Introduction
Myocardial ischemia/reperfusion (I/R) injury is
common following acute coronary syndrome and heart transplantation.
Although reperfusion is essential for the survival of ischemic
myocardial tissue, reperfusion causes additional cellular injury.
I/R may result in local myocardial inflammation, accompanied by
apoptosis, which may cause cardiomyocyte damage (1,2).
Cardiomyocyte damage is thought to occur as a result of an intense
inflammatory response initiated by the infiltration of leukocytes
and the production of pro-inflammatory cytokines. High mobility
group box 1 protein (HMGB1), a non-chromosomal nuclear protein, has
been identified as a novel pro-inflammatory cytokine that functions
as a late mediator of inflammation in sepsis, acute lung injury,
autoimmune disease and coronary artery diseases (3–6).
Previous studies have shown that HMGB1 acts as an early mediator of
inflammation and promotes cell injury during myocardial I/R. HMGB1
also promotes the release of early pro-inflammatory cytokines,
including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6).
Inhibition of HMGB1 by HMGB1 A box peptide (a specific HMGB1
antagonist) has been shown to have a protective effect against
myocardial I/R injury and to inhibit the release of TNF-α and IL-6
(7,8). These results suggest that HMGB1 may
have an important role in myocardial I/R injury.
Sodium butyrate, an inhibitor of histone
deacetylase, has been previously shown to have an anti-inflammatory
effect and to inhibit the expression of HMGB1 in an ischemic model
of stroke (9,10). Therefore, in the present study, it
was hypothesized that sodium butyrate may protect against
myocardial I/R injury by inhibiting the expression of HMGB1. In the
present study the effect of sodium butyrate preconditioning on
myocardial I/R injury in a rat myocardial I/R model was
investigated.
Materials and methods
Animal preparation and experimental
design
The experimental protocol was in accordance with the
Guidelines for the Care and Use of Laboratory Animals published by
the US National Institutes of Health (Bethesda, MD, USA) and was
approved by the Institutional Animal Care and Use Committee (Renmin
Hospital of Wuhan University, Wuhan, China). Male Sprague-Dawley
rats (250–300 g) were randomly divided into four groups receiving
the following treatments: group 1, sham-operated control (SO;
n=10): rats were subjected to surgical manipulation without the
induction of myocardial ischemia; group 2, I/R group (n=15): rats
were subjected to left anterior descending coronary artery (LAD)
occlusion for 30 min followed by reperfusion for 4 h; group 3, SB1
+ I/R (SB1-I/R; n=15): rats were administered sodium butyrate (100
mg/kg) dissolved in sterile saline intraperitoneally 30 min prior
to LAD occlusion; and group 4, SB2 + I/R (SB2-I/R; n=15): rats were
administered sodium butyrate (300 mg/kg) intraperitoneally 30 min
prior to LAD occlusion.
Following anesthetization with sodium pentobarbital
(45 mg/kg, intraperitoneally), the rats were ventilated
artificially using a volume-controlled rodent respirator at 70
strokes/min. The rats were placed on an electric heating pad to
maintain their body temperature at 37°C. Heparin (200 IU/kg) was
then administered intravenously prior to the induction of ischemia.
Lead-II of an electrocardiogram was monitored with subcutaneous
stainless steel electrodes. The electrocardiogram was monitored
using a computer-based EP system (LEAD2000B; Jinjiang Ltd.,
Chengdu, China).
A thoracotomy through a left parasternal incision
was performed. The pericardium was incised, and the anterior wall
of the left ventricle was exposed. A 4-0 silk suture on a small
curved needle was passed through the myocardium beneath the middle
segment of the LAD branch coursing down the middle of the anterior
wall of the left ventricle. A small vinyl flake was passed into the
ends of the suture, which was then fixed by clamping the tube with
a mosquito hemostat. A successful myocardial I/R model was
confirmed by changes in the ST segment elevation in Leads-II and
regional cyanosis of the myocardial surface. The rats then
underwent a 30-min LAD occlusion, followed by a 4-h
reperfusion.
Assessment of myocardial injury
To assess the lactate dehydrogenase (LDH) and
creatine kinase (CK) activities, blood samples were collected,
centrifuged and stored at −20°C until analysis. The samples were
analyzed using standard techniques using an LDH Assay kit and CK
Assay kit in accordance with the manufacturer’s instructions
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Values were expressed as international units (IU) per liter.
Assessment of infarct size
Following the 4-h reperfusion, the LAD was again
occluded and 1.5% Evans blue dye (2 ml) was injected via the
femoral vein. The risk area was analyzed using negative staining
with Evans blue. The rats were then sacrificed and their hearts
were excised and frozen overnight. The atria and right ventricle
were removed and the left ventricle was sectioned into 2-mm thick
transverse slices from the apex to base. The risk area was
separated from the colored nonischemic area (blue) and then
incubated with a 1% solution of 2,3,5-triphenyltetrazolium chloride
(TTC, in 0.2 M Tris buffer, pH 7.4) stain for 20 min at 37°C.
Viable myocardium was stained red by TTC, whilst necrotic
myocardium was not stained red. In each slice, the infarct size and
the risk area (left ventricular areas) were determined using a
computer-assisted image analysis system (Image-Pro Plus 3.0, Media
Cybernetics Inc., Rockville, MD, USA) and multiplied by the
thickness of the slice to calculate the volume of the risk area.
The infarct size was expressed as a percentage of the risk area
volume (infarct size/risk area).
Analysis of myocardial TNF-α and IL-6
expression
The expression levels of TNF-α and IL-6 in
myocardial tissue supernatants were determined using a commercial
enzyme-linked immunosorbent assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) in accordance with the
manufacturer’s instructions. The sensitivity of the assay was 1
pg/ml for TNF-α and IL-6.
Measurement of myocardial malondialdehyde
(MDA) levels and superoxide dismutase (SOD) activity
The concentration of MDA and the activity of SOD in
myocardial tissue were measured using an MDA Assay kit and SOD
Assay kit in accordance with the manufacturer’s instructions
(Nanjing Jiancheng Bioengineering Institute) as previously
described (11). The MDA
concentration and SOD activity were used to indicate the amount of
oxygen free radicals and the lipid superoxide level in the
myocardium, respectively.
Western blot analysis
The protein expression of HMGB1 in the pulverized
frozen ischemic area of the left ventricle or cultured myocardium
was analyzed using quantitative immunoblotting using an antibody
against HMGB1 (Santa Cruz, Santa Cruz Biotechnology, Inc., CA, USA)
as previously described (11). The
expression level was normalized against glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) expression.
Statistical analysis
All values are presented as the mean ± standard
deviation. The student t-test was used for comparisons between the
groups. A one-way analysis of variance (ANOVA) or Welch ANOVA was
used for comparisons among the groups and the Student-Newman-Keuls
or Dunnett T3 test was used for post-hoc multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Infarct size
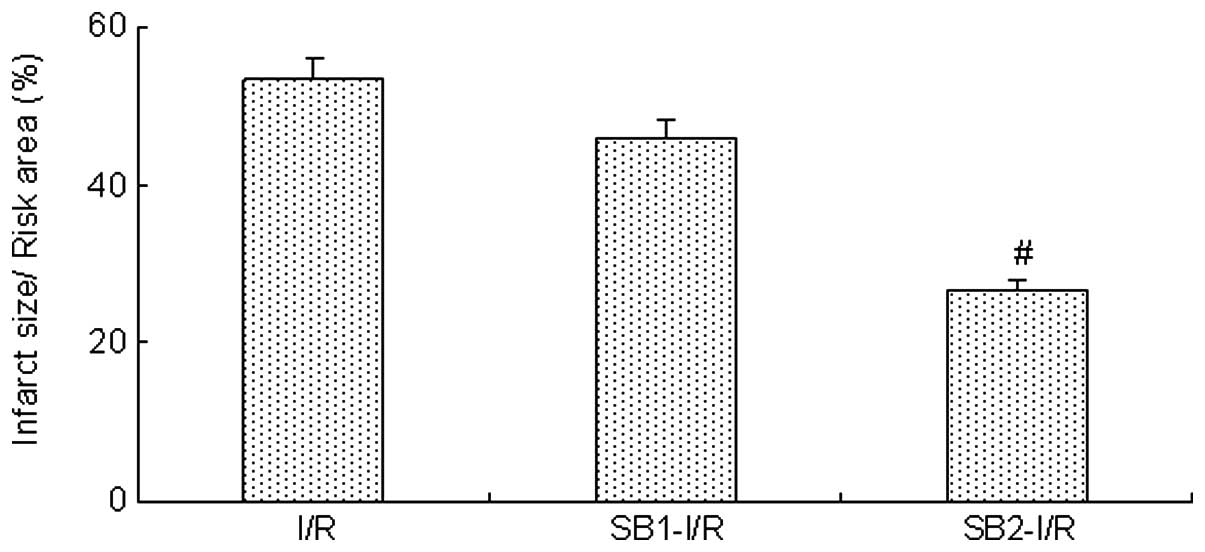
Following the 4-h reperfusion, treatment with sodium
butyrate (300 mg/kg) was found to reduce the infarct size induced
by myocardial I/R compared with that in the I/R group (26.8±3.8 vs.
53.4±4.9%; P<0.05). However, a lower dose of sodium butyrate
(100 mg/kg) did not have an inhibitory effect (P>0.05; Fig. 1)
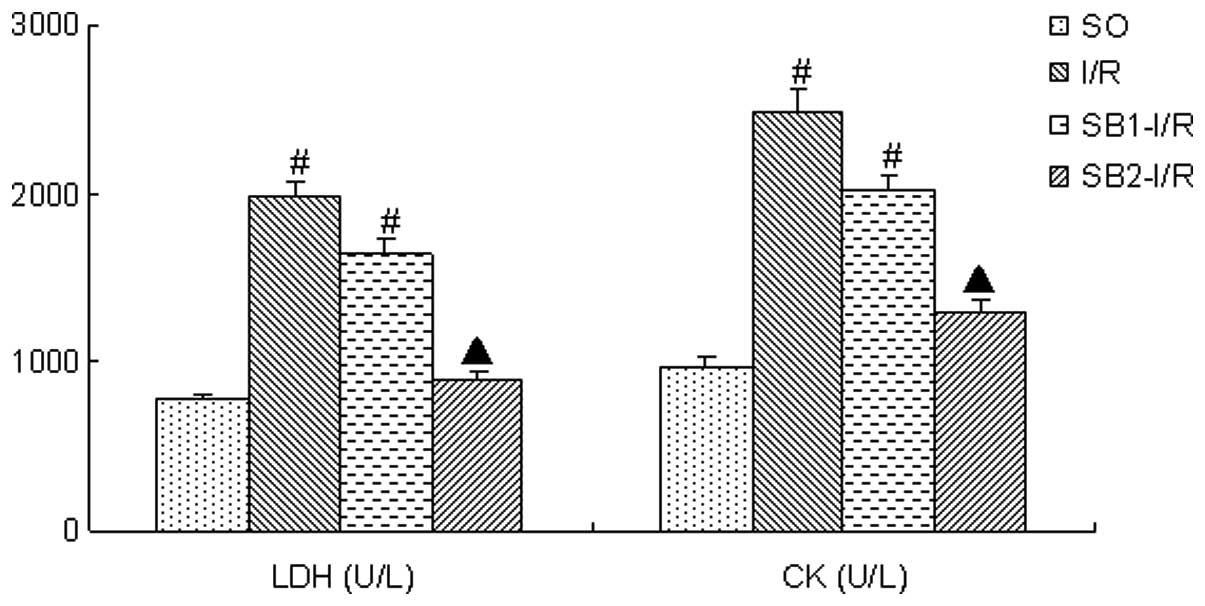
LDH and CK activities
Following the 4-h reperfusion, the LDH and CK
activities in the I/R group were significantly increased compared
with those in the SO group (P<0.05). Treatment with a high dose
of sodium butyrate (300 mg/kg) significantly inhibited the increase
of LDH and CK levels (P<0.05); however, treatment with 100 mg/kg
sodium butyrate did not provide an inhibitory effect (P>0.05;
Fig. 2)
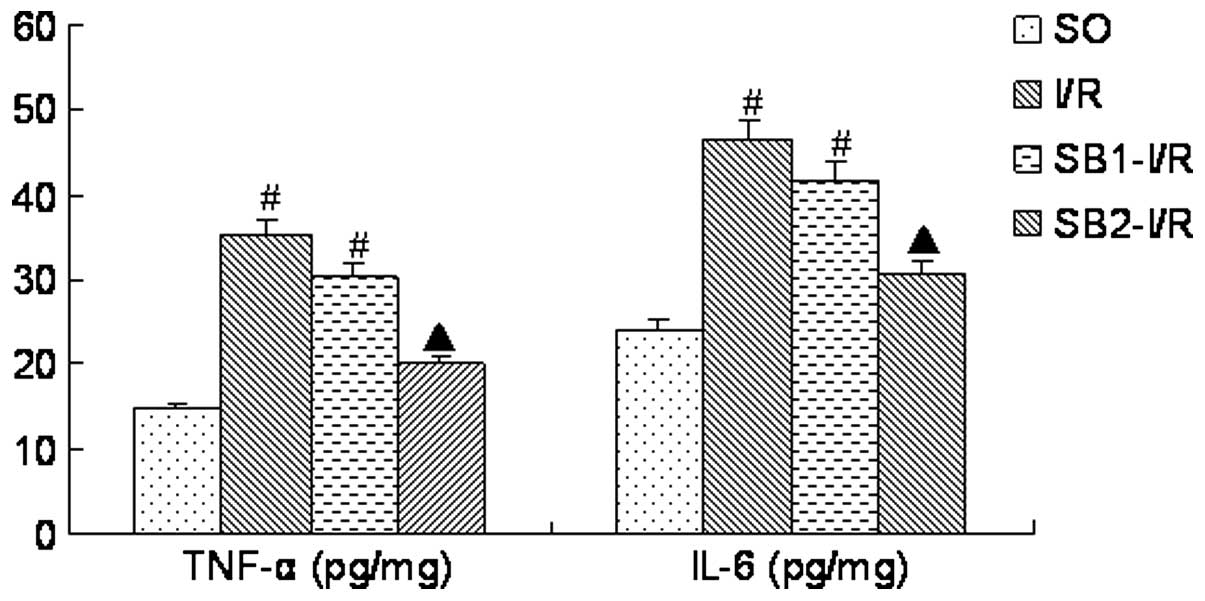
TNF-α and IL-6 levels
Following the 4-h reperfusion, the levels of TNF-α
and IL-6 in the I/R group were significantly increased compared
with those in the SO group (P<0.05). Treatment with a high dose
of sodium butyrate (300 mg/kg) significantly inhibited the
increases of the TNF-α and IL-6 levels (P<0.05), whilst
treatment with 100 mg/kg sodium butyrate did not provide a
protective effect (P>0.05; Fig.
3).
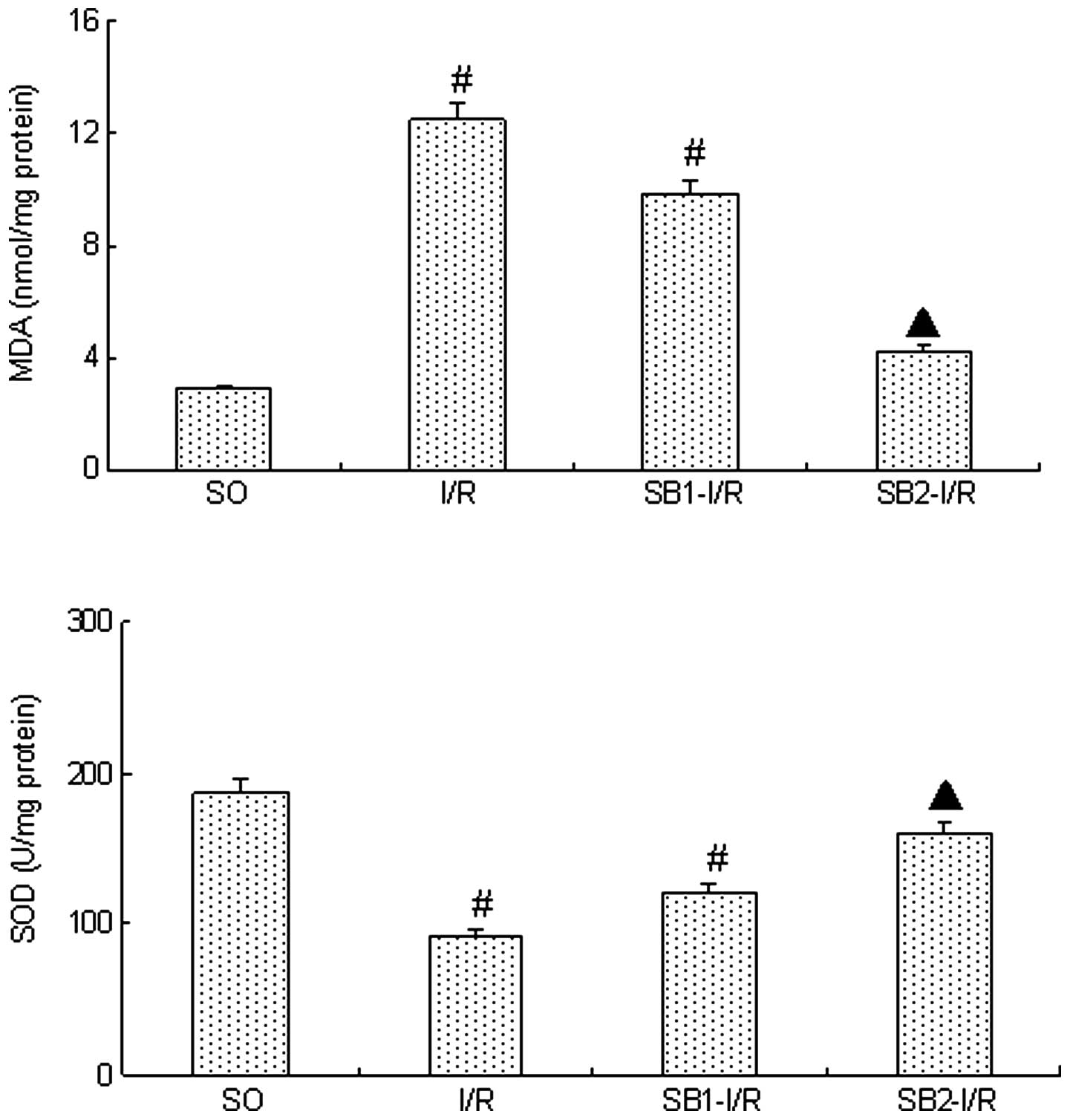
MDA and SOD levels
Following the 4-h reperfusion, the level of MDA in
the I/R group was significantly increased whilst the level of SOD
decreased significantly compared with those in the SO group
(P<0.05). Sodium butyrate (300 mg/kg) was found to significantly
inhibit the increase in the MDA levels and the reduction in the SOD
levels (P<0.05). However, treatment with a lower dose of sodium
butyrate (100 mg/kg) did not provide a protective effect
(P>0.05; Fig. 4).
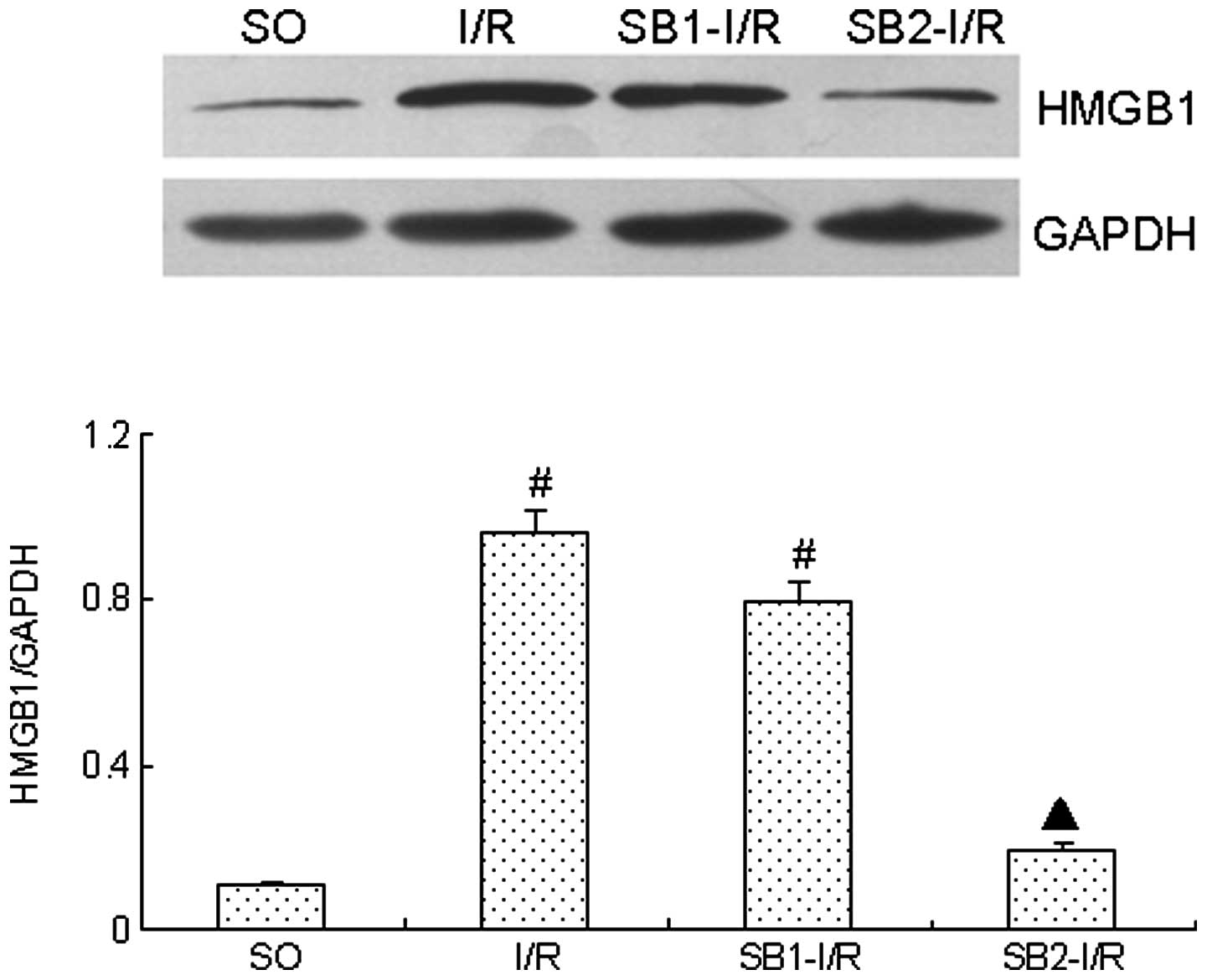
Effect of sodium butyrate on HMGB1
expression
Following the 4-h reperfusion, HMGB1 expression was
markedly increased compared with that in SO group (P<0.05).
However, treatment with sodium butyrate (300 mg/kg) significantly
inhibited the expression of HMGB1 (P<0.05). By contrast,
treatment with a lower dose of sodium butyrate (100 mg/kg) did not
provide an inhibitory effect (P>0.05; Fig. 5).
Discussion
HMGB1 has been shown to function as a novel
pro-inflammatory cytokine with an important role in myocardial I/R
injury (7,8). Previous studies have shown that there
is cross-talk between HMGB1 and other pro-inflammatory cytokines,
including TNF-α, IL-6 and C-reactive protein (CRP) (7,12–15).
HMGB1, when it is released from necrotic cells, apoptotic cells,
macrophages or monocytes, upregulates the levels of IL-1, IL-6,
TNF-α, CRP and macrophage inflammatory proteins (MIP-1α and
MIP-1β). Other pro-inflammatory cytokines may in turn promote the
release of HMGB1 (7,12–15),
indicating that this mechanism reinforces the inflammatory process.
In addition, our previous study demonstrated that HMGB1 may promote
the apoptosis of myocardium in a dose-dependent manner (11). Inflammation and apoptosis have a
critical role in myocardial I/R injury (1,2). In
the present study, pretreatment with sodium butyrate (300 mg/kg)
was found to significantly attenuate myocardial I/R injury, as well
as downregulate the expression of TNF-α, IL-6 and HMGB1. Previous
studies have shown that sodium butyrate is also able to inhibit
HMGB1 expression in other diseases (9,10).
Therefore, in the present study, it was hypothesized that sodium
butyrate may protect against myocardial I/R injury and inflammation
by inhibiting HMGB1 expression.
In addition, the present study demonstrated that
pretreatment with sodium butyrate (300 mg/kg) decreases the levels
of MDA (a reactive oxygen species) and increases the levels of SOD
(a key antioxidant enzyme). Previous studies have shown that
reactive oxygen species may be involved in the release of the
pro-inflammatory cytokine HMGB1. Tang et al (16) demonstrated that hydrogen peroxide,
a reactive oxygen species, stimulates the release of HMGB1 from
macrophages and monocytes. Furthermore, Tsung et al
(17) showed that HMGB1 released
from cultured hepatocytes was an active process regulated by
reactive oxygen species. Zhang et al (18) showed that antioxidants inhibit
HMGB1 expression and reduce pancreatic injury in rats with severe
acute pancreatitis, and this was mainly attributed to the release
of HMGB1 (19,20), further indicating that inhibiting
reactive oxygen species may inhibit HMGB1 expression. These results
suggest that pretreatment with sodium butyrate inhibits HMGB1
expression, which may be associated with the inhibition of reactive
oxygen species induced by myocardial I/R injury.
In conclusion, the results from the present study
suggest that preconditioning with sodium butyrate (300 mg/kg) is
able to attenuate myocardial I/R injury, which may be associated
with the inhibition of the expression of inflammatory mediators
during myocardial I/R.
Acknowledgments
This study was partially supported by grants from
the National Natural Science foundation of China (nos. 81100146 and
81370308), grant 111023 from the Fundamental Research Funds for the
Central Universities and the Specialized Research Fund for the
Doctoral Program of Higher Education of China (no. 20110141120060)
and the Fundamental Research Funds of Wuhan City (no.
2013070104010044).
References
|
1
|
Gottlieb RA and Engler RL: Apoptosis in
myocardial ischemia-reperfusion. Ann N Y Acad Sci. 874:12–26. 1999.
View Article : Google Scholar
|
|
2
|
Frangoginis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar
|
|
3
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, et al: HMG-1 as a late mediator of endotoxin
lethality in mice. Science. 285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu X, Jiang H, Bai Q, Zhou X, Xu C, Lu Z,
et al: Increased serum HMGB1 is related to the severity of coronary
artery stenosis. Clin Chim Acta. 406:139–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andrassy M, Volz HC, Igwe JC, Funke B,
Eichberger SN, Kaya Z, et al: High-mobility group box-1 in
ischemia-reperfusion injury of the heart. Circulation.
117:3216–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu X, Fu W and Jiang H: HMGB1: a potential
therapeutic target for myocardial ischemia and reperfusion injury.
Int J Cardiol. 155:4892012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang LT, Yao YM, Lu JQ, et al: Sodium
butyrate prevents lethality of severe sepsis in rats. Shock.
27:672–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HJ, Rowe M, Ren M, et al: Histone
deacetylase inhibitors exhibit anti-inflammatory and
neuroprotective effects in a rat permanent ischemic model of
stroke: multiple mechanisms of action. J Pharmacol Exp Ther.
321:892–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du X, Hu X and Wei J: Postconditioning
with rosuvastatin reduces myocardial ischemia-reperfusion injury by
inhibiting high mobility group box 1 protein expression. Exp Ther
Med. 7:117–120. 2014.PubMed/NCBI
|
|
12
|
Andersson U, Wang H, Palmblad K, Aveberger
AC, Bloom O, Erlandsson-Harris H, et al: High mobility group 1
protein (HMG-1) stimulates proinflammatory cytokine synthesis in
human monocytes. J Exp Med. 192:565–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erlandsson Harris H and Andersson U: The
nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol.
34:1503–1512. 2004.
|
|
14
|
Inoue K, Kawahara K, Biswas KK, Ando K,
Mitsudo K, Nobuyoshi M and Maruyama I: HMGB1 expression by
activated vascular smooth muscle cells in advanced human
atherosclerosis plaques. Cardiovasc Pathol. 16:136–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawahara K, Biswas KK, Unoshima M, Ito T,
Kikuchi K, Morimoto Y, et al: C-reactive protein induces high
mobility group box-1 protein release through activation of p38MAPK
in macrophage RAW264.7 cells. Cardiovasc Pathol. 17:129–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang D, Shi Y, Kang R, Li T, Xiao W, Wang
H and Xiao X: Hydrogen peroxide stimulates macrophages and
monocytes to actively release HMGB1. J Leukoc Biol. 81:741–747.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsung A, Klune JR, Zhang X, Jeyabalan G,
Cao Z, Peng X, et al: HMGB1 release induced by liver ischemia
involves Toll-like receptor 4-dependent reactive oxygen species
production and calcium-mediated signaling. J Exp Med.
204:2913–2923. 2007. View Article : Google Scholar
|
|
18
|
Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen
TK, Zhu YF and Wu L: Antioxidant inhibits HMGB1 expression and
reduces pancreas injury in rats with severe acute pancreatitis. Dig
Dis Sci. 55:2529–2536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yasuda T, Ueda T, Takeyama Y, Shizeki M,
Sawa H, Nakajima T, et al: Significant increase of serum
high-mobility group box chromosomal protein 1 levels in patients
with severe acute pancreatitis. Pancreas. 33:359–363. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sawa H, Ueda T, Takeyama Y, Yasuda T,
Shinzeki M, Nakajima T and Kuroda Y: Blockade of high mobility
group box-1 protein attenuates experimental severe acute
pancreatitis. World J Gastroenterol. 12:7666–7670. 2006.PubMed/NCBI
|