Introduction
Green tea (Camellia sinensis) is one of the
most popular beverages worldwide, and contains a large amount of
flavonoids, predominantly catechins, including epicatechin, its
hydroxyl derivative epigallocatechin, and their gallic acid esters,
epicatechin-3-gallate and epigallocatechin-3-gallate (EGCg;
Fig. 1). Among these catechins,
EGCg is an abundant constituent of green tea (leaf) and has been
shown to exhibit antioxidative, anticarcinogenic and anticancer
effects in vitro. The polyphenolic structure of these
compounds exerts antioxidative effects by trapping reactive oxygen
species (ROS). It was previously demonstrated that daily intake of
green tea reduced oxidative stress in vivo (1). In addition, against a background of
increasing public health concerns, it has been hypothesized that
green tea consumption has beneficial effects against various
pathological conditions, including cardiovascular disease, diabetes
and cancer.
Green tea catechins, in particular, have attracted
attention as cancer-preventive agents due to their low toxicity and
ready availability to the general population, as well as exerting
preventive effects against cancers in humans (2–5). A
prospective cohort study on a Japanese population demonstrated that
green tea has a strong potency in preventing cancers in a variety
of organs (6). Additional
epidemiological or clinical studies revealed that green tea
consumption is inversely associated with the progression of
prostate cancer, the risk of hematological malignancies and the
risk of breast cancer recurrence, among others (7–9). In
cells cultured for in vitro experiments on green tea
catechins, growth inhibition and apoptosis induction have been
observed in a variety of cell lines (10,11).
Previously, using two cell lines, peripheral blood T lymphocytes of
adult T-cell leukemia patients and human T-cell leukemia virus type
1 (HTLV-1)-infected T-cell line, it was demonstrated that EGCg
inhibited cell growth concomitant with the induction of apoptosis,
and was responsible for suppressing the expression of HTLV-1 pX
mRNA, which encodes the oncoprotein, Tax (12). Tax protein plays an important role
in HTLV-1-infected T-cell leukemogenesis by mediating interactions
with transcription factors, including nuclear factor (NF)-κB. In
the CTLL-2 Tax-expressing mouse T-cell line, constitutive
expression of B-cell lymphoma-extra large (Bcl-xL) via the NF-κB
pathway has been shown to contribute to the inhibition of apoptosis
(13).
Several cell culture studies have focused on one of
the hallmarks of the decrease in cell growth by green tea
catechins, namely, the suppression of NF-κB activation and the
subsequent induction of apoptosis. However, in vivo evidence
remains limited and no definitive conclusions have yet been drawn.
Ahmad et al revealed that EGCg reversed the degradation of
IκBα protein, which specifically inhibits NF-κB activation, and
subsequently downregulated cell cycle deregulation and the
induction of apoptosis in A431 human epidermoid carcinoma cells
(14). An in vivo
interventional study revealed that intake of green tea extract
capsules diminished the HTLV-1 provirus load in peripheral blood
lymphocytes of asymptomatic HTLV-1 carriers. Therefore, it was
hypothesized that the decrease in HTLV-1 provirus load was caused
by EGCg stabilizing IκB and abrogating NF-κB activation in HTLV-1
carrier lymphocytes following the intake of capsules (15).
An increase in the level of nuclear translocation or
constitutive activation of NF-κB has been attributed to the
induction of prosurvival gene products, including Bcl-2 and Bcl-xL
(16,17). Bcl-xL, a member of the Bcl-2
family, inhibits apoptosis by blocking the release of cytochrome
c from the mitochondria. A decrease in Bcl-xL gene
expression may lead to the promotion of cell death. However, the
events downstream of NF-κB inactivation by catechins are not
clear.
Among green tea catechins, EGCg has been shown to
exhibit optimal anticancer activity, which is associated with the
number of -OH groups. Therefore, in the present study, EGCg was
adopted as a well-characterized model catechin. The aim of the
present study was to identify the major molecule that mediates
proapoptotic cell death by EGCg. To achieve this objective, the
A549 human non-small-cell lung cancer cell line was used and the
effect of EGCg on cell proliferation and the induction of mRNA that
modulates apoptotic cell death was evaluated.
Materials and methods
Chemicals and reagents
EGCg was purchased from Funakoshi Co., Ltd. (Tokyo,
Japan). RPMI-1640 medium and 100X Antibiotic-Antimycotic were
obtained from Invitrogen Life Technologies (Carlsbad, CA, USA),
while fetal bovine serum (FBS) was purchased from Thermo Scientific
Fisher (Waltham, MA, USA). Reverse transcription polymerase chain
reaction (RT-PCR) was performed with the SuperScript One-Step
RT-PCR with Platinum Taq kit (Invitrogen Life Technologies)
and total RNA was extracted using TRIzol reagent (Invitrogen Life
Technologies). MTT assay kit was obtained from Roche Diagnostics
(Indianapolis, IN, USA). EGCg was dissolved in phosphate-buffered
saline (PBS) as a 2 mM stock solution and then stored at −30°C.
Cell lines and cell culture
A human non-small-cell lung cancer cell line, A549,
was provided by Professor Akiyama from the Department of Molecular
Oncology at the Graduate School of Medical and Dental Sciences
(Kagoshima University, Kagoshima, Japan). A549 cells were grown in
RPMI-1640 medium supplemented with 10% FBS and
Antibiotics-Antimycotics in a 5% CO2 humidified
atmosphere at 37°C.
Determination of cell survival using the
MTT assay
Chemosensitivity was measured in vitro using
the MTT colorimetric assay, which was performed in 96-well plates
(18). To determine the effect of
EGCg, A549 cells (2.5×103) in 90 μl culture medium were
inoculated into each well. Following 24 h incubation, 10-μl samples
of various concentrations of EGCg and the vehicle were added and
the plate was incubated for 72 h. Next, 0.5 mg/ml MTT (final
concentration) was added to each well and the plate was incubated
for a further 4 h at 37°C. The resulting formazan was dissolved in
100 μl solubilization solution (10% SDS in 0.01 M HCl) and the
plate was re-incubated overnight at 37°C. The optical density (OD)
at 550 nm was determined using an ARVO SX model 1420 Multilabel
Counter (PerkinElmer, Waltham, MA, USA). The control wells were set
as zero absorbance. The percentage of cell survival was calculated
using the background-corrected absorbance as follows: Cell survival
(%) = (ODexperiment/ODcontrol) × 100. The
data represent the mean and standard deviation from triplicate
determination.
RT-PCR
Total cellular RNA was extracted using TRIzol
reagent, according to the manufacturer’s instructions. RT-PCR was
performed with the SuperScript One-Step RT-PCR system and
gene-specific primers, according to the manufacturer’s
instructions. The reaction mixture contained 500 ng total RNA, 0.2
mM dNTPs, 0.2 μM each primer and the enzyme mixture, including
SuperScript II RT, Platinum Taq DNA polymerase and 1X buffer
with 1.2 mM MgSO4. The mixture was maintained at 50°C
for 20 min, 94°C for 2 min and then PCR was performed as follows:
30 cycles at 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec.
The primers for RT-PCR were designed on the basis of the human
sequences in GenBank. These sequences used the following primers:
Bcl-xL primer forward, 5′-CGGTGAATGGAGCCACTGACCA-3′ and reverse,
5′-GCCATCCAAGCTGCGATCCGAC-3′; GAPDH forward,
5′-AGAACATCATCCCTGCCTCTACTGG-3′ and reverse,
5′-AAAGGTGGAGGAGTGGGTGTCGCTG-3′.
Statistical analysis
Data from MTT assay were presented as the mean ±
standard deviation of triplicate determinations. Statistical
difference was analyzed using a one-way analysis of variance
(ANOVA) followed by Dunett’s test. SPSS software (SPSS Inc.,
Chicago, IL, USA) was used and P<0.05 was considered to indicate
a statistically significant result.
Results
Effect of EGCg on the proliferation of
A549 cells
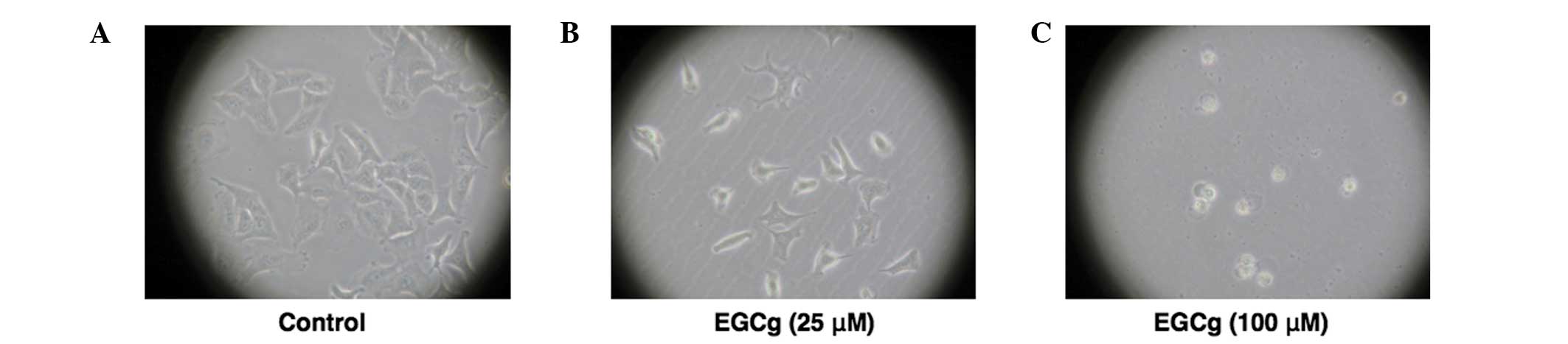
Morphological changes in A549 cells were shown to be
dependent on the EGCg concentration. Cells exhibited a shape
representative of A549 cells in the control (24 h incubation),
whereas the cells lost their adhesion ability when treated with 25
μM EGCg. When treated with 100 μM ECGg, the cells were observed to
float in the medium, exhibiting cell death (Fig. 2). The MTT assay was performed at 48
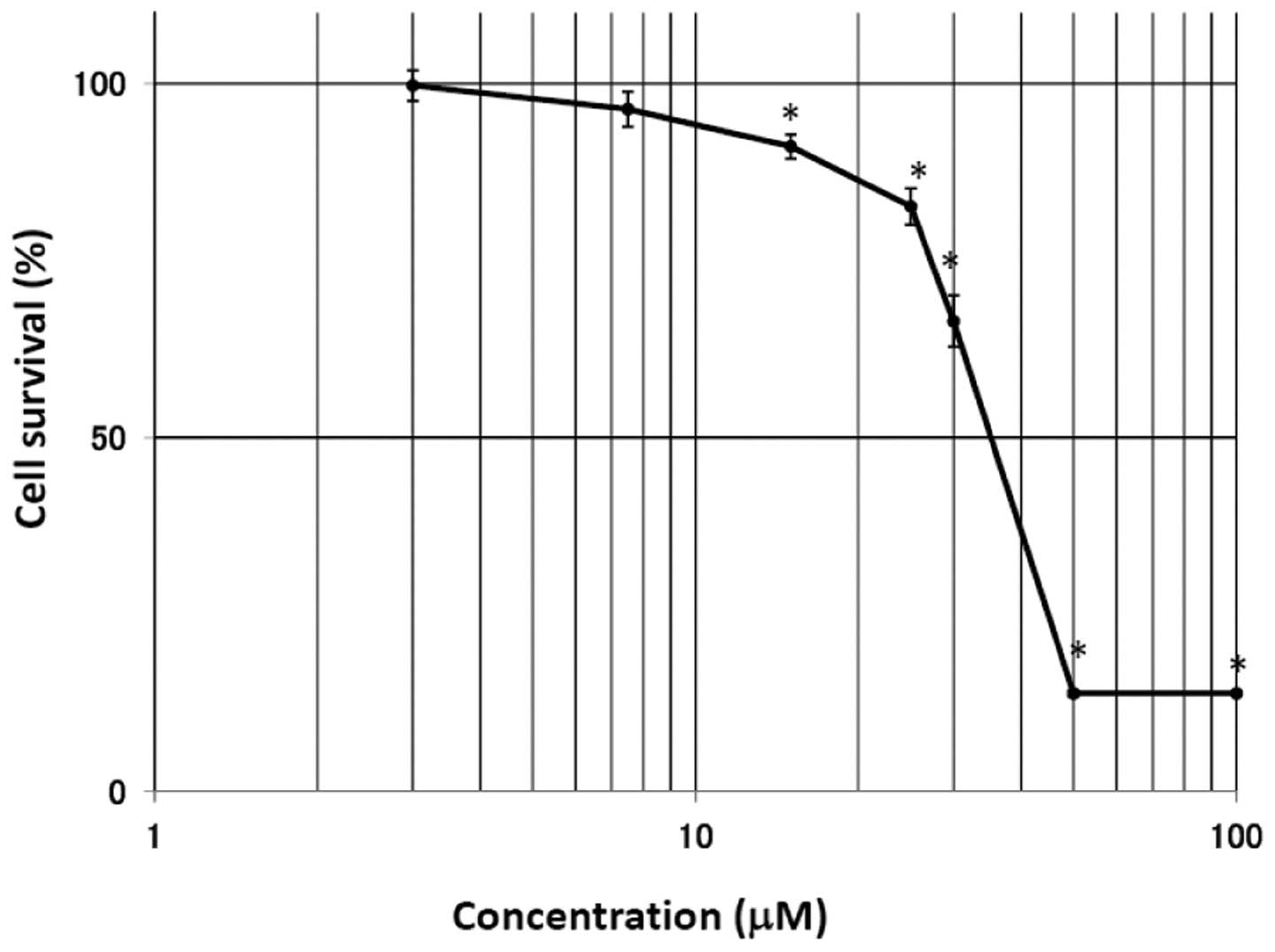
h after treatment with EGCg in the A549 cells. As shown in Fig. 3, the survival rate in the A549
cells was significantly suppressed by treatment with EGCg. The cell
viability rate was markedly reduced at EGCg concentrations >25
μM, reaching a plateau at 50 μM. The IC50 (50%
inhibition of cell growth) for EGCg in the A549 cells was 36.0
μM.
Effect of EGCg on the mRNA expression of
Bcl-xL in A549 cells
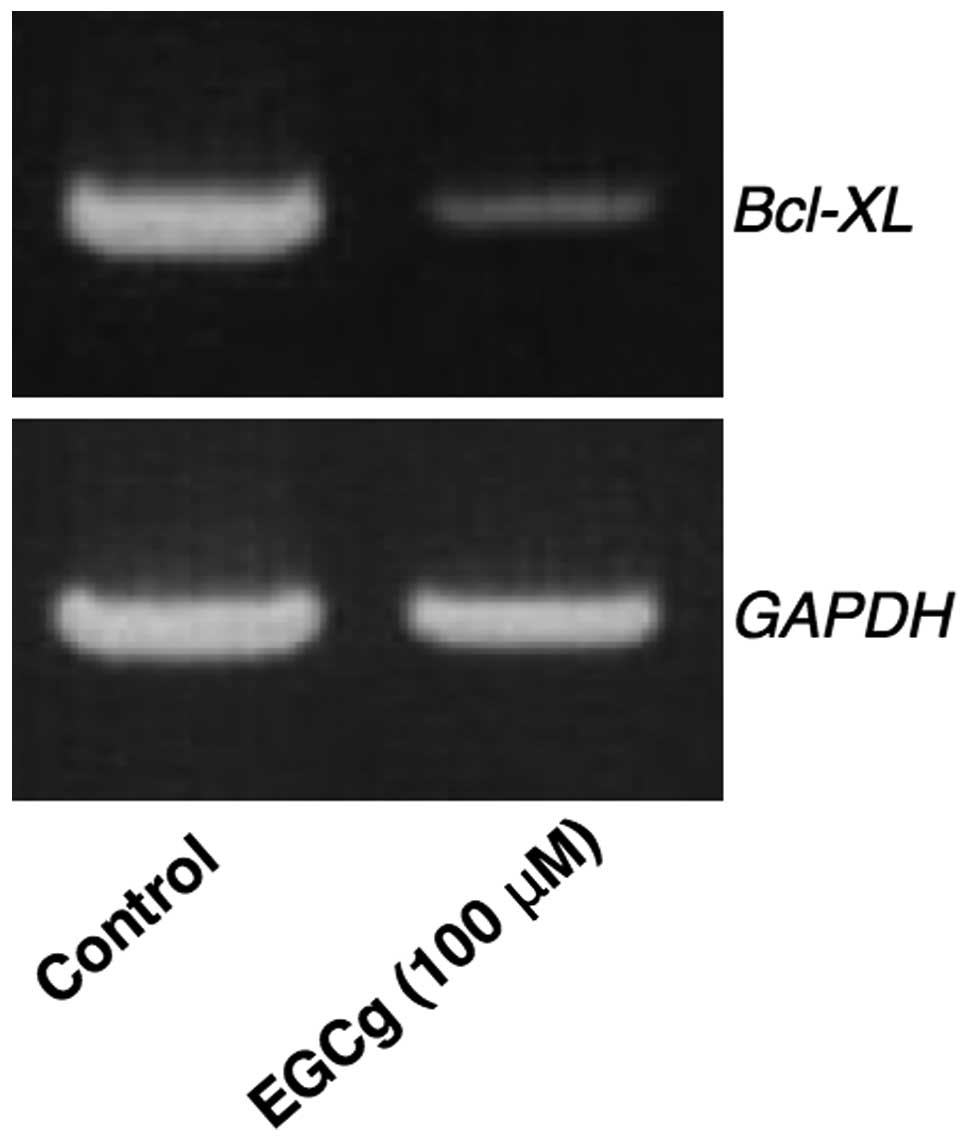
Intracellular Bcl-xL expression was analyzed since
this protein strongly inhibits apoptosis. If cytosolic Bcl-xL mRNA
expression was suppressed by EGCg, the target cells should be
induced to undergo apoptosis. The effect of EGCg on the expression
of Bcl-xL in A549 cells is shown in Fig. 4. EGCg (100 μM) was shown to
suppress the mRNA expression of Bcl-xL in A549 cells at 24 h
following administration.
Discussion
In the present study, EGCg was demonstrated to
markedly inhibit cell proliferation at concentrations between 25
and 100 μM (Fig. 3), and decrease
Bcl-xL mRNA expression under the same conditions at 100 μM
(Fig. 4) in A549 cells. EGCg has
been reported to inhibit the activation of NF-κB (14), and the activation of NF-κB leads to
the inhibition of apoptosis. NF-κB is a heterodimer consisting of
two proteins, p65 and p50. In unstimulated cells, NF-κB is located
in the cytoplasm and is bound to IκBα and IκBb, which prevents the
molecule from entering the nucleus. External stimuli modulate
signal transduction pathways leading to IκB phosphorylation,
causing its rapid degradation by proteasomes. The release of NF-κB
from IκB results in translocation to the nucleus, where NF-κB binds
to a specific sequence in the promoter regions of target genes of
antiapoptotic proteins, including Bcl-xL. Therefore, the results of
the present study indicate that EGCg reduces the expression of the
death-inhibiting gene, Bcl-xL, consequently inducing apoptosis in
A549 cells.
Although green tea catechins have been shown to
reduce the risk of cardiovascular disease and certain types of
cancer, as well as promote physiological functions, including body
weight control and antihypertensive, antibacterial, antiviral and
neuroprotective effects (19), the
present study focused on the anticancer effect of EGCg. On the
basis of recent observations, green tea catechins were assumed to
exhibit three beneficial properties against cancer.
Firstly, green tea catechins, predominantly EGCg,
have potent antioxidant activity and may reduce adverse events
associated with pro-oxidant anticancer agents. Generally,
anthracyclins and a platinum agent (cisplatin) are considered to
release ROS and cause unique side effects, namely, cardiac toxicity
and renal dysfunction, respectively. Green tea catechins have been
shown to protect against normal cell damage from ROS. Previously,
it was demonstrated that daily intake of green tea tablets
containing 474 mg catechins significantly reduced the oxidative
stress induced by hepatic arterial infusion of cisplatin and
5-fluorouracil in patients with metastatic liver cancer or
hepatocellular carcinoma (1). It
has also been indicated that administration of EGCg together with
pro-oxidant anticancer agents is useful in minimizing adverse
effects (20,21). In Japan, cisplatin combination
regimens have been considered as standard chemotherapy for
non-small cell lung cancer (NSCLC) (22). We hypothesized that conventional
chemotherapy combined with green tea catechins may be useful for
enhancing their anticancer effectiveness and reducing their adverse
drug reactions. Therefore the A549 cell line, which is derived from
NSCLC, was adopted in this study assuming lung cancer therapy.
Secondly, EGCg has shown the reverse property
against multidrug resistance (MDR). Upon exposure to one
chemotherapeutic agent in a clinical context, cancer cells may
acquire resistance to chemotherapy. Overexpression of efflux
transporters, including P-glycoprotein (P-gp),
multidrug-resistance-associated protein 1 and breast cancer
resistance protein, has been shown to be a major cause of MDR.
Green tea catechins are one type of candidate agent for an
effective MDR modulator since they exhibit few side effects and are
consumed routinely by a number of people, as a therapeutic aid. A
previous study demonstrated that EGCg reversed a
doxorubicin-resistant model of hepatocellular carcinoma by
inhibiting P-gp pump function (23).
Finally, the most crucial feature is that green tea
catechins themselves possess anticancer activity. The present study
demonstrated that EGCg significantly reduced A549 cell
proliferation at a concentration of 100 μM. Numerous studies on a
wide variety of histological types of cancer, including prostate,
breast, colorectal, esophageal, stomach and pancreatic cancer, have
also documented the anticancer effects of green tea catechins in
experimental and clinical studies, as well as in population-based
studies (24–32).
Numerous cell-culture studies have revealed that
green tea catechins, particularly EGCg, exert growth inhibition and
apoptosis induction effects. Apoptotic cell death is mediated by
regulator proteins, including Fas ligand, tumor necrosis factor-α,
CD95, NF-κB, apoptotic protease activating factor 1 (Apaf-1),
caspases and the Bcl-2 family (Bcl-2, Bcl-xL, Bax and Bad). In
human cancer cell lines, it has been demonstrated that
[3H]EGCg or fluorescein isothiocyanate-conjugated EGCg
is incorporated into the cytosol and the nucleus in a
time-dependent manner (33,34).
The structure of EGCg was shown to be preserved in the cytosol
following identification with a high performance liquid
chromatography-electrochemical detector with a reversed-phase
column, and the retention time of cytosolic EGCg matched that of
standard EGCg (35). Thus,
antiapoptotic function evoked by EGCg may be localized in the
cytosol, and EGCg may interact with intracellular proteins,
including the IκB/NF-κB complex, caspases, Apaf-1 and the Bcl-2
family (Bcl-xL, Bcl-2, Bax and Bad). Certain studies have
demonstrated that EGCg inactivates NF-κB, which consequently
induces apoptosis (14,36). The present study attempted to
identify a common molecule that is closely associated with
apoptosis induction by EGCg, and we hypothesize that the decrease
in Bcl-xL expression levels is accompanied with the downstream
inactivation of NF-κB. The expression levels of antiapototic
protein, Bcl-xL, have been shown to be regulated by the NF-κB
transcription factor in a wide spectrum of cells (10,13,37).
In the present study, Bcl-xL mRNA expression levels were shown to
be reduced following treatment with EGCg in A549 cells.
In conclusion, the results of the present study
demonstrate that the inhibition of cell proliferation by EGCg may
occur via the suppression of cell death-inhibiting gene expression.
Bcl-xL mRNA expression levels decreased following EGCg
administration in non-small-cell lung cancer A549 cells. The
observations indicate that green tea may be useful as an antitumor
agent to enhance the efficacy of cancer therapy. Although the
balance between the expression levels of death-inhibiting genes
(Bcl-xL and Bcl-2) and death-promoting genes (Bax and Bad) is
critically important in the regulation of apoptosis, whether EGCg
affects Bcl-2, Bax or Bad gene expression is unclear at present.
Thus, further studies investigating whether EGCg regulates the gene
expression of Bcl-2 family members other than Bcl-xL are
required.
Acknowledgements
The authors thank Dr Hisahiro Kai and Dr Tomohiro
Shinya (Kyushu University of Health and Wealthfare) for their
technical assistance.
References
|
1
|
Baba Y, Sonoda JI, Hayashi S, et al:
Reduction of oxidative stress in liver cancer patients by oral
green tea polyphenol tablets during hepatic arterial infusion
chemotherapy. Exp Ther Med. 4:452–458. 2012.PubMed/NCBI
|
|
2
|
Lee IP, Kim YH, Kang MH, et al:
Chemopreventive effect of green tea (Camellia sinensis)
against cigarette smoke-induced mutations (SCE) in humans. J Cell
Biochem Suppl. 27:68–75. 1997.
|
|
3
|
Shimizu M, Fukutomi Y, Ninomiya M, et al:
Green tea extracts for the prevention of metachronous colorectal
adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev.
17:3020–3025. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan N, Adhami VM and Mukhtar H: Review:
green tea polyphenols in chemoprevention of prostate cancer:
preclinical and clinical studies. Nutr Cancer. 61:836–841. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suganuma M, Saha A and Fujiki H: New
cancer treatment strategy using combination of green tea catechins
and anticancer drugs. Cancer Sci. 102:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imai K, Suga K and Nakachi K:
Cancer-preventive effects of drinking green tea among a Japanese
population. Prev Med. 26:769–775. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue M, Tajima K, Mizutani M, et al:
Regular consumption of green tea and the risk of breast cancer
recurrence: follow-up study from the Hospital-based Epidemiologic
Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer
Lett. 167:175–182. 2001. View Article : Google Scholar
|
|
8
|
Bettuzzi S, Brausi M, Rizzi F, et al:
Chemoprevention of human prostate cancer by oral administration of
green tea catechins in volunteers with high-grade prostate
intraepithelial neoplasia: a preliminary report from a one-year
proof-of-principle study. Cancer Res. 66:1234–1240. 2006.
|
|
9
|
Naganuma T, Kuriyama S, Kakizaki M, et al:
Green tea consumption and hematologic malignancies in Japan: the
Ohsaki study. Am J Epidemiol. 170:730–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishikawa T, Nakajima T, Moriguchi M, et
al: A green tea polyphenol, epigalocatechin-3-gallate, induces
apoptosis of human hepatocellular carcinoma, possibly through
inhibition of Bcl-2 family proteins. J Hepatol. 44:1074–1082. 2006.
View Article : Google Scholar
|
|
11
|
Yamauchi R, Sasaki K and Yoshida K:
Identification of epigallocatechin-3-gallate in green tea
polyphenols as a potent inducer of p53-dependent apoptosis in the
human lung cancer cell line A549. Toxicol In Vitro. 23:834–839.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li HC, Yashiki S, Sonoda J, et al: Green
tea polyphenols induce apoptosis in vitro in peripheral blood T
lymphocytes of adult T-cell leukemia patients. Jap J Cancer Res.
91:34–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsukahara T, Kannagi M, Ohashi T, et al:
Induction of Bcl-x(L) expression by human T-cell leukemia virus
type 1 Tax through NF-kappaB in apoptosis-resistant T-cell
transfectants with Tax. J Virol. 73:7981–7987. 1999.PubMed/NCBI
|
|
14
|
Ahmad N, Gupta S and Mukhtar H: Green tea
polyphenol epigallocatechin-3-gallate differentially modulates
nuclear factor κB in cancer cells versus normal cells. Arch Biochem
Biophys. 376:338–346. 2000.PubMed/NCBI
|
|
15
|
Sonoda J, Koriyama C, Yamamoto S, et al:
HTLV-1 provirus load in peripheral blood lymphocytes of HTLV-1
carriers is diminished by green tea drinking. Cancer Sci.
95:596–601. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khoshnan A, Tindell C, Laux I, et al: The
NF-kappa B cascade is important in Bcl-xL expression and for the
anti-apoptotic effects of the CD28 receptor in primary human CD4+
lymphocytes. J Immunol. 165:1743–1754. 2000.PubMed/NCBI
|
|
17
|
Bui NT, Livolsi A, Peyron JF and Prehn JH:
Activation of nuclear factor kappaB and Bcl-x survival gene
expression by nerve growth factor requires tyrosine phosphorylation
of IkappaBalpha. J Cell Bio. 152:753–764. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carmichael J, De Graff WG, Gazdar AF,
Minna JD and Mitchell JB: Evaluation of a tetrazolium-based
semiautomated colorimetric assay: assessment of chemosensitivity
testing. Cancer Res. 47:936–942. 1987.PubMed/NCBI
|
|
19
|
Cabrera C, Artacho R and Giménez R:
Beneficial effects of green tea - a review. J Am Coll Nutr.
25:79–99. 2006. View Article : Google Scholar
|
|
20
|
Yamamoto T, Staples J, Wataha J, et al:
Protective effects of EGCG on salivary gland cells treated with
gamma-radiation or cis-platinum(II)diammine dichloride. Anticancer
Res. 24:3065–3073. 2004.PubMed/NCBI
|
|
21
|
Zheng J, Lee HC, Bin Sattar MM, Huang Y
and Bian JS: Cardioprotective effects of epigallocatechin-3-gallate
against doxorubicin-induced cardiomyocyte injury. Eur J Pharmacol.
652:82–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang G, Tang A, Lin X, et al: Green tea
catechins augment the antitumor activity of doxorubicin in an in
vivo mouse model for chemoresistant liver cancer. Int J Oncol.
37:111–123. 2010.PubMed/NCBI
|
|
24
|
Kurahashi N1, Sasazuki S, Iwasaki M, Inoue
M and Tsugane S; JPHC Study Group. Green tea consumption and
prostate cancer risk in Japanese men: a prospective study. Am J
Epidemiol. 167:71–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paschka AG, Butler R and Young CY:
Induction of apoptosis in prostate cancer cell lines by the green
tea component, (−)-epigallocatechin-3-gallate. Cancer Lett.
130:1–7. 1998.
|
|
26
|
Fujiki H: Two stages of cancer prevention
with green tea. J Cancer Res Clin Oncol. 125:589–597. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inoue M, Tajima K, Mizutani M, Iwata H,
Iwase T, Miura S, Hirose K, Hamajima N and Tominaga S: Regular
consumption of green tea and the risk of breast cancer recurrence:
follow-up study from the Hospital-based Epidemiologic Research
Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett.
167:175–182. 2001. View Article : Google Scholar
|
|
28
|
Orner GA, Dashwood WM, Blum CA, Díaz GD,
Li Q and Dashwood RH: Suppression of tumorigenesis in the Apc(min)
mouse: down-regulation of beta-catenin signaling by a combination
of tea plus sulindac. Carcinogenesis. 24:263–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai
Q and Fraumeni JF Jr: Reduced risk of esophageal cancer associated
with green tea consumption. J Natl Cancer Inst. 86:855–858. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hibasami H, Komiya T, Achiwa Y, Ohnishi K,
Kojima T, Nakanishi K, Akashi K and Hara Y: Induction of apoptosis
in human stomach cancer cells by green tea catechins. Oncol Rep.
5:527–529. 1998.PubMed/NCBI
|
|
31
|
Takada M, Nakamura Y, Koizumi T, Toyama H,
Kamigaki T, Suzuki Y, Takeyama Y and Kuroda Y: Suppression of human
pancreatic carcinoma cell growth and invasion by
epigallocatechin-3-gallate. Pancreas. 25:45–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujimoto N, Sueoka N, Sueoka E, Okabe S,
Suganuma M, Harada M and Fujiki H: Lung cancer prevention with
(−)-epigallocatechin gallate using monitoring by heterogeneous
nuclear ribonucleoprotein B1. Int J Oncol. 20:1233–1239. 2002.
|
|
33
|
Okabe S, Suganuma M, Hayashi M, Sueoka E,
Komori A and Fujiki H: Mechanisms of growth inhibition of human
lung cancer cell line, PC-9, by tea polyphenols. Jap J Cancer Res.
88:639–643. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee MH, Han DW, Hyon SH and Park JC:
Apoptosis of human fibrosarcoma HT-1080 cells by
epigallocatechin-3-O-gallate via induction of p53 and caspases as
well as suppression of Bcl-2 and phosphorylated nuclear factor-κB.
Apoptosis. 16:75–85. 2011.PubMed/NCBI
|
|
35
|
Hong J, Lambert JD, Lee SH, Sinko PJ and
Yang CS: Involvement of multidrug resistance-associated proteins in
regulating cellular levels of (−)-epigallocatechin-3-gallate and
its methyl metabolites. Biochem Biophys Res Commun. 310:222–227.
2003.
|
|
36
|
Ahn SC, Kim GY, Kim JH, et al:
Epigallocatechin-3-gallate, constituent of green tea, suppresses
the LPS-induced phenotypic and functional maturation of murine
dendritic cells through inhibition of mitogen-activated protein
kinases and NF-kappaB. Biochem Biophys Res Commun. 313:148–155.
2004. View Article : Google Scholar
|
|
37
|
Lee HH, Dadgostar H, Cheng Q, Shu J and
Cheng G: NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is
required for CD40 survival signaling in B lymphocytes. Proc Natl
Acad Sci U S A. 96:9136–9141. 1999. View Article : Google Scholar : PubMed/NCBI
|