.866;
P=1.6×10−3), and the frequency of the G allele was also
significantly increased in the patients with relapse history
(P=1.4×10−3; OR, 2.628; 95% CI, 1.427–4.840). However,
no associations were observed between the other clinical phenotypes
and rs907715 SNPs, including age at initial diagnosis of GD,
presence or absence of goiter by palpation, presence or absence of
ophthalmopathy and family history (Table II).
 | Table IICorrelation analyses between the
genotypes and alleles of IL-21/rs907715 and the clinical
characteristics of GD. |
Table II
Correlation analyses between the
genotypes and alleles of IL-21/rs907715 and the clinical
characteristics of GD.
|
Genotype/allele | Age at onset, n
(%) | Thyroid size, n
(%) | Family history, n
(%) | Ophthalmopathy, n
(%) | Relapse history, n
(%) |
|---|
|
|
|
|
|
|---|
| ≤30 years | ≥31 years | ≤I° | ≥II° | (+) | (−) | (+) | (−) | (+) | (−) |
|---|
| Genotype |
| GG | 33 (39.8) | 43 (33.6) | 15 (46.9) | 76 (42.5) | 16 (39.0) | 71 (41.8) | 11 (38.0) | 75 (41.2) | 22 (64.7) | 57 (32.2) |
| AG | 29 (34.9) | 61 (47.7) | 12 (37.5) | 62 (34.6) | 18 (43.9) | 67 (39.4) | 9 (31.0) | 63 (34.6) | 9 (26.5) | 89 (50.3) |
| AA | 21 (25.3) | 24 (18.7) | 5 (15.6) | 41 (22.9) | 7 (17.1) | 32 (18.8) | 9 (31.0) | 44 (24.2) | 3 (8.8) | 31 (17.5) |
| χ2 | 3.453 | 0.846 | 0.280 | 0.628 | 12.866 |
| P-value | 0.178 | 0.655 | 0.869 | 0.730 |
1.6×10−3 |
| Allele |
| G | 95 (57.2) | 147 (57.4) | 42 (65.6) | 214 (59.8) | 50 (61.0) | 209 (61.5) | 31 (53.4) | 213 (58.5) | 53 (77.9) | 203(57.3) |
| A | 71 (42.8) | 109 (42.6) | 22 (34.4) | 144 (40.2) | 32 (39.0) | 131 (38.5) | 27 (46.6) | 151 (41.5) | 15 (22.1) | 151(42.7) |
| χ2 |
1.5×10−3 | 0.778 |
6.8×10−3 | 0.527 | 10.141 |
| P-value | 0.969 | 0.378 | 0.934 | 0.468 |
1.4×10−3 |
| OR (95% CI) | | | | | 2.628
(1.427–4.840) |
Allele and genotype frequencies of
IL-21/rs907715 in GD patients with and without relapse history and
controls
Compared with the controls, the GG genotype and G
allele of IL-21/rs907715 were markedly increased in the patients
with and without relapse history. As shown in Table III, the frequencies of the G
allele in the relapse and control groups were 77.9 and 44.6%,
respectively (P=1.0×10−6; OR, 4.393; 95% CI,
2.401–8.040). Similarly, the G allele exhibited an increased
frequency in the non-relapse patients when compared with the
controls (P=3.9×10−4; OR, 1.672; 95% CI, 1.257–2.222).
The frequency of the AA genotype in the relapse group was much
lower compared with the control group (χ2=25.588;
P=2.8×10−6). A significant correlation in AA genotype
distributions was also observed between the non-relapse patients
and healthy controls (χ2=13.910;
P=9.5×10−4).
 | Table IIIAllele and genotype frequencies of
IL-21/rs907715 in GD patients with and without relapse history and
controls. |
Table III
Allele and genotype frequencies of
IL-21/rs907715 in GD patients with and without relapse history and
controls.
| Genotype
/allele | GD, n (%) | (3) Control n
(%) | χ2 | P-value | OR (95% CI) |
|---|
|
|
|
|
|---|
| (1) Relapse | (2)
Non-relapse | (1) vs. (3) | (2) vs. (3) | (1) vs. (3) | (2) vs. (3) | (1) vs. (3) | (2) vs. (3) |
|---|
| Genotype |
| GG | 22 (64.7) | 57 (32.2) | 49 (23.0) | | | | | | |
| AG | 9 (26.5) | 89 (50.3) | 91 (43.0) | 25.588 | 13.910 |
2.8×10−6 |
9.5×10−4 | | |
| AA | 3 (8.8) | 31 (17.5) | 72 (34.0) | | | | | | |
| Allele |
| G | 53 (77.9) | 203 (57.3) | 189 (44.6) | 26.103 | 12.583 |
1.0×10−6 |
3.9×10−4 | 4.393 | 1.672 |
| A | 15 (22.1) | 151 (42.7) | 235 (55.4) | | | | | (2.401–8.040) | (1.257–2.222) |
Discussion
IL-21 is a potent immunomodulatory
four-α-helical-bundle type I cytokine that was initially identified
via functional cloning as the ligand for IL-21R (22). The IL-21 gene consists of five
exons spanning ~8.44 kb genomic DNA and is located on human
chromosome 4q26-27, which is close to IL-2, a region known to be a
common risk locus for multiple autoimmune diseases (35,36).
IL-21 activity is mediated via binding to a compound receptor
consisting of IL-21R and γc, and exerts the
corresponding biological effects primarily through the
Janus-activated kinase/signal transducers and activators of
transcription pathway or the phosphatidylinositol
3-kinase/mitogen-activated protein kinase pathway (37,38).
The signaling pathways can promote T cell activation and memory,
stimulate B cell differentiation and antibody production, as well
as enhance the differentiation and activation of NK and Th17 cells
(15,39). Thus, IL-21 may be regarded as a key
cytokine linking innate and adaptive immune responses. Numerous
polymorphisms of IL-21 have been identified, and it has been
reported that they may directly affect the abundance of IL-21,
thus, contribute to diseases (14,40).
In mouse models of SLE and diabetes, increased IL-21 production was
shown to be associated with autoimmunity (24,41).
Furthermore, IL-21 was detected at high levels in the gut of
patients with Crohn’s disease and ulcerative colitis (42,43).
These observations indicate that IL-21 may be involved in the
progression of multiple autoimmune diseases.
Previous study found that GD patients have markedly
elevated serum levels of IL-21 compared with healthy controls
(31), indicating that IL-21 may
have a role in the pathogenesis of GD. In the present study, the
association between polymorphisms of IL-21/rs907715 and GD was
analyzed in a Southern Chinese population. The G allele of rs907715
was demonstrated to be significantly associated with an increased
risk of GD development. With regard to genotype distribution
analysis, the GG genotype frequencies of rs907715 in the case and
control subjects were 35.1 and 23.0%, respectively
(P=6.7×10−3). Thus, the GG genotype was significantly
higher in the GD group. Similarly, the G allele of rs907715 was
markedly increased in the GD cohorts, and the OR for carrying the G
allele in the GD patients was 1.807 greater than the controls.
These results indicated that the GG genotype and G allele of
IL-21/rs907715 increased the susceptibility to GD and may be
associated with the development of GD in a Cantonese population. By
contrast, the AA genotype frequency of rs907715 was significantly
lower in GD patients compared with the controls. The OR for
carrying the AA genotype between the patients and control subjects
was 0.387 (P=4.0×10−5; 95% CI, 0.244–0.613). Therefore,
the A allele of IL-21/rs907715 may lower the risk of suffering from
GD in a Cantonese population. In order to investigate whether
IL-21/rs907715 is associated with a particular clinical
manifestation in GD patients, correlation analyses between
genotypes/alleles and clinical characteristics were performed.
Notably, the GG genotype was found to be significantly increased in
the relapse group when compared with the non-relapse group. In
addition, the frequencies of the GG genotype in the two groups were
higher than in the controls (P=2.8×10−6 and
P=9.5×10−4, respectively). The G allele was
significantly more frequent in the relapse group than in
non-relapse group with an OR of 2.628. Thus, the G allele at the
IL-21/rs907715 locus may be a significant risk factor in the
susceptibility to relapse in GD patients. However, a much larger
collection of cases and controls is required to confirm this
association.
The observations of the present study were
consistent with other studies. According to a case-control cohort
study conducted in Shanghai (Eastern China), which involved 633 GD
patients and 612 healthy controls, the IL-21/rs907715 SNP is
significantly associated with GD, with rs907715 G allele
frequencies of 54.4 and 46.3% in the GD patients and controls,
respectively (χ2=16.05; P=6×10−5) (8). These results may provide further
evidence for the hypothesis that IL-21/rs907715 contributes to
susceptibility to GD in a Southern Chinese population. Furthermore,
a statistically significant association between IL-21/rs907715 and
SLE was observed in a European-American sample set, where the
rs907715 SNP had a minor allele frequency of 35% in the patients as
compared with 39% in the controls (χ2=11.55;
P=6.8×10−4) (27).
However, a Chinese population study found no association between
IL-21/rs907715 polymorphisms and SLE (44). This may be due to a smaller sample
size of patients, ethnic diversity and/or other various factors,
including environmental and socioeconomic factors.
In addition, several studies have reported a
correlation between IL-21 gene polymorphisms and a number of
diseases in disparate populations (27,30,36,44,45).
An association study in a Japanese population showed that the
(T)7-IL-21 allele within the IL-21 gene was significantly more
frequent in patients with type 1 diabetes than in control subjects
(20.4 vs. 13.6%; P=0.03), indicating that the allele may be
positively associated with type 1 diabetes and possible involved in
the IL-21 pathway in the pathogenesis of the disease (30). Furthermore, a previous study
identified that three SNPs from chromosome 4q27, containing genes
for IL-2 and IL-21, were involved in the genetic susceptibility to
psoriasis and psoriatic arthritis. The most significant of these
was rs13151961, and the frequency of the associated T allele was
significantly higher in the cases than in the controls (P=0.003)
(45). Increasing evidence is
supporting the role of IL-21 as a susceptibility gene contributing
to multiple autoimmune diseases, including GD. However, the
specific mechanisms involved require further investigation.
The results of the present study indicated that
rs907715 in intron 3 of the IL-21 gene correlates with GD
susceptibility. Individuals carrying risk-associated G alleles
tended to be much more susceptible to GD. Furthermore, in GD
patients, the G allele carriers were more susceptible to relapse,
which may provide further support for the hypothesis that the G
allele of IL-21/rs907715 is a risk factor of GD in a Cantonese
population. However, the biological roles that the G allele may
play remain poorly understood. The intronic SNP may not be the
actual risk mutation, but is likely to be a surrogate marker for a
mutation with functional consequences. It is possible that the
associated SNP may be in linkage disequilibrium with a variant
correlated with the translation of mRNA, thus, contribute to the
change of protein expression or autoantibody production or the
other immunological derangements in GD. However, more detailed
studies are required to confirm this hypothesis.
The results of the present study provide new
evidence for the presence of GD susceptibility loci in the IL-21
gene among a Southern Chinese population. Different autoimmune
diseases may share similar susceptibility alleles and genetic
etiologies; consequently, to a certain degree, the current results
may be helpful in further clarifying variants accounting for
susceptibility to other autoimmune diseases in this region.
However, there are several limitations to the present study.
Firstly, the number of cases and controls is insufficient. Studies
with a larger sample size are required to validate the results.
Secondly, GD is a complex autoimmune disease associated with other
genetic and environmental factors. GD is considered to be
associated with a multiple network of various susceptible loci,
thus, each locus may play a small role (46). Therefore, clarifying the underlying
mechanisms of IL-21 SNPs involved in the genetic predisposition to
GD is necessary in further research.
In conclusion, the present study indicated that
rs907715 in the IL-21 gene was significantly associated with GD in
a Cantonese population from Southern China. The G allele of
IL-21/rs907715 demonstrated a positive effect on the susceptibility
to GD and may be a risk factor for the susceptibility to relapse in
GD patients. In addition, patients carrying the A allele may have a
reduced risk of suffering from GD. However, further functional
studies are required to elucidate the roles in GD development at a
molecular level, which may aid the identification of a potential
therapeutic intervention for GD.
Acknowledgements
The authors thank the patients who consented to take
part in the study and the doctors and nurses for recruitment. The
study was supported by grants from the Guangdong Medical Science
and Technology Research Foundation (no. A2010166) and the Guangdong
Province Science and Technology Project (no. 2011B031800162).
References
|
1
|
Di Paola R, Menzaghi C, De Filippis V,
Corda D and Di Cerbo A: Cyclooxygenase-dependent thyroid cell
proliferation induced by immunoglobulins from patients with Graves’
disease. J Clin Endocrinol Metab. 82:670–673. 1997.PubMed/NCBI
|
|
2
|
Chistiakov DA: Thyroid-stimulating hormone
receptor and its role in Graves’ disease. Mol Genet Metab.
80:377–388. 2003.
|
|
3
|
Weetman AP: Graves’ disease. N Engl J Med.
343:1236–1248. 2000.
|
|
4
|
Farid NR and Balazs C: The genetics of
thyroid associated ophthalmopathy. Thyroid. 8:407–409. 1998.
View Article : Google Scholar
|
|
5
|
Falgarone G, Heshmati HM, Cohen R and
Reach G: Mechanisms in endocrinology. Role of emotional stress in
the pathophysiology of Graves’ disease. Eur J Endocrinol.
168:R13–R18. 2012.PubMed/NCBI
|
|
6
|
Jacobson EM and Tomer Y: The CD40, CTLA-4,
thyroglobulin, TSH receptor, and PTPN22 gene quintet and its
contribution to thyroid autoimmunity: back to the future. J
Autoimmun. 28:85–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brand OJ, Barrett JC, Simmonds MJ, et al:
Association of the thyroid stimulating hormone receptor gene (TSHR)
with Graves’ disease. Hum Mol Genet. 18:1704–1713. 2009.
|
|
8
|
Jia HY, Zhang ZG, Gu XJ, et al:
Association between interleukin 21 and Graves’ disease. Genet Mol
Res. 10:3338–3346. 2011.
|
|
9
|
Simmonds MJ, Howson JM, Heward JM, et al:
A novel and major association of HLA-C in Graves’ disease that
eclipses the classical HLA-DRB1 effect. Hum Mol Genet.
16:2149–2153. 2007.PubMed/NCBI
|
|
10
|
Simmonds MJ, Howson JM, Heward JM, et al:
Regression mapping of association between the human leukocyte
antigen region and Graves disease. Am J Hum Genet. 76:157–163.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heward JM, Brand OJ, Barrett JC, et al:
Association of PTPN22 haplotypes with Graves’ disease. J Clin
Endocrinol Metab. 92:685–690. 2007.
|
|
12
|
Vaidya B, Imrie H, Perros P, et al: The
cytotoxic T lymphocyte antigen-4 is a major Graves’ disease locus.
Hum Mol Genet. 8:1195–1199. 1999.PubMed/NCBI
|
|
13
|
Ban Y, Tozaki T, Taniyama M, Tomita M and
Ban Y: Association of a CTLA-4 3′ untranslated region (CT60) single
nucleotide polymorphism with autoimmune thyroid disease in the
Japanese population. Autoimmunity. 38:151–153. 2005.
|
|
14
|
Ettinger R, Kuchen S and Lipsky PE:
Interleukin 21 as a target of intervention in autoimmune disease.
Ann Rheum Dis. 67(Suppl 3): iii83–iii86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spolski R and Leonard WJ: Interleukin-21:
basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Habib T, Senadheera S, Weinberg K and
Kaushansky K: The common gamma chain (gamma c) is a required
signaling component of the IL-21 receptor and supports
IL-21-induced cell proliferation via JAK3. Biochemistry.
41:8725–8731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caruso R, Fina D, Peluso I, et al: A
functional role for interleukin-21 in promoting the synthesis of
the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells.
Gastroenterology. 132:166–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jüngel A, Distler JH, Kurowska-Stolarska
M, et al: Expression of interleukin-21 receptor, but not
interleukin-21, in synovial fibroblasts and synovial macrophages of
patients with rheumatoid arthritis. Arthritis Rheum. 50:1468–1476.
2004.
|
|
19
|
Distler JH, Jüngel A, Kowal-Bielecka O, et
al: Expression of interleukin-21 receptor in epidermis from
patients with systemic sclerosis. Arthritis Rheum. 52:856–864.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davis ID, Skak K, Smyth MJ, et al:
Interleukin-21 signaling: functions in cancer and autoimmunity.
Clin Cancer Res. 13:6926–6932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Bleakley M and Yee C: IL-21
influences the frequency, phenotype, and affinity of the
antigen-specific CD8 T cell response. J Immunol. 175:2261–2269.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parrish-Novak J, Dillon SR, Nelson A, et
al: Interleukin 21 and its receptor are involved in NK cell
expansion and regulation of lymphocyte function. Nature. 408:57–63.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng R, Spolski R, Finkelstein SE, et al:
Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and
function. J Exp Med. 201:139–148. 2005.PubMed/NCBI
|
|
24
|
Ozaki K, Spolski R, Ettinger R, et al:
Regulation of B cell differentiation and plasma cell generation by
IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol.
173:5361–5371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ettinger R, Sims GP, Fairhurst AM, et al:
IL-21 induces differentiation of human naive and memory B cells
into antibody-secreting plasma cells. J Immunol. 175:7867–7879.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozaki K, Spolski R, Feng CG, et al: A
critical role for IL-21 in regulating immunoglobulin production.
Science. 298:1630–1634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sawalha AH, Kaufman KM, Kelly JA, et al:
Genetic association of interleukin-21 polymorphisms with systemic
lupus erythematosus. Ann Rheum Dis. 67:458–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Shen W, Kong K and Liu Z:
Interleukin-21 induces T-cell activation and proinflammatory
cytokine secretion in rheumatoid arthritis. Scand J Immunol.
64:515–522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coenen MJ and Gregersen PK: Rheumatoid
arthritis: a view of the current genetic landscape. Genes Immun.
10:101–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asano K, Ikegami H, Fujisawa T, et al:
Molecular scanning of interleukin-21 gene and genetic
susceptibility to type 1 diabetes. Hum Immunol. 68:384–391. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Zeng H, Ren M, et al:
Interleukin-21 is associated with disease activity in patients with
Graves’ disease. Endocrine. Nov 28–2013.(Epub ahead of print).
|
|
32
|
Meller J, Jauho A, Hüfner M, Gratz S and
Becker W: Disseminated thyroid autonomy or Graves’ disease:
reevaluation by a second generation TSH receptor antibody assay.
Thyroid. 10:1073–1079. 2000.
|
|
33
|
Liu L, Wu HQ, Wang Q, Zhu YF, et al:
Association between thyroid stimulating hormone receptor gene
intron polymorphisms and autoimmune thyroid disease in a Chinese
Han population. Endocr J. 59:717–723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simmonds MJ, Heward JM, Carr-Smith J, et
al: Contribution of single nucleotide polymorphisms within FCRL3
and MAP3K7IP2 to the pathogenesis of Graves’ disease. J Clin
Endocrinol Metab. 91:1056–1061. 2006.PubMed/NCBI
|
|
35
|
Plagnol V, Howson JM, Smyth DJ, et al:
Genome-wide association analysis of autoantibody positivity in type
1 diabetes cases. PLoS Genet. 7:e10022162011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Festen EA, Goyette P, Scott R, et al:
Genetic variants in the region harbouring IL2/IL21 associated with
ulcerative colitis. Gut. 58:799–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeng R, Spolski R, Casas E, et al: The
molecular basis of IL-21-mediated proliferation. Blood.
109:4135–4142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Konforte D and Paige CJ: Identification of
cellular intermediates and molecular pathways induced by IL-21 in
human B cells. J Immunol. 177:8381–8392. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coquet JM, Kyparissoudis K, Pellicci DG,
et al: IL-21 is produced by NKT cells and modulates NKT cell
activation and cytokine production. J Immunol. 178:2827–2834. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rahman M, Nara H, Onoda T, et al: Cloning
and characterization of an isoform of interleukin-21. FEBS Lett.
581:4001–4009. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
King C, Ilic A, Koelsch K and Sarvetnick
N: Homeostatic expansion of T cells during immune insufficiency
generates autoimmunity. Cell. 117:265–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Monteleone G, Monteleone I, Fina D, et al:
Interleukin-21 enhances T-helper cell type I signaling and
interferon-gamma production in Crohn’s disease. Gastroenterology.
128:687–694. 2005.PubMed/NCBI
|
|
43
|
di Carlo E, de Totero D, Piazza T, Fabbi M
and Ferrini S: Role of IL-21 in immune-regulation and tumor
immunotherapy. Cancer Immunol Immunother. 56:1323–1334.
2007.PubMed/NCBI
|
|
44
|
Ding L, Wang S, Chen GM, et al: A single
nucleotide polymorphism of IL-21 gene is associated with systemic
lupus erythematosus in a Chinese population. Inflammation.
35:1781–1785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Y, Helms C, Liao W, et al: A
genome-wide association study of psoriasis and psoriatic arthritis
identifies new disease loci. PLoS Genet. 4:e10000412008. View Article : Google Scholar
|
|
46
|
Leonard WJ and Spolski R: Interleukin-21:
a modulator of lymphoid proliferation, apoptosis and
differentiation. Nat Rev Immunol. 5:688–698. 2005. View Article : Google Scholar : PubMed/NCBI
|