Introduction
Triptolide is a diterpene triepoxide antibiotic
compound that can be isolated from extracts of the medicinal plant,
Tripterygium wilfordii Hook F, which has been used for a
number of years in traditional Chinese medicine (1,2).
Tripterygium wilfordii Hook F and triptolide have
immunosuppressive and anti-inflammatory properties (3,4).
Triptolide causes apoptosis by inducing the activation of caspases
(5–8). Proliferation of rheumatoid synovial
fibroblasts associated with rheumatoid arthritis has also been
shown to decrease with triptolide treatment through a mechanism of
increasing caspase-3 activity (9).
The Wnt/β-catenin signaling pathway plays important
roles during the development of malignancies by regulating
proliferation, migration, tissue polarity and organogenesis
(10,11). In the canonical Wnt/β-catenin
pathway, β-catenin functions as the central component (12). Receptor binding of Wnt inhibits the
formation of a protein complex that includes axin, glycogen
synthase kinase-3 and adenomatous polyposis coli (13). This inhibition leads to the
accumulation of β-catenin in the cytoplasm, which then translocates
to the nucleus (14). In the
nucleus, β-catenin binds to T-cell factor, resulting in the
transcription of target genes (15). Such aberrant β-catenin signaling
has been identified in a number of types of human cancers,
including melanomas, and colorectal and prostate cancers (16). β-catenin is hypothesized to affect
the metastatic potential of malignant cells by changing chromatin
remodeling (17) and altering the
oxidative stress response (18).
Therefore, reducing the constitutive activation of the
Wnt/β-catenin signaling pathway is an attractive target for the
treatment of cancers.
In the present study, the effects of triptolide
treatment were determined on multiple breast cancer cell lines,
specifically, the highly metastatic MDA-MB-231, human epidermal
growth factor receptor (HER2)-positive BT-474 and estrogen receptor
(ER)-positive MCF7 cell lines. Whether the anti-tumor effects of
triptolide on breast cells are associated with the inhibition of
β-catenin expression was also investigated. The potential of using
triptolide as an effective approach for the treatment of breast
cancers in the future was thus evaluated
Materials and methods
Reagents and cell lines
Triptolide (molecular formula,
C20H24O6; molecular weight, 360.4
g/mol) was purchased from Santa Cruz Biotechnology, Inc.
(sc-200122; Santa Cruz, CA, USA). Triptolide was dissolved in
dimethyl sulfoxide (DMSO). Three breast cancer cell lines,
specifically, the highly metastatic MDA-MB-231, HER2-positive
BT-474 and ER-positive MCF7 cell lines, were provided by the
Department of Oncology at the Hospital of Traditional Chinese
Medicine (Yantai, China). MDA-MB-231, BT-474 and MCF-7 cells were
cultured in Dulbecco’s modified Eagle’s medium, RPMI and α-minimal
essential medium (Sigma-Aldrich, St. Loius, MO, USA), respectively.
Cells were cultured at 37°C with 5% CO2 and 100%
humidity. The medium was supplemented with 10% fetal bovine serum
(FBS; HyClone Laboratories, Inc., Logan, UT, USA), 100 U/ml
penicillin and 100 μg/ml streptomycin.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells at a density of 1×105 cells/well in
medium were placed into 6-well plates and were continuously
cultured for 24 h. The cells were then treated with triptolide (0,
10, 25 and 50 nM). Cells treated with DMSO only (0 nM triptolide)
were used as a control. After 48 h, cells were incubated with 0.5
mg/ml MTT (Sigma-Aldrich) for 4 h according to the manufacturer’s
instructions. The cell viability of the treated cells was expressed
relative to that of the cells treated with DMSO only (relative
viability).
Apoptosis assay
Cells at a density of 1×105 cells/well
were cultured in six-well plates in medium supplemented with 10%
FBS for 24 h. This was followed by the addition of triptolide (0,
10, 25 or 50 nM). After 48 h, cells were harvested by
centrifugation, rinsed twice with phosphate-buffered saline (PBS),
fixed by incubation in 4% paraformaldehyde for 30 min at room
temperature and then rinsed again with PBS to remove the fixative.
Fixed cells were resuspended in PBS that contained 5 μg/ml Hoechst
33258 and incubated at room temperature for 15 min in the dark. The
cells were placed on glass slides and examined to record the
percentages of apoptotic cells with apoptotic morphology by
determining the nuclear condensation and chromatin fragmentation
via fluorescence microscopy (Olympus IX81, Olympus, Tokyo, Japan).
To quantify the apoptotic rate, 250 nuclei from random microscopic
fields were examined. Data are presented as the mean percentage of
apoptotic cells relative to the total cell number.
Western blot analysis
Cells were harvested by centrifugation and rinsed
twice with PBS. Total proteins were harvested from cells, separated
on 10% SDS-PAGE gels and then subjected to immunoblot analyses.
Primary antibodies against β-catenin (~90 kDa) and β-actin were
purchased from Santa Cruz Biotechnology, Inc. (anti-β-catenin,
sc-7963; 1:200; anti-β-actin, sc-130301; 1:10,000). The secondary
antibody, goat anti-mouse IgG-horseradish peroxidase, was purchased
from Santa Cruz Biotechnology, Inc. (sc-2005; 1:5,000). Antibodies
bound to the blots were detected using an enhanced
chemiluminescence system (Pierce Biotechnology, Inc., Rockford, IL,
USA). The mean optical densities (ODs) of β-catenin protein bands
were normalized against the OD of the β-actin band from the same
treatment. Quantity One software (Bio-Rad Laboratories, Hercules,
CA, USA) was used to quantify the OD levels. Immunoblotting
experiments were repeated at least 3 times.
Statistical analysis
Experimental data are expressed as mean ± SEM.
Statistical software (SPSS version 12.0; SPSS, Inc., Chicago, IL,
USA) was used to perform independent sample t-tests, followed by
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Triptolide has a toxic effect on breast
cancer cells
To determine whether triptolide (Fig. 1A) exhibited toxic effects on breast
cancer cells, highly metastatic MDA-MB-231, HER2-positive BT-474
and ER-positive MCF7 cells were treated with DMSO only (0 nM
triptolide) or triptolide at a concentration of 10, 25 or 50 nM.
Cell viability was determined using the MTT assay following
treatment with triptolide. DMSO only treatment served as a control
since triptolide was tested as a solution in DMSO. The cells were
analyzed for differences in cell viability following the various
treatments by counting the number of living cells in the presence
or absence of triptolide using the MTT assay.
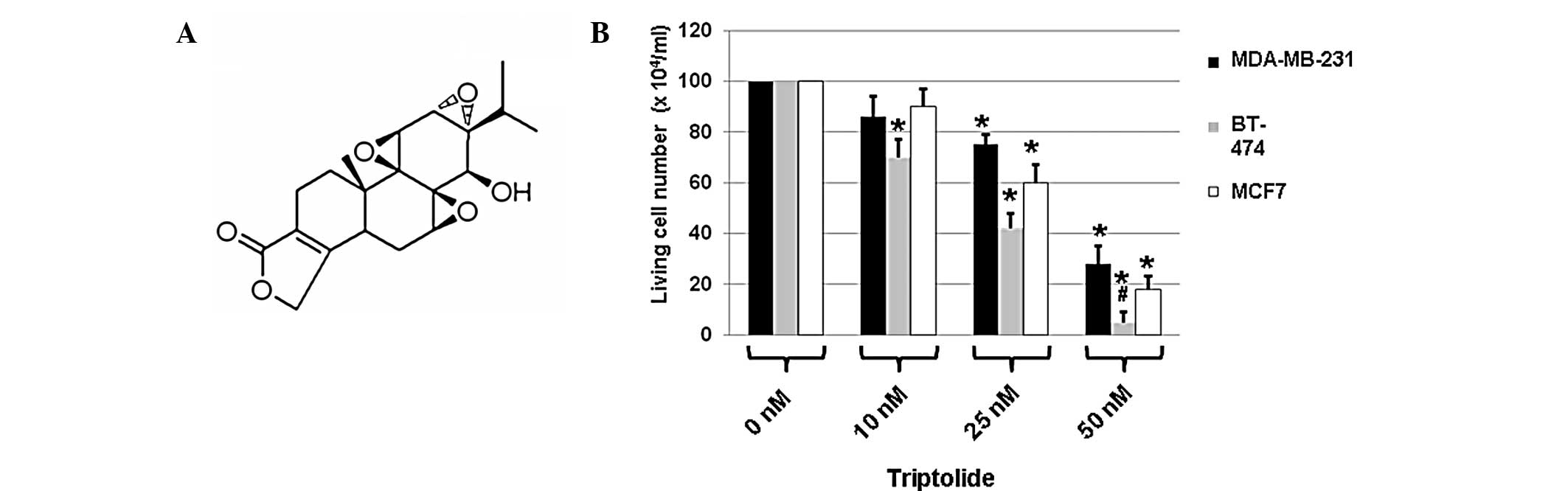 | Figure 1(A) Chemical structure of triptolide
(molecular formula, C20H24O6;
molecular weight, 360.4 g/mol). (B) Three breast cancer cell lines
(MDA-MB-231, BT-474 and MCF7) were treated with DMSO only (0 nM
triptolide) or triptolide at a concentration of 10, 25 or 50 nM.
Cell viability was measured using the MTT assay after 2 days of
incubation with triptolide. Values are expressed as mean ± SEM and
were obtained from three independent experiments. DMSO, dimethyl
sulfoxide; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
*P<0.05, compared with the untreated cells (0 nm);
#P<0.05, compared with the MDA-MB-231 and MCF7 cells
that were treated with triptolide at a concentration of 50 nM. |
Results demonstrated that in comparison with the
cells treated with DMSO only, 48 h-treatment with triptolide
reduced the cell viability of all three types of tumor cells
(Fig. 1B). Treatment with 10 nM
triptolide for 48 h exhibited an inhibitory effect on the cell
viability of BT-474 cells (Fig.
1B). However, treatment with 25 nM triptolide for 48 h had
marked inhibitory effects on the cell viability of all three cell
types, with greater effects observed in BT-474 cells compared with
the other two types of cell (Fig.
1B). In addition, 50 nM triptolide treatment resulted in
greater significant inhibitory effects on cell numbers (Fig. 1B). Among the three types of cell,
BT-474 cells were more sensitive compared with the other two cell
types (Fig. 1B). These results
indicate that triptolide has significant toxic effects on breast
cancer cells.
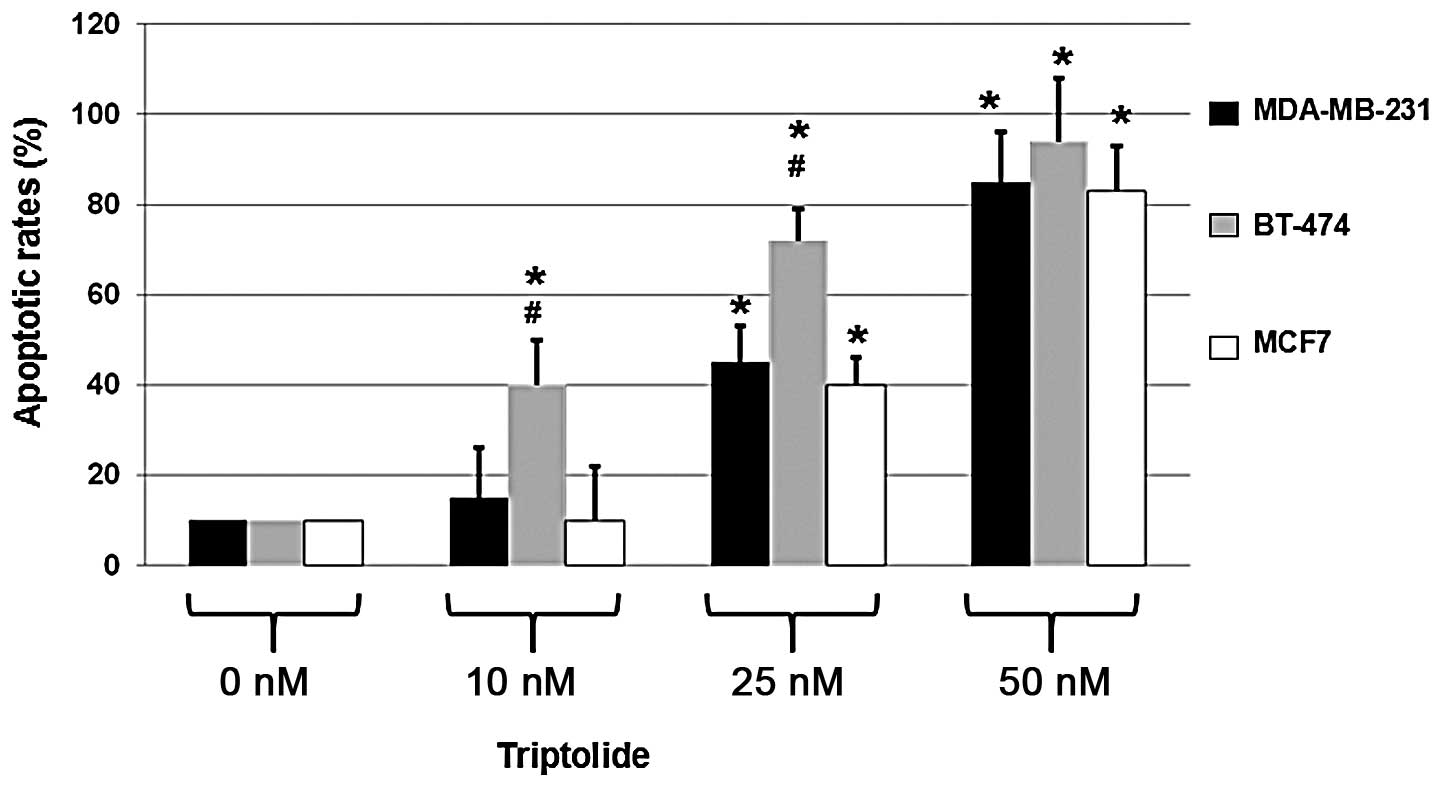
Triptolide induces apoptosis of breast
cancer cells
Since triptolide exhibited toxic effects on the
highly metastatic MDA-MB-231, HER2-positive BT-474 and ER-positive
MCF7 cells, the apoptotic effects of triptolide were determined in
the three cell types. Cells were treated with DMSO only (0 nM
triptolide) or 10, 25 or 50 nM triptolide for 48 h. To determine
the apoptotic rates, a fluorescence microscopic assay was performed
following the staining of the triptolide- or DMSO-treated cells
with Hoechst 33258.
As shown in Fig. 2,
triptolide treatment resulted in an increase in apoptosis in all
three cell types. When compared with the cells not treated with
triptolide, 50 nM triptolide treatment resulted in the apoptosis of
MDA-MB-231, BT-474 and MCF7 cells with an apoptotic rate of ~80%.
These results indicate that the rate of apoptosis is significantly
increased in triptolide-treated cells.
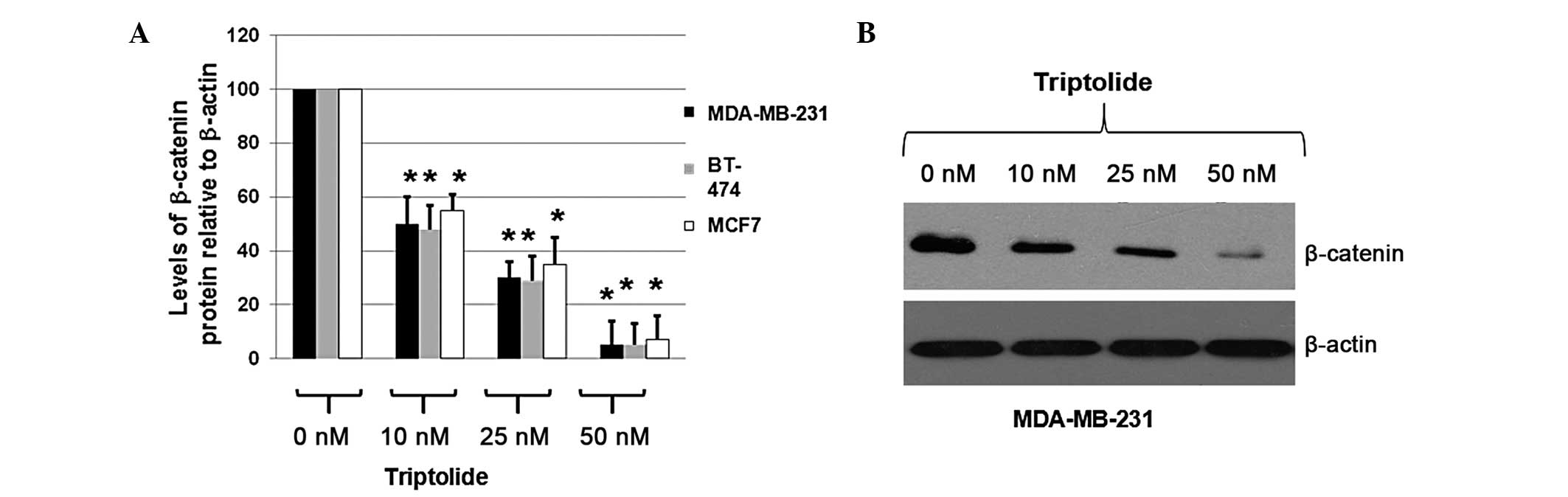
Triptolide treatment results in the
degradation of β-catenin
To determine whether triptolide inhibited the
expression of β-catenin in MDA-MB-231, BT-474 and MCF7 cells, the
cells were treated with DMSO only (0 nM triptolide) or 10, 25 or 50
nM triptolide for 48 h. Total proteins were isolated and the
expression levels of β-catenin were determined by immunoblot
analysis. Cellular β-actin protein was used as a loading control.
The mean OD values of β-catenin protein bands, normalized against
the OD of the β-actin band from the same treatment, were calculated
and subjected to statistical analyses. The calculated ratios of the
levels of β-catenin proteins relative to β-actin levels were
obtained and are shown in Fig.
3A.
As shown in Fig.
3A, triptolide treatment of MDA-MB-231, BT-474 and MCF7 cells
decreased the expression levels of β-catenin to 5–10% of the
expression levels observed in the cells treated with DMSO only,
according to the calculated OD values of the β-catenin bands
relative to the β-actin bands. A representative blot is shown in
Fig. 3B. These results indicate
that triptolide significantly decreased β-catenin expression in the
breast cancer cells, suggesting that triptolide effectively
inhibits the proliferation of breast cancer cells via a mechanism
associated with the Wnt/β-catenin signaling pathway.
Discussion
Triptolide is reported to have multiple functions,
including immunosuppressive and anti-inflammatory properties and
the induction of apoptosis via the activation of caspases (3–8).
Triptolide functions by regulating multiple cellular signaling
pathways, including the NF-κB pathway (19). In the present study, the inhibitory
effects of triptolide were investigated in three breast cancer cell
lines, specifically, highly metastatic MDA-MB-231, HER2-positive
BT-474 and ER-positive MCF7 cell lines.
In the present study, triptolide treatment was found
to induce apoptosis in the three types of malignant cells.
Treatment with 25 nM triptolide for 48 h exhibited marked
inhibitory effects on the cell viability of all three cell types,
with greater effects observed in BT-474 cells compared with the
other two cell types. When compared with the cells not treated with
triptolide, 50 nM triptolide treatment resulted in the apoptosis of
MDA-MB-231, BT-474 and MCF7 cells with apoptotic rates of ~80%.
Western blot analysis indicated that triptolide treatment in
MDA-MB-231, BT-474 and MCF7 cells decreased the expression levels
of β-catenin to 5–10% of the expression levels observed in the
cells treated with DMSO only. Therefore, the results of the present
study indicate that triptolide induces apoptosis in breast cancer
cells via a mechanism associated with the Wnt/β-catenin signaling
pathway.
Triptolide is involved in multiple cellular
signaling pathways. Recently, triptolide was shown to induce
extracellular signal-regulated kinase activation and affect the
generation of reactive oxygen species and the induction of
endoplasmic reticulum stress via the PERK-eIF2α pathway (20). In addition, a previous study has
demonstrated that triptolide induces cell cycle arrest and
apoptosis in human tumor cells by inducing DNA damage and the
expression of repair-associated genes (21). Therefore, the observations of the
present study indicate that triptolide induces apoptosis in breast
cancer cells by a mechanism associated with the Wnt/β-catenin
signaling pathway, further improving the understanding of the role
of triptolide as a potential treatment for breast cancer.
References
|
1
|
Chen F and Huang K: Effects of the Chinese
medicine matrine on experimental C. parvum infection in
BALB/c mice and MDBK cells. Parasitol Res. 111:1827–1832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jong TT, Lee MR, Chiang YC and Chiang ST:
Using LC/MS/MS to determine matrine, oxymatrine, ferulic acid,
mangiferin, and glycyrrhizin in the Chinese medicinal preparations
Shiau-feng-saan and Dang-guei-nian-tong-tang. J Pharm Biomed Anal.
40:472–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen HL, Liang ZS, Zhang R and Yang K:
Anti-inflammatory effects of triptolide improve left ventricular
function in a rat model of diabetic cardiomyopathy. Cardiovasc
Diabetol. 12:502013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue M, Jiang ZZ, Liu JP, et al:
Comparative study on the anti-inflammatory and immune suppressive
effect of Wilforlide A. Fitoterapia. 81:1109–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong YY, Chen HP, Tan BZ, Yu HH and Huang
XS: Triptolide avoids cisplatin resistance and induces apoptosis
via the reactive oxygen species/nuclear factor-κB pathway in
SKOV3PT platinum-resistant human ovarian cancer cells. Oncol Lett.
6:1084–1092. 2013.PubMed/NCBI
|
|
6
|
Rousalova I, Banerjee S, Sangwan V, et al:
Minnelide: a novel therapeutic that promotes apoptosis in non-small
cell lung carcinoma in vivo. PLoS One. 8:e774112013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai YY, Lin WP, Li AP and Xu JY: Combined
effects of curcumin and triptolide on an ovarian cancer cell line.
Asian Pac J Cancer Prev. 14:4267–4271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma JX, Sun YL, Wang YQ, Wu HY, Jin J and
Yu XF: Triptolide induces apoptosis and inhibits the growth and
angiogenesis of human pancreatic cancer cells by downregulating
COX-2 and VEGF. Oncol Res. 20:359–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu C, Zhang Y, Kong X, et al: Triptolide
prevents bone destruction in the collagen-induced arthritis model
of rheumatoid arthritis by targeting RANKL/RANK/OPG signal Pathway.
Evid Based Complement Alternat Med. 2013:6260382013.PubMed/NCBI
|
|
10
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: a
review. Gynecol Oncol. 131:772–779. 2013.
|
|
11
|
Huang GL, Luo Q, Rui G, Zhang W, Zhang QY,
Chen QX and Shen DY: Oncogenic activity of retinoic acid receptor γ
is exhibited through activation of the Akt/NF-κB and Wnt/β-catenin
pathways in cholangiocarcinoma. Mol Cell Biol. 33:3416–3425.
2013.
|
|
12
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minde DP, Radli M, Forneris F, Maurice MM
and Rüdiger SG: Large extent of disorder in adenomatous polyposis
coli offers a strategy to guard Wnt signalling against point
mutations. PLoS One. 8:e772572013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rahmani M, Read JT, Carthy JM, et al:
Regulation of the versican promoter by the beta-catenin-T-cell
factor complex in vascular smooth muscle cells. J Biol Chem.
280:13019–13028. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin T, George Fantus I and Sun J: Wnt and
beyond Wnt: multiple mechanisms control the transcriptional
property of beta-catenin. Cell Signal. 20:1697–1704. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tarapore RS, Siddiqui IA and Mukhtar H:
Modulation of Wnt/β-catenin signaling pathway by bioactive food
components. Carcinogenesis. 33:483–491. 2012.
|
|
17
|
Kim JH, Kim B, Cai L, et al:
Transcriptional regulation of a metastasis suppressor gene by Tip60
and beta-catenin complexes. Nature. 434:921–926. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Essers MA, de Vries-Smits LM, Barker N, et
al: Functional interaction between beta-catenin and FOXO in
oxidative stress signaling. Science. 308:1181–1184. 2005.
View Article : Google Scholar
|
|
19
|
Park SW and Kim YI: Triptolide induces
apoptosis of PMA-treated THP-1 cells through activation of
caspases, inhibition of NF-κB and activation of MAPKs. Int J Oncol.
43:1169–1175. 2013.PubMed/NCBI
|
|
20
|
Tan BJ and Chiu GN: Role of oxidative
stress, endoplasmic reticulum stress and ERK activation in
triptolide-induced apoptosis. Int J Oncol. 42:1605–1612.
2013.PubMed/NCBI
|
|
21
|
Chueh FS, Chen YL, Hsu SC, et al:
Triptolide induced DNA damage in A375.S2 human malignant melanoma
cells is mediated via reduction of DNA repair genes. Oncol Rep.
29:613–618. 2013.PubMed/NCBI
|