Introduction
Human embryonic stem cells (ESCs) are self-renewing
pluripotent cells that can differentiate into a wide variety of
cell types under specific in vitro culture conditions
(1). These cells offer a promising
source to treat a number of human diseases by providing an
unlimited supply of different cell types, including endothelial
cells (ECs) for therapeutic neovascularization and cardiomyocytes
for repairing heart failure damage (2–5).
Although human ESC-derived ECs and cardiomyocytes have been
reported, the underlying mechanisms of differentiation and
maintenance of cell fate remain unclear. Understanding the
molecular biology of human ESCs may help to identify the factors
promoting a cardiovascular fate and improve ESC culture conditions,
which may lead to novel therapeutic methods for the treatment of
cardiovascular diseases.
Several cytokines have been shown to facilitate the
differentiation of ESCs into ECs. One such cytokine is transforming
growth factor (TGF)-β1, a highly conserved protein produced by a
variety of cells (6). TGF-β1 is a
member of the TGF-β superfamily, which plays a key role in the
regulation of human ESCs (6,7).
TGF-β1 activates its receptors through ligand binding, which is
followed by oligomerization of serine/threonine receptor kinases
and the phosphorylation of the cytoplasmic signaling SMAD proteins
that regulate the transcription of targeted genes (8). Targeted gene deletions of TGF-β1 and
its receptors, TGF-βR1/2, result in abnormal vascular
development of mouse embryos, particularly of the yolk sac, leading
to embryonic lethality (9–11). A number of studies have commonly
indicated a requirement for TGF-β1 to support self-renewing
cultures of human ESCs. For example, the inhibition of TGF
receptors with a compound resulted in rapid differentiation of
human ESCs (12–14).
When allowed to differentiate in suspension, ESCs
develop cystic embryoid bodies (EBs) that have specific features of
early post-implantation embryos. Notably, EB formation from mouse
ESCs has already been extensively utilized and validated as an
in vitro model of early mouse development (15–18).
Mouse ESCs transfected with a 1.7 kb cDNA of porcine TGF-β1 were
shown to be able to differentiate into EBs with outspread tubular
structures and increased endothelial marker expression (19). However, human and mouse ESCs have
disparate responses to extracellular stimuli (7). In a study of human ESCs collected
from the yolk sac, Poon et al found that TGF-β1 inhibited
the expression of endodermal and hematopoietic markers, which is in
contrast to the observations with mouse ESCs (7). To date, the roles of TGF-β1 in human
ESCs derived from inner cell mass (ICM) remain unclear.
In previous research, human ESCs were isolated from
the ICM of human blastocysts and were shown to have a normal
karyotype, express specific pluripotent markers and propagate for
extended periods of time (20). In
the present study, these human ESCs were cultured, differentiated
into EBs in suspension culture and transferred into medium
containing TGF-β1. The structures of the EBs were examined by light
and electron microscopy, while the cellular composition of these
structures was analyzed via the expression levels of several
endothelial-specific markers.
Materials and methods
Cell culture
A human ESC line was previously established
(21), with approval from the
Internal Review Board on Human Subjects Research and Ethics
Committees at Shanghai Ninth People’s Hospital (Shanghai, China).
Briefly, human ESCs were isolated from day 6 blastocysts and
transferred onto a feeder layer of mitomycin-C- (Sigma-Aldrich, St.
Louis, MO, USA) inactivated mouse embryonic fibroblasts (MEFs) in
human ESC culture medium. The medium consisted of Dulbecco’s
Modified Eagle’s medium (DMEM; Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 20% KnockOut Serum Replacement
(Invitrogen Life Technologies), 1% non-essential amino acids, 1 mM
L-glutamine, 0.1 mM β-mercaptoethanol and 4 ng/ml basic fibroblast
growth factor (bFGF; R&D Systems, Minneapolis, MN. USA). Cells
were incubated at 37°C with 5% CO2, and colonies were
passed every 5–7 days using 1 mg/ml IV collagenase.
Induction and formation of EBs
To induce EB formation, human ESC colonies were
dissociated into small cell mass mechanically and grown in dishes
covered with 1% agar in ESC culture medium containing
1×109 mol/l retinoic acid (R2625; Sigma-Aldrich).
Following culturing for 3–5 days, the cells aggregated and formed
EBs. The EBs were then transferred into dishes covered with 0.1%
gelatin and cultured in serum-free DMEM containing 3 ng/ml TGF-β1
(240-B-010; R&D Systems) or as controls with serum-free DMEM
only. The culture medium was changed every 24 h, and the
morphological changes of the EBs were examined daily under a
phase-contrast microscope. The images of beating cells of the EBs
were stored on a videotape using a Nikon CCD camera (Nikon
Corporation, Tokyo, Japan).
Semiquantitative polymerase chain
reaction (PCR) analysis
Total RNA isolation from the EBs was performed using
TRIzol reagent (Invitrogen Life Technologies), according to the
manufacturer’s instructions. An aliquot of 2 μg total RNA from each
sample was used for the synthesis of cDNA using a High-Capacity
cDNA Reverse Transcription kit (Applied Biosystems, Inc., Foster
City, CA, USA). The first-strand cDNA (equivalent of 40 ng
reverse-transcribed RNA) was amplified in a final volume of 20 μl
with 1 unit TaqDNA polymerase (Invitrogen Life Technologies)
and 10-pmol samples of each primer. The oligonucleotide primers are
listed on Table I. The
thermal-cycle program was as follows: 95°C for 5 min (one cycle);
94°C for 1 min, 58°C for 1 min and 72°C for 1 min (30 cycles); and
72°C for 5 min (one cycle). To ensure the accuracy of the
quantitative results, the number of PCR cycles for each set of
primers was validated in the linear range of amplification, and all
cDNA samples were adjusted to yield equal amplifications of
β-actin, which was used as the internal control. The PCR products
were visualized by ethidium bromide staining following 1.2% agarose
gel electrophoresis.
 | Table IOligonucleotide primers used for
semiquantitative PCR analysis. |
Table I
Oligonucleotide primers used for
semiquantitative PCR analysis.
| Gene | Primer
sequence | Annealing
temperature, °C | Cycles, n | Size, bpa |
|---|
| FLK1 |
5′-CCTACCCCACACATTACATGG-3′
5′-TTTTCCTGGGCACCTTCTATT-3′ | 58 | 30 | 200 |
| CD31 |
5′-AGGAAAGCCAAGGCCAGG-3′
5′-CCTTGCTGTCTAAGTCCT-3′ | 58 | 30 | 354 |
| β-actin |
5′-AGGTGACAGCATTGCTTCTG-3′
5′-GCTGCCTCAACACCTCAAC-3′ | 58 | 30 | 188 |
Immunofluorescence staining
EBs were fixed in 4% paraformaldehyde for 10 min,
washed with phosphate-buffered saline (PBS) containing 0.25% Triton
and incubated with rabbit anti-von Willebrand factor (vWF)
antibodies (Dako, Ely, UK) at a working concentration of 1:100 for
1 h at 37°C. Following washing with PBS, the EBs were incubated
with goat anti-rabbit IgG (H&L) secondary antibodies conjugated
with fluorescein (Abnova Corporation, Taipei City, Taiwan) at a
working concentration of 1:50 for 45 min at 37°C. The samples were
then mounted using glycerol and photographed with a fluorescent
microscope. The negative control was prepared using the same
protocol without primary antibody incubation.
Electron microscopy
Observations with a scanning electron microscope
(SEM) were firstly conducted. The EBs were fixed with 2% glutaric
dialdehyde, rehydrated in PBS, fixed again with osmic acid and
washed in PBS. Next, the EBs were dehydrated by gradient alcohol
with amyl acetate, dried with a Critical Point Dryer,
sputter-coated by an ionic sprayer meter and observed with a SEM
(S450; Hitachi, Ltd., Tokyo, Japan). Observations were also
performed with a transmission electron microscope (TEM). The EBs
were fixed with 2% glutaric dialdehyde for 1 h and osmic acid for 1
h, dehydrated by gradient alcohol and incubated with 1:1 acetone
embedding liquid in infiltration. The EBs were embedded with EPON
and cut into ultra-thin sections, which were then observed with a
TEM (JEM-1200EX; Jeol Ltd., Tokyo, Japan).
Results
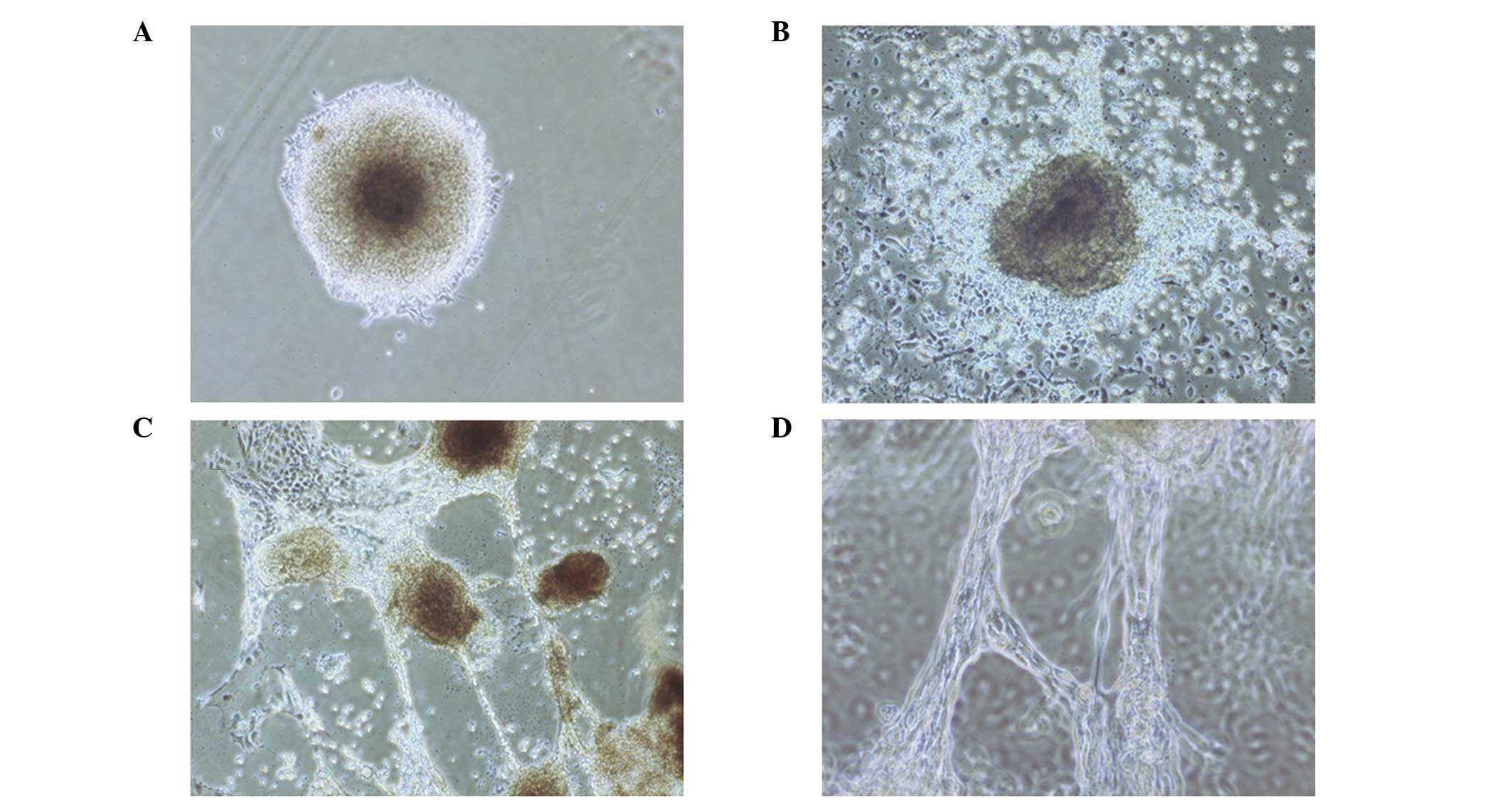
Formation of EBs with tubular structures
from human ESCs stimulated by TGF-β1
Colonies of human ESCs were dissociated into small
cell mass and grown on 1% agar. Following culturing in media
containing retinoic acid for 4 days, the cell mass formed EBs,
which adhered to the dishes and became semispheres. The EBs were
then cultured in serum-free DMEM containing 3 ng/ml TGF-β1, and
epithelial-like and round cells appeared around the EBs after 1
day. On day 3, tube-like structures deriving from the round cells
were observed, while the cells gradually transformed from round to
flat shapes (Fig. 1A). Tube-like
structures radiated from EBs to the outside (Fig. 1B). These tubular structures
connected, extended and joined with each other to form a network
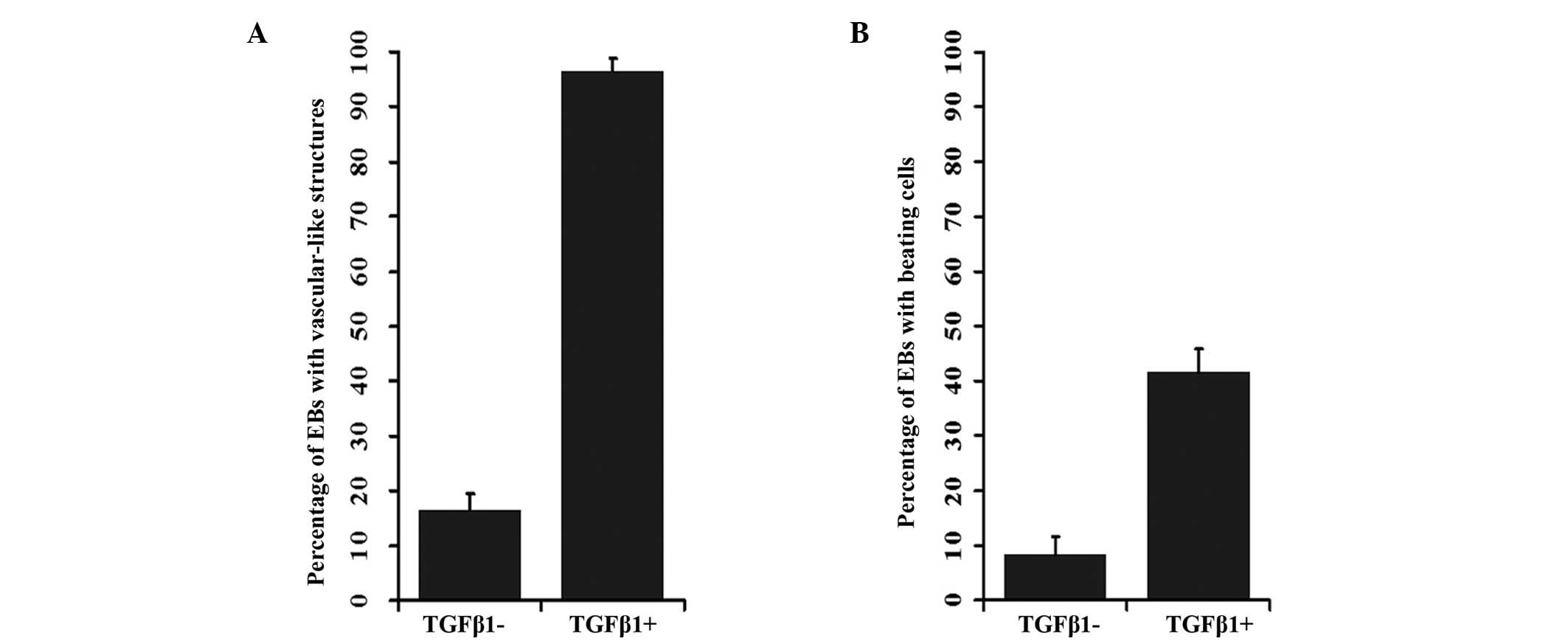
(Fig. 1C and D). The frequencies
of these structures in the control and TGF-β1 treated groups were
12.77 and 84.31%, respectively (P<0.001; Fig. 2A; Table II). At day 8, cardiomyocyte-like
beating cells were observed in the EBs that had been treated with
TGF-β1. In addition, at day 10, beating cells were observed in
37.25% of the EBs treated with TGF-β1, while only in 8.51% of the
EBs in the control (P<0.001; Fig.
2B, Table III).
 | Table IINumber of EBs with vascular-like
structures in the control and TGF-β1-treated groups. |
Table II
Number of EBs with vascular-like
structures in the control and TGF-β1-treated groups.
| Control | TGF-β1 |
|---|
|
|
|
|---|
| Group | EBs formed, n | EBs with VS, n
(%) | EBs formed, n | EBs with VS, n
(%) |
|---|
| 1 | 15 | 2 (13.33) | 18 | 15 (83.33) |
| 2 | 16 | 3 (18.75) | 13 | 13 (100) |
| 3 | 16 | 1 (6.25) | 20 | 15 (75) |
| Total | 47 | 6 (12.77) | 51 | 43 (84.31) |
 | Table IIINumber of EBs with beating cells in
the control and TGF-β1-treated groups. |
Table III
Number of EBs with beating cells in
the control and TGF-β1-treated groups.
| Control | TGF-β1 |
|---|
|
|
|
|---|
| Group | EBs formed, n | EBs with beating
cells, n (%) | EBs formed, n | EBs with beating
cells, n (%) |
|---|
| 1 | 15 | 2 (13.33) | 18 | 6 (33.33) |
| 2 | 16 | 1 (6.25) | 13 | 4 (30.77) |
| 3 | 16 | 1(6.25) | 20 | 9 (45) |
| Total | 47 | 4 (8.51) | 51 | 19 (37.25) |
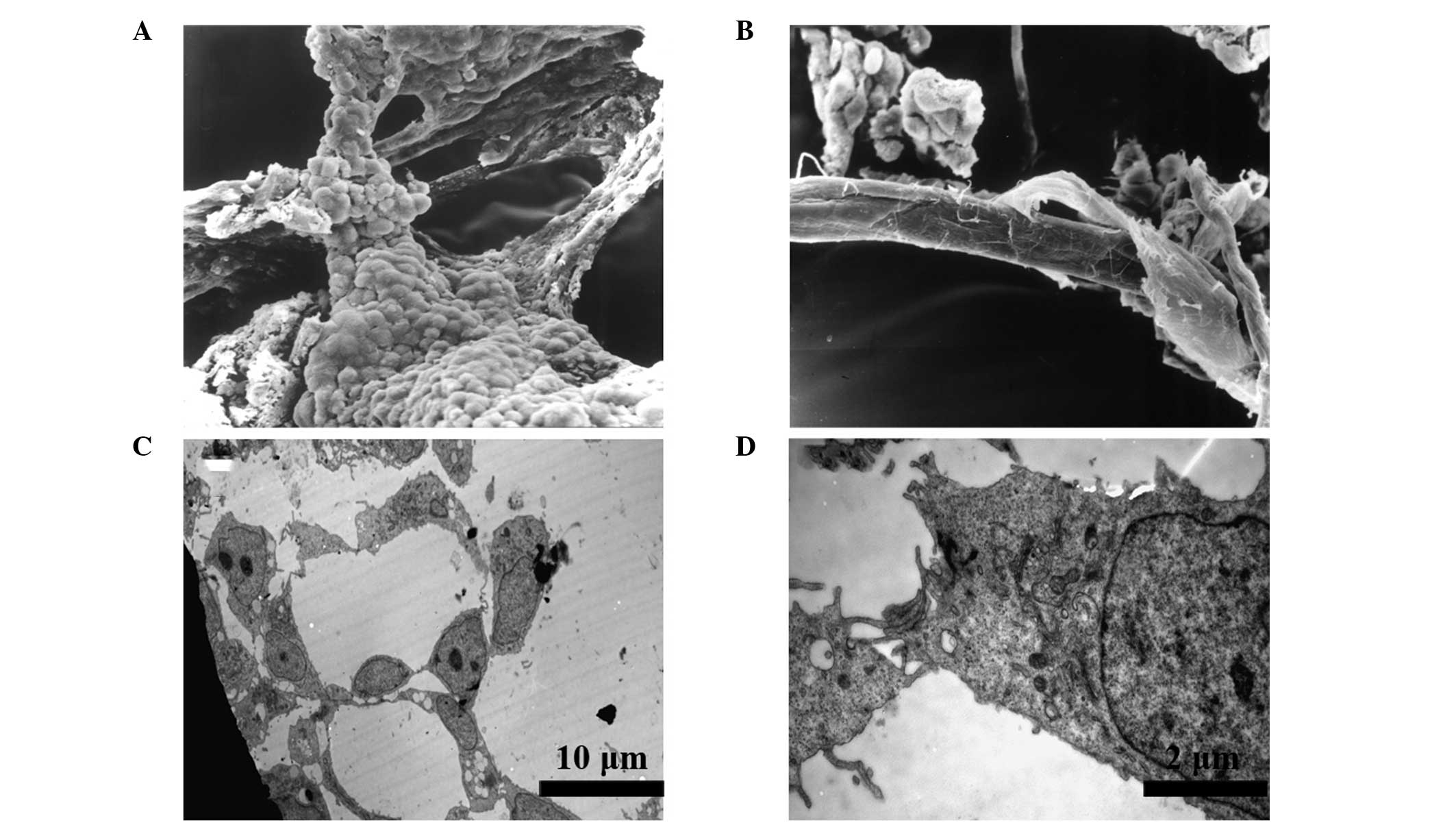
Characterization of the vascular-like
structures by electron microscopy
SEM images exhibited a three-dimensional morphology
of the EBs. The tubular structures were composed of round and flat
cells, which were morphologically similar to ECs (Fig. 3A). These structures joined with
each other and constituted a network of tubes with a smooth surface
(Fig. 3B). Further observations
using a TEM revealed the presence of lumens and gap junctions in
the section of tubular structures (Fig. 3C). The lumens were surrounded by
several round and flat cells, which were connected by tight
junctions (Fig. 3D). These
structures were similar to those of capillaries during early embryo
development.
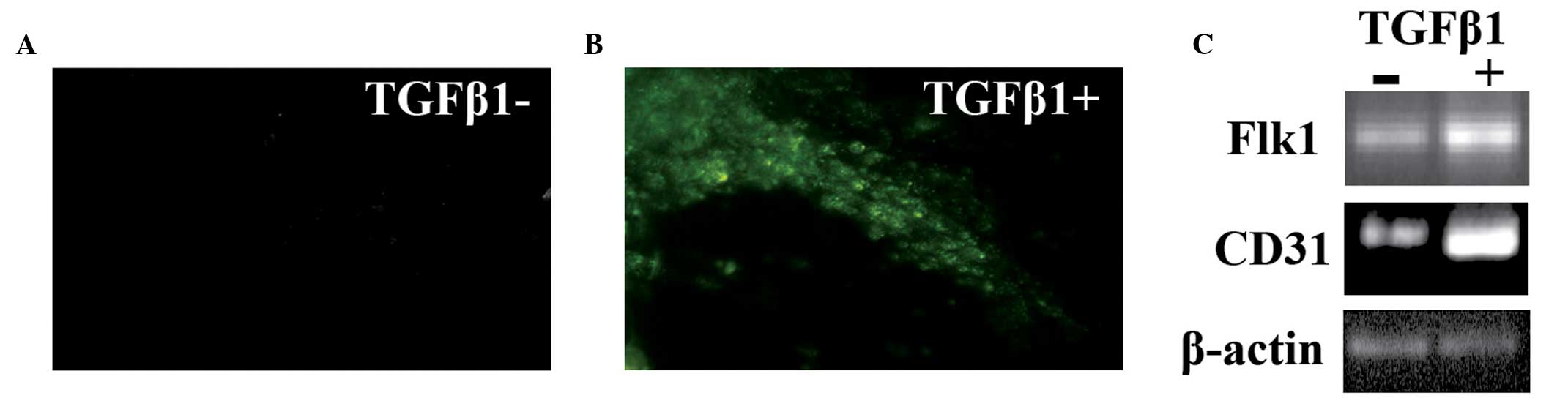
Examination of the endothelial-like cells
in the EBs derived from human ESCs
Expression levels of vWF, an established endothelial
marker (22), were analyzed on the
EBs by immunofluorescence staining. On the tubular structures of
the EBs stimulated by TGF-β1, marked vWF staining was observed,
while only a limited number of vWF positive cells were observed on
the tubular structures from the control group (Fig. 4A and B). In addition, the EBs were
harvested and total RNA was extracted for semiquantitative PCR
analysis. The two markers of endothelial cells, CD31 and FLK1,
exhibited higher expression levels in the EBs treated with TGF-β1
compared with those from the control group (Fig. 4C), indicating that TGF-β1 induced
an increase in differentiated cells with endothelial cell
characteristics.
Discussion
Previously, a human ESC line derived from ICM was
established and characterized. In the current study, these ESCs
were employed and the development of EBs stimulated by TGF-β1 was
investigated. Light and electron microscopic observations
demonstrated that TGF-β1 induced the in vitro
differentiation of embryonic stem cells into ECs, as well as the
formation of vascular-like structures. The vascular identity of the
cells in the EBs was validated by the expression of endothelial
cell markers. In addition, TGF-β1 promoted the differentiation of
cardiomyocyte-like beating cells in EBs.
Human HSCs require coculture on mitotically
inactivated MEFs, which cannot be substituted by the addition of
leukemia inhibitory factor (1,23).
However, the addition of TGF-β1 and other soluble factors,
including bFGF and insulin-like growth factor, demonstrated a
supportive role in the maintenance and propagation of human ESCs in
culture (12,14,24).
In addition, TGF-β1 has been shown to affect the cell fate
decisions during epithelial-mesenchymal transition by upregulating
surviving-associated proteins (25). In this study, TGF-β1 was shown to
differentially regulate the differentiation of human ESCs and
promote an endothelial cell fate. Although the mechanisms are not
clear, upregulation of FLK1 expression may play an important role
in this process. The FLK1 gene encodes vascular endothelial growth
factor receptor 2 (VEGFR2), the major functional receptor of VEGF
in ECs (26). Upon stimulation by
TGF-β1, SMAD3 becomes phosphorylated and forms a complex with
SMAD4. These SMAD complexes translocate into the nucleus, where
they bind to the promoter region of the FLK1 gene, thereby
activating transcription (27). In
the current study, EBs stimulated by TGF-β1 exhibited marked mRNA
expression of FLK1, supporting the role in mediating
TGF-β1-regulated cell fate decisions.
TGF-β1 and its isoforms regulate a variety of
diverse biological functions, and the role of TGF-βl in vascular
development is complicated. TGF-β1 stimulates in vivo
angiogenesis (28–30), but directly inhibits the
proliferation of ECs in vitro (31–34).
TGF-β1-stimulated induction of angiogenesis requires EC apoptosis,
which occurs via autocrine/paracrine stimulation of VEGF expression
and signaling via VEGFR2 (35).
In vitro, TGF-β1 induces tube formation when ECs are
cultured inside three-dimensional collagen gels (36,37).
In the current study, TGF-β1 was shown to promote vascular tube
formation in EBs. Tube formation is a key process during vascular
development, and vascular endothelial cadherin (VE-cadherin) is the
cell adhesion molecule essential for this process (38,39).
VE-cadherin facilitates homotypic interactions between ECs and is
strictly required for the polarization of ECs (40,41).
TGF-β1 induces the rearrangement of the adherens junction complex
by separating FLK1 from VE-cadherin and increasing the associations
between β-catenin and FLK1 or VE-cadherin (42). Therefore, TGF-β1 may promote cells
with an endothelial identity to interact with each other and form
tubular-like structures in EBs.
A previous study demonstrated that transfection of
ESCs with the porcine TGF-β1 gene permits vascular development from
murine ESCs in culture (19).
Under electron microscopy, the tubular structures observed in
transfected murine EBs were similar to those from the human EBs
stimulated by extracellular TGF-β1 in the present study. All these
structures were composed by the cells expressing EC markers.
Therefore, extracellular TGF-β1 and the transfected TGF-β1 gene can
induce vascular-like structures in EBs, possibly by activating
downstream TGF-β1 signaling in ESCs. The results indicated that
TGF-β1 is closely associated with the formation of vascular
structure.
Cardiomyocyte-like cells derived from human ESCs
have been actively pursued as a novel therapeutic to repair regions
of damaged hearts. Studies on mouse embryonic development
identified the TGF-β superfamily member, activin A, and bone
morphogenic protein 4, as key inducers of mesoderm and
cardiovascular differentiation (43). In addition, TGF-β1 induces the
differentiation of human cardiac progenitor cells into beating
cardiomyocytes with characteristic cross striations (44). In the present study, TGF-β1 was
shown to promote the differentiation of human ESCs to
cardiomyocyte-like beating cells in EBs, consistent with the role
of TGF-β1 in supporting stem cell differentiation towards
functional cardiomyocytes. Therefore, components of the TGF-β
signaling pathway may be used to manipulate human ESCs to
regenerate myocardium for cell-based therapy.
In conclusion, the present study has demonstrated
that extracellular TGF-β1 promotes a cardiovascular fate of human
ESCs in culture. The results indicate that TGF-β1 can be used as a
stimulator for the production of human ESC-derived ECs,
cardiomyocytes and forming vascular tube structures. These
observations may help to improve the methods of propagating
specific cell types for the clinical therapy of cardiovascular
diseases. Further studies are required to dissect the molecular
mechanisms underlying the function of TGF-β1 on cell fate decisions
and vascular tube formation.
Acknowledgements
The study was supported by grants from the Natural
Science Foundation of Shandong Province (no. ZR2009CQ001), the
Science and Technology Development Plan of Shandong Province (no.
2011GSF11839) and the Medical Science and Technology Development
Plan of Shandong Province (no. 2009-QW016). The authors are
grateful for the support received from Shandong Taishan Scholarship
(Ju Liu).
References
|
1
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
et al: Embryonic stem cell lines derived from human blastocysts.
Science. 282:1145–1147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Irion S, Nostro MC, Kattman SJ and Keller
GM: Directed differentiation of pluripotent stem cells: from
developmental biology to therapeutic applications. Cold Spring Harb
Symp Quant Biol. 73:101–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murry CE and Keller G: Differentiation of
embryonic stem cells to clinically relevant populations: lessons
from embryonic development. Cell. 132:661–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murry CE, Reinecke H and Pabon LM:
Regeneration gaps: observations on stem cells and cardiac repair. J
Am Coll Cardiol. 47:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Freund C and Mummery CL: Prospects for
pluripotent stem cell-derived cardiomyocytes in cardiac cell
therapy and as disease models. J Cell Biochem. 107:592–599. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Avery S, Zafarana G, Gokhale PJ and
Andrews PW: The role of SMAD4 in human embryonic stem cell
self-renewal and stem cell fate. Stem Cells. 28:863–873.
2010.PubMed/NCBI
|
|
7
|
Poon E, Clermont F, Firpo MT and Akhurst
RJ: TGFbeta inhibition of yolk-sac-like differentiation of human
embryonic stem-cell-derived embryoid bodies illustrates differences
between early mouse and human development. J Cell Sci. 119:759–768.
2006. View Article : Google Scholar
|
|
8
|
Massagué J and Wotton D: Transcriptional
control by the TGF-beta/Smad signaling system. EMBO J.
19:1745–1754. 2000.PubMed/NCBI
|
|
9
|
Doetschman T, Shull M, Kier A and Coffin
JD: Embryonic stem cell model systems for vascular morphogenesis
and cardiac disorders. Hypertension. 22:618–629. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shull MM, Ormsby I, Kier AB, et al:
Targeted disruption of the mouse transforming growth factor-beta 1
gene results in multifocal inflammatory disease. Nature.
359:693–699. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oshima M, Oshima H and Taketo MM: TGF-beta
receptor type II deficiency results in defects of yolk sac
hematopoiesis and vasculogenesis. Dev Biol. 179:297–302. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vallier L, Alexander M and Pedersen RA:
Activin/Nodal and FGF pathways cooperate to maintain pluripotency
of human embryonic stem cells. J Cell Sci. 118:4495–4509. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Besser D: Expression of nodal, lefty-a,
and lefty-B in undifferentiated human embryonic stem cells requires
activation of Smad2/3. J Biol Chem. 279:45076–45084. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
James D, Levine AJ, Besser D and
Hemmati-Brivanlou A: TGFbeta/activin/nodal signaling is necessary
for the maintenance of pluripotency in human embryonic stem cells.
Development. 132:1273–1282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ng YS, Ramsauer M, Loureiro RM and D’Amore
PA: Identification of genes involved in VEGF-mediated vascular
morphogenesis using embryonic stem cell-derived cystic embryoid
bodies. Lab Invest. 84:1209–1218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gendron RL, Tsai FY, Paradis H and Arceci
RJ: Induction of embryonic vasculogenesis by bFGF and LIF in vitro
and in vivo. Dev Biol. 177:332–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirashima M, Kataoka H, Nishikawa S, et
al: Maturation of embryonic stem cells into endothelial cells in an
in vitro model of vasculogenesis. Blood. 93:1253–1263.
1999.PubMed/NCBI
|
|
18
|
Goumans MJ, Zwijsen A, van Rooijen MA, et
al: Transforming growth factor-beta signalling in extraembryonic
mesoderm is required for yolk sac vasculogenesis in mice.
Development. 126:3473–3483. 1999.PubMed/NCBI
|
|
19
|
Zhang XJ, Tsung HC, Caen JP, Li XL, Yao Z
and Han ZC: Vasculogenesis from embryonic bodies of murine
embryonic stem cells transfected by Tgf-beta1 gene. Endothelium.
6:95–106. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang WJ and Cao YL: Stem cell, the basis
for tissue and organ reconstruction. Zhongguo Yi Xue Ke Xue Yuan
Xue Bao. 27:662–664. 2005.(In Chinese).
|
|
21
|
Wu CF, Tsung HC, Zhang WJ, et al: Improved
cryopreservation of human embryonic stem cells with trehalose.
Reprod Biomed Online. 11:733–739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wall RT, Counts RB, Harker LA and Striker
GE: Binding and release of factor VIII/von Willebrand’s factor by
human endothelial cells. Br J Haematol. 46:287–298. 1980.
|
|
23
|
Dahéron L, Opitz SL, Zaehres H, et al:
LIF/STAT3 signaling fails to maintain self-renewal of human
embryonic stem cells. Stem Cells. 22:770–778. 2004.PubMed/NCBI
|
|
24
|
Bendall SC, Stewart MH, Menendez P, et al:
IGF and FGF cooperatively establish the regulatory stem cell niche
of pluripotent human cells in vitro. Nature. 448:1015–1021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JS, Seo TW, Yi JH, et al: CHIP has a
protective role against oxidative stress-induced cell death through
specific regulation of endonuclease G. Cell Death Dis. 4:e6662013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibuya M: Tyrosine kinase receptor
Flt/VEGFR family: Its characterization related to angiogenesis and
cancer. Genes Cancer. 1:1119–1123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeon SH, Chae BC, Kim HA, et al:
Mechanisms underlying TGF-beta1-induced expression of VEGF and
Flk-1 in mouse macrophages and their implications for angiogenesis.
J Leukoc Biol. 81:557–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leibovich SJ and Wiseman DM: Macrophages,
wound repair and angiogenesis. Prog Clin Biol Res. 266:131–145.
1988.PubMed/NCBI
|
|
29
|
Park C, Kim TM and Malik AB:
Transcriptional regulation of endothelial cell and vascular
development. Circ Res. 112:1380–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roberts AB, Sporn MB, Assoian RK, et al:
Transforming growth factor type beta: rapid induction of fibrosis
and angiogenesis in vivo and stimulation of collagen formation in
vitro. Proc Natl Acad Sci USA. 83:4167–4171. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carmeliet P, Ferreira V, Breier G, et al:
Abnormal blood vessel development and lethality in embryos lacking
a single VEGF allele. Nature. 380:435–439. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baird A and Durkin T: Inhibition of
endothelial cell proliferation by type beta-transforming growth
factor: interactions with acidic and basic fibroblast growth
factors. Biochem Biophys Res Commun. 138:476–482. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heimark RL, Twardzik DR and Schwartz SM:
Inhibition of endothelial regeneration by type-beta transforming
growth factor from platelets. Science. 233:1078–1080. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maharaj AS, Saint-Geniez M, Maldonado AE
and D’Amore PA: Vascular endothelial growth factor localization in
the adult. Am J Pathol. 168:639–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferrara N and Bunting S: Vascular
endothelial growth factor, a specific regulator of angiogenesis.
Curr Opin Nephrol Hypertens. 5:35–44. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ingber DE, Madri JA and Jamieson JD: Role
of basal lamina in neoplastic disorganization of tissue
architecture. Proc Natl Acad Sci USA. 78:3901–3905. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Merwin JR, Anderson JM, Kocher O, Van
Itallie CM and Madri JA: Transforming growth factor beta 1
modulates extracellular matrix organization and cell-cell
junctional complex formation during in vitro angiogenesis. J Cell
Physiol. 142:117–128. 1990. View Article : Google Scholar
|
|
38
|
Cavallaro U, Liebner S and Dejana E:
Endothelial cadherins and tumor angiogenesis. Exp Cell Res.
312:659–667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lammert E and Axnick J: Vascular lumen
formation. Cold Spring Harb Perspect Med. 2:a0066192012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lampugnani MG, Orsenigo F, Rudini N, et
al: CCM1 regulates vascular-lumen organization by inducing
endothelial polarity. J Cell Sci. 123:1073–1080. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Strilić B, Kucera T, Eglinger J, et al:
The molecular basis of vascular lumen formation in the developing
mouse aorta. Dev Cell. 17:505–515. 2009.PubMed/NCBI
|
|
42
|
Cook BD, Ferrari G, Pintucci G and
Mignatti P: TGF-beta1 induces rearrangement of
FLK-1-VE-cadherin-beta-catenin complex at the adherens junction
through VEGF-mediated signaling. J Cell Biochem. 105:1367–1373.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mignone JL, Kreutziger KL, Paige SL and
Murry CE: Cardiogenesis from human embryonic stem cells. Circ J.
74:2517–2526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Goumans MJ, de Boer TP, Smits AM, et al:
TGF-beta1 induces efficient differentiation of human cardiomyocyte
progenitor cells into functional cardiomyocytes in vitro. Stem Cell
Res. 1:138–149. 2007. View Article : Google Scholar : PubMed/NCBI
|