Introduction
Anterior cruciate ligament (ACL) rupture is a common
injury among young sportsmen and women, with a reported annual
incidence in the general population of 0.8 per 1,000 (1). The ACL is a common site of knee joint
injury. With the wide use of allogeneic tendons in cruciate
ligament reconstruction, research into acellular tendons and
acellular tissue-engineered tendons has become increasingly
studied. Ideal tissue-engineered ligaments retain the basic
histological structure of the ligaments and provide a suitable
environment for stem cell proliferation and differentiation,
forming connections between ligament collagen fibers. Acellular
tissue-engineered ligaments provide a suitable alternative, while
preventing ligament immunogenicity and the spread of disease
(2). Therefore, further research
is required to establish more effective and physiological graft
options with a greater understanding of anatomical graft placement
and graft tension (3). At present,
tendon decellularization has been studied intensively with a
variety of chemical reagents. The removal of ligament cell
components may reduce immunogenicity, preserve the integrity of
ligament structure and preserve the biological characteristics of
the ligament. There are a number of decellularization methods,
including physical, chemical and enzymatic, as well as combinations
of these techniques (4). The ideal
method is to remove cell residue completely, while maintaining
collagen structure, in order to maximize the retention of the
biomechanical characteristics of the tendon, leaving the original
extracellular matrix (ECM) structure and composition intact. ECM
materials may also encourage angiogenesis, which is required to
deliver cells to repair the tissue and prevent infection (5). In previous years, tissue engineering
has emerged as a promising approach for tendon repair or
regeneration (6–9). The decelluarization tissue
engineering tendon for ACL repair has beep reported as one of
important method (10), however to
find the most effective and least destructive decelluarization
method was the important step.
Comparisons between singular and different
combinations of decellularization methods have not yet been
conducted, thus, in the present study, a variety of combinations
were investigated, including t-octyl-phenoxypolyethoxyethanol
(Triton-X 100), sodium dodecyl sulfate (SDS) and tri-n-butyl
phosphate (TnBP), in order to determine which combination exhibited
the greatest decellularization effect. In the present study, rabbit
semitendinosus (ST) and flexor digitorum (DT) muscles were treated
with a variety of decellularization methods and the histological
and biomechanical changes were evaluated. Through comparing the
effects of various methods on the collagen structure and
biomechanics, the aim of the study was to identify an the most
effective decellularization method.
Materials and methods
Animal grouping
In total, 28 male standard New Zealand rabbits (the
experimental animals were provided and maitanied by the
Experimental Animal Center of Urumqi Gengeral Hospital), weighing
3.0–3.5 kg, were randomly divided into control and experimental
groups. A total of eight ST and DT sections from the hindlimb
flexor muscles were harvested from each group and immediately
prepared for testing. Four rabbits comprised the control group, and
the experimental group was further divided into six subgroups of
four animals each. A total of 48 ST and DT samples were collected
and the tendons were treated with six decellularization methods.
The study was conducted in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health, and the animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of Ürümqi General Hospital (Ürümqi, China).
Tissue extraction and storage
Tendons were collected from the rabbits following
the administration of anesthesia. All the tendons and accessory
tissues were removed and stored at 4°C in phosphate-buffered saline
(PBS) with 5% penicillin/streptomycin (Mediatech, Herndon, VA, USA)
and 0.02% EDTA (Sigma-Aldrich, St. Louis, MO, USA) to prevent
tissue degradation and inhibit bacterial growth. All the specimens
were washed at 4°C with PBS for 12 h prior to the experiments.
Decellularization methods
Tendons were treated with the following six chemical
reagents for 24 h: i) 1% Triton-X 100 (Yuanye Biotechnology Co.,
Shanghai, China); ii) 0.5% SDS (Yuanye Biotechnology Co.); iii) 1%
TnBP (Yuanye Biotechnology Co.); iv) 1% Triton-X 100 + 0.5% SDS; v)
1% TnBP + 0.5% SDS; and vi) 1% TnBP + 1% Triton-X 100. The control
group was treated with PBS (Cellchip Biotechnology Co., Beijing,
China) only.
Following the decellularization treatment, the
tendons were washed in distilled water at 4°C for 24 h. Next, the
tendons were incubated in PBS containing 100 μg/ml DNase
(Sigma-Aldrich) and 150 IU/ml RNase (Sigma-Aldrich) at 37°C for 24
h, and then placed in PBS containing 0.02% EDTA at 4°C for 24 h.
All the specimens were stored in a gauze at 4°C with PBS until
required for mechanical testing and pathological analysis.
Histopathological analysis
Gross observations analyzing the tendon morphology
and color were conducted. A number of the tendons from the various
groups were selected to assess the extent of decellularization and
collagen changes using light microscopy (Olympus Corporation,
Tokyo, Japan).
Measurements
Cross-sectional dimensions were measured using
vernier calipers. The tendons were extended straight with no
tension and the cross-sectional area was calculated, assuming an
elliptical cross-section. The two ends of the tendon were placed at
the two fixtures of the MTS 858 Mini Bionix test system (MTS
Systems Corporation, Eden Prairie, MN, USA) and maintained at an
upright position, straightening without tension by adjusting the
test system. The distance between the two fixtures was measured and
this was considered to be the length of the tested tendons. For
rupture testing, a load was applied directly to tendons at constant
velocity of 10 mm/min until the tendon broke. The test system
recorded the load-displacement curve, the peak load of the tendon
damage and the tensile displacement at the peak load. The rigidity
and elastic modulus of the tendon graft were then calculated.
Statistical analysis
Data were analyzed using SPSS 12.0 software (SPSS,
Inc., Chicago, IL, USA) and the results are presented as the mean ±
standard deviation. Biomechanical differences among the treatment
groups and between the treatment and control groups were analyzed
using the t-test, where P<0.05 was considered to indicate a
statistically significant difference.
Results
Gross anatomy and pathology
observations
In the group of untreated rabbit tendon specimens,
the hematoxylin and eosin staining was normal, with tendon cells
and normally arranged collagen structures observed, as shown in
Fig. 1A. In the 1% Triton-X
100-treated group, as shown in Fig.
1B, a number of residual tendon cells were observed in the
rabbit flexor DT muscles, while a small number of cells were
observed in the ST muscles. In addition, the collagen structure was
abnormal in the ST samples. In the 0.5% SDS-treated group, as shown
in Fig. 1C, there was a small
number of residual tendon cells, and the collagen arrangement and
nuclear structures in the flexor DT muscles were normal. A small
number of tendon cells and a small amount of vascular epithelial
cell debris were observed in the ST muscles, with the arrangement
of collagen largely normal with the exception of a few local
ruptures. In the 1% TnBP-treated group, as shown in Fig. 1D, a small number of residual cells
was observed in the flexor DT muscles, while in the ST samples,
there were no cells remaining and the collagen gap had widened,
revealing a small number of ruptures. In the 1% TnBP + 0.5%
SDS-treated group, as shown in Fig.
1E, no cells were observed in the flexor DT or ST muscles.
Collagen structures remained intact with a small number of ruptures
and the collagen gap had widened slightly. In the 1% TnBP + 1%
Triton-X 100-treated group, as shown in Fig. 1F, the treatment was effective.
There were no clear cell remnants in the flexor DT or ST muscles,
and the collagen arrangement was slightly disordered, particularly
in the ST samples. In the 1% Triton-X 100 + 0.5% SDS-treated group,
as shown in Fig. 1G, there were no
clear residual cells in the flexor DT or ST muscles, however, the
two areas exhibited disorganized collagen and evident collagen
ruptures. Following the decellularization treatment, the two types
of tendon exhibited a degree of damage to the collagen structures,
particularly in the 1% Triton-X 100- and 1% Triton-X 100 + 0.5%
SDS-treated groups. The three groups treated with a single reagent
all showed residual cells, particularly in the DT muscle, which is
bigger and thicker compared with the ST muscle. In the groups
treated with two reagents, the treatment was more effective and
there were no residual cells observed in the tendons. In the 1%
TnBP-treated group, little damage to the collagen structure was
observed. In the 1% TnBP + 0.5% SDS-treated group, the collagen
structure remained intact and only a small amount of collagen
fracturing was observed in the ST muscles. In the 1% Triton-X 100 +
0.5% SDS-treated group, the treatment was effective, however,
significant collagen damage was observed. Therefore, the 1% TnBP +
0.5% SDS treatment preserved the integrity of the tendon collagen
arrangement and structure, while successfully removing all the
nuclei.
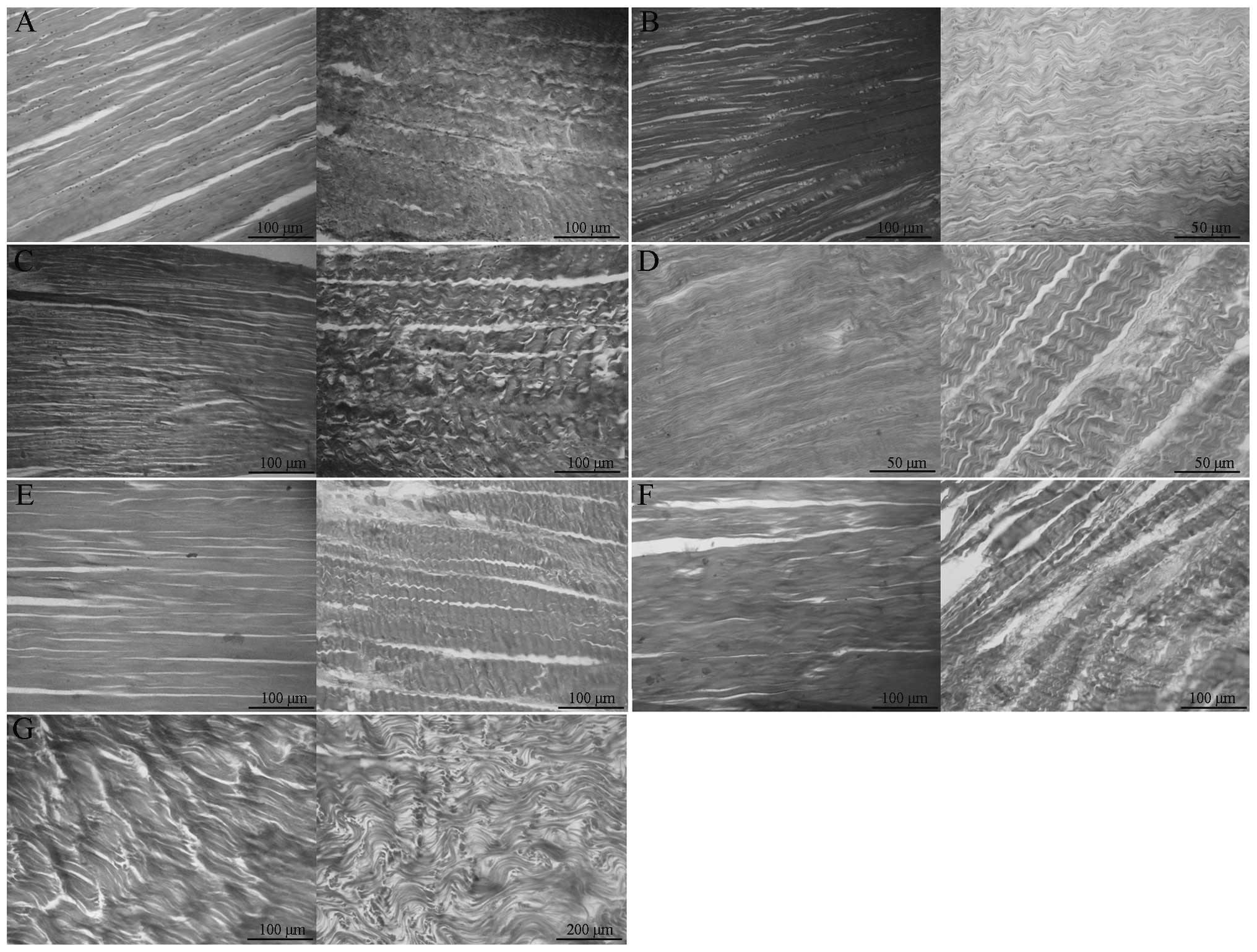 | Figure 1Rabbit limb flexor DT muscle (left,
DT; right, ST) arrangement following treatment with (A) PBS
(control), (B) 1% Triton-X 100, (C) 1% SDS, (D) 1% TnBP, (E) 1%
TnBP + 0.5% SDS, (F) 1% TnBP + 1% Triton-X 100 and (G) 1% Triton-X
100 + 0.5% SDS decellularization methods. ST, semitendinosus ; DT,
digitorum; Triton-X 100, t-octyl-phenoxypolyethoxyethanol; SDS,
sodium dodecyl sulfate; TnBP, tri-n-butyl phosphate; PBS,
phosphate-buffered saline. |
Tensile biomechanics
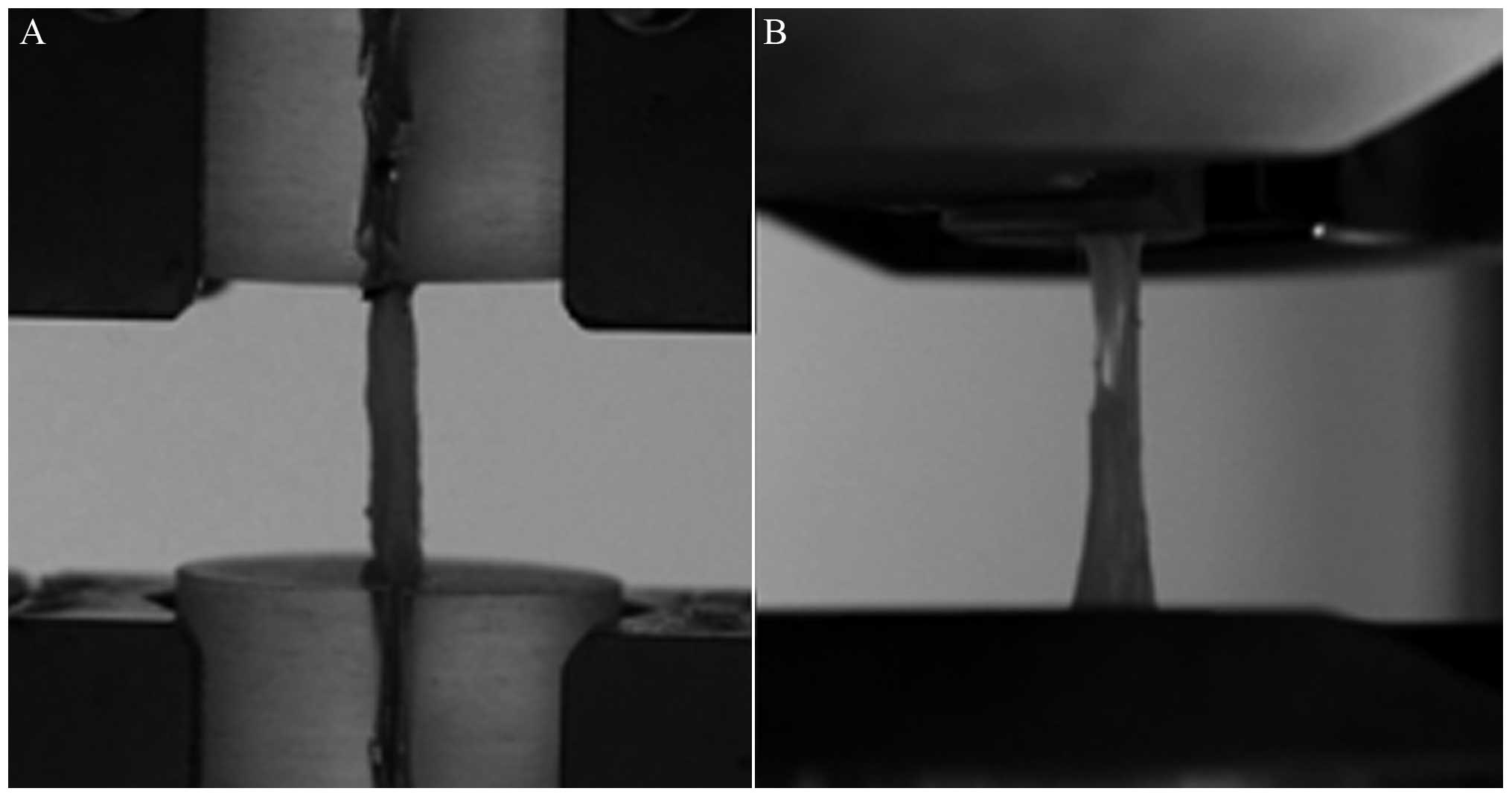
Tendons were subjected to tensile mechanical testing
(Fig. 2). All the specimens failed
at the ligament section. No damage was observed at the tendon or
clip surface, however, tendon ruptures were located at the body of
the tendon, close to the flat end of the tendon.
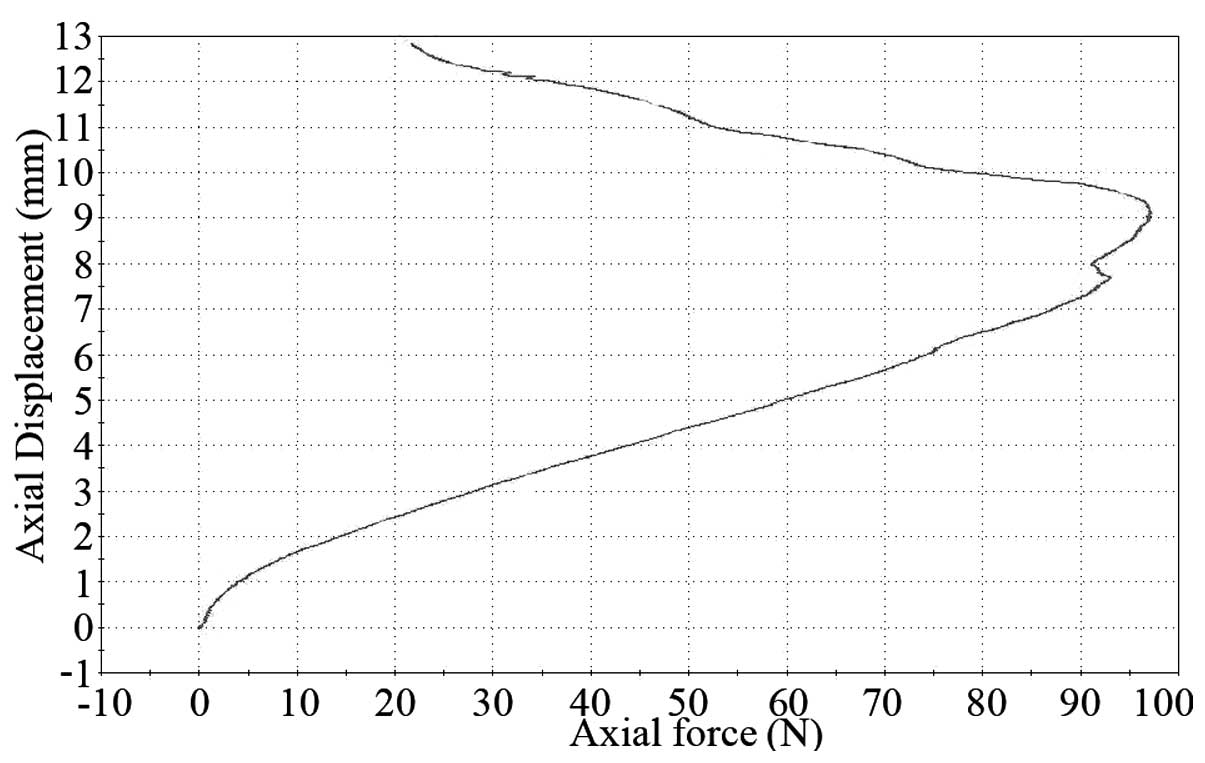
A typical stress-strain curve at the tendon stretch
is shown in Fig. 3. The
association between the stress and strain that a particular
material displays is known as that particular material’s
stress-strain curve. It is unique for each material and is
calculated by recording the amount of deformation (strain) at
distinct intervals of tensile or compressive loading (stress). It
shows certain properties of the tendon.
No statistically significant difference was detected
in the morphological parameters between the ST and flexor DT
muscles (Table I).
 | Table IComparison of cross-sectional areas
and lengths of the tendons in the various decellularization groups
(mean ± SD). |
Table I
Comparison of cross-sectional areas
and lengths of the tendons in the various decellularization groups
(mean ± SD).
| Cross-sectional area
(mm2) | Length (mm) |
|---|
|
|
|
|---|
| Group | ST | DT | ST | DT |
|---|
| 1 | 1.02±0.15 | 3.02±0.68 | 20.65±1.23 | 20.20±0.84 |
| 2 | 0.85±0.18 | 2.12±2.34 | 18.67±0.14 | 20.91±1.13 |
| 3 | 1.22±0.75 | 2.42±1.25 | 20.07±0.63 | 18.87±1.89 |
| 4 | 0.94±0.67 | 2.48±1.70 | 20.24±1.08 | 18.43±1.68 |
| 5 | 0.73±0.36 | 2.53±1.94 | 19.98±0.35 | 19.56±1.05 |
| 6 | 0.89±1.04 | 2.47±0.88 | 20.16±0.76 | 20.00±0.81 |
| 7 | 0.92±1.31 | 2.82±1.53 | 19.38±1.28 | 20.12±1.32 |
Final mechanical measurements are shown in Table II. No statistically significant
differences were observed among the three types of structural
failure loads (normal ST, 101.40±6.34 N; normal flexor DT,
253.78±12.36 N). The 1% Triton-X 100 + 0.5% SDS group exhibited a
lower failure load, however, no statistically significant
difference was detected among the groups. With regard to
extensibility, statistically significant differences were observed
between the TnBP and control groups, as well as between the TnBP
and other treatment groups. TnBP treatment was found to
significantly increase the extensibility. No statistically
significant difference was observed in extensibility among the
other groups. The results indicated that the extensibility of the
tendons increased following TnBP treatment, and the increase was
statistically significant when compared with the other groups.
Therefore, TnBP treatment may cause the relaxation of tendons. No
statistically significant difference with regard to failure load or
elastic modulus was observed between the TnBP treatment group and
the other groups. The elastic modulus of the 1% Triton-X 100 + 0.5%
SDS-treated group was significantly different when compared with
the control group (P<0.05), however, statistically significant
differences were not observed when compared with the other
treatment groups (P>0.05).
 | Table IIFailure load, extensibility and
elastic modulus of the tendons in the various decellularization
groups (mean ± SD). |
Table II
Failure load, extensibility and
elastic modulus of the tendons in the various decellularization
groups (mean ± SD).
| Failure load (N) | Elastic modulus
(MPa) | Extensibility
(%) |
|---|
|
|
|
|
|---|
| Group | ST | DT | ST | DT | ST | DT |
|---|
| 1 | 88.63±8.70 | 225.80±16.05 | 317.25±2.03 | 492.76±3.47 | 11.98±4.24 | 9.27±2.83 |
| 2 | 92.02±7.76 | 233.4±23.92 | 331.43±5.14 | 503.76±5.86 | 12.71±1.52 | 10.63±1.42 |
| 3 | 85.74±10.13 | 234.58±28.31 | 285.67±11.73 | 483.76±2.64 | 25.52±8.23a | 22.78±4.18a |
| 4 | 81.36±7.63 | 198.24±21.05 | 313.52±7.76b | 493.76±7.55b | 13.56±3.03 | 10.36±3.86 |
| 5 | 93.23±7.04 | 238.77±27.13 | 332.76±2.19 | 538.76±4.62 | 13.52±2.16 | 12.76±0.93 |
| 6 | 90.82±7.85 | 235.82±12.59 | 330.34±5.33 | 529.76±6.32 | 14.75±1.89 | 11.66±2.53 |
| 7 | 101.40±6.34 | 253.78±12.36 | 356.52±16.94 | 560.09±2.73 | 10.73±3.21 | 8.76±2.76 |
Discussion
The present study analyzed the decellularization
effects of two rabbit tendons treated with several singular and
combination decellularization methods, with the aim of determining
the most effective decellularization method. These observations may
provide rational for the future study of tendon tissue engineering
applications. At present, decellularization methods focus on a
number of chemical reagents. These decellularization methods are
frequently used in research, however, the most effective method of
decellularization for the rabbit tendon has not yet been reported
in literature. Thus, the present study compared the effects of a
variety of decellularization methods on the histological structures
and biomechanical properties of rabbit flexor DT and ST
muscles.
Decellularizated tendons, as compared with
allografts or synthetic materials, have a number of promising
advantages. Firstly, the immunogenicity and antigenicity of the
tissue is reduced, whereas only minimal antigenic ECM is preserved
(11). Secondly, an ideal
environment is provided for the cells to undergo incorporation,
metabolism and matrix synthesis, by preserving other macromolecules
in addition to the collagenous structure. Furthermore, by
preserving the natural structure of the tendon, the initial
biomechanical strength should be preserved, with a biomechanical
strength similar to that of the tendon itself (12).
The results of the present study demonstrated that
all the decellularization methods have the capacity to remove cells
from the tendon. Histological results showed intact cells and cell
debris following decellularization with a single reagent. Fewer
cells remained in the ST muscle, as compared with the flexor DT
muscle, which may be due to the smaller diameter of the ST.
Combinations of two reagents exhibited excellent decellularization
effects and there were fewer intact cells and cell debris when
compared with the single reagent methods. The 1% TnBP + 1% Triton-X
100 and 1% TnBP + 0.5% SDS treatments exhibited excellent
decellularization effects with a predominantly intact collagen
structure. In particular, the 1% TnBP + 0.5% SDS treatment
demonstrated little damage to the collagen structure, with the
collagen showing a normal arrangement. Tendons treated with 1%
Triton-X 100 + 0.5% SDS exhibited more ruptures in the collagen
structure and more disordered arrangements when compared with the
other treatment groups. Biomechanical analysis indicated that all
the decellularization treatments maintained a good biomechanical
performance. The 1% TnBP + 0.5% SDS group exhibited the least
mechanical reduction, while the 1% Triton-X 100 + 0.5% SDS group
demonstrated the most biomechanical reduction. This observation may
be due to the intensive collagen damage associated with this
method. No statistically significant difference was observed with
regard to biomechanics between each treatment group and the
control. The results also demonstrated that the extensibility of
the ST and DT muscles increased following TnBP treatment, with the
difference between the control and TnBP treatment group being
statistically significant (P<0.05). No statistically significant
difference in extensibility was observed between the other
treatment groups and the control group. The study conducted by
Deeken et al, which used TnBP (1%) to treat pig
diaphragmatic tendon specimens, demonstrated TnBP to have excellent
decellularization effects, but also increased tendon extensibility
(13). The increased extensibility
associated with TnBP treatment may be due to the effects of TnBP on
collagen crosslinking. TnBP treatment also makes it easier for
collagen fibers to slide and cause the entire tissue to extend
easily, which may also be due to the decreased degree of
crosslinking. The increased tendon extensibility associated with
TnBP treatment may lead to tendon laxity following reconstruction,
which may affect the long-term biomechanical properties of the
tendon. Therefore, TnBP treatment is not an ideal decellularization
method and the present study does not recommend TnBP treatment for
the preparation of tendon scaffolds. However, TnBP treatment may be
combined with other methods in order to mitigate the effects on
tendon extensibility.
Tendon damage and cruciate ligament injury are the
main causes of lost and limited motor functions (14,15).
Ligaments have a very limited ability to self-heal following injury
and often require surgical intervention. This is particularly true
of cruciate ligament injury following knee joint torsion. For young
patients, cruciate ligament reconstruction using autologous tendons
is an ideal method to restore function, however, this method may
cause donor site complications, including sustained patellar pain,
a lack of muscle strength around the knee joints and a decline in
joint stability. Allografts are an alternative material for
surgical reconstruction, however, these may cause immune responses
and spread disease. The long-term results associated with
allogeneic tendons are not good. For example, they involve risks of
ligament laxity and extension and decreased mechanical properties.
Acellular tissue-engineered ligaments are becoming increasingly
studied (16) and research has
shown that tissue-engineered ligaments are the most promising
method of solving this problem. The success of tissue engineering
relies on the selection of optimal biological materials, and
acellular ligaments may be the most effective. Decellularization
technology includes physical methods, such as ultrasound, freezing
and agitation; chemical methods, such as organ-dissolving solutions
and acid, ionic, nonionic and zwitterionic detergents; and enzymes,
such as proteases, nucleases and combinations thereof. Ideal
decellularization techniques successfully dissolve the cell
membrane, cytoplasm and nuclear remnants, while removing the
residual chemical and cellular components, leaving the original ECM
scaffolds and their optimal biomechanical properties undamaged
(17). The results of previous
studies have led researchers to question whether the acellular
tendon is necessary for ligament tissue engineering. The removal of
zymogen clusters from cell surfaces using antigenic determinants
can reduce allograft immunogenicity (18). Inner cells can cause necrosis
following transplantation and delay host cell invasion and
religamentization. Decellularization may reduce the allogeneic
spread of disease. This evidence cannot be ignored (19). Therefore, the decellularization of
tendon scaffolds continues to be necessary for ligament tissue
engineering (20).
Decellularization using SDS, an ionic detergent, Triton-X 100, a
nonionic detergent, and TnBP, a disinfecting and bacteriostatic
agent, has been successfully applied in scaffold preparation for
skin grafts, heart valves, blood vessels, tendons, ligaments and
cartilage (21,22).
Studies conducted by Cartmell and Dunn on patellar
tendon grafts demonstrated that TnBP and SDS decellularization
treatment removed 70–90% of cells (23,24).
Only a few cells remained, although a number of changes to the
collagen morphology were observed. Similar results were obtained
when the authors used SDS to treat rat tail tendons. Specimens
treated with Triton-X 100 exhibited numerous broken cells, cell
debris and mild collagen damage. However, a study conducted by
Courtman et al demonstrated that Triton-X 100 successfully
removed cells in other tissues, including bovine pericardium,
without damaging the collagen structure or reducing the collagen
strength (25). In the present
study, the results showed that all the decellularization methods
exhibited a certain capacity to remove cells, with the methods
involving a single reagent showing better retention of the collagen
structure. Among the methods involving two reagents, the 1% TnBP +
0.5% SDS treatment exhibited the least collagen damage.
The study conducted by Woods and Gratzer focused on
the effects of Triton-X combined with SDS (26). The results demonstrated that this
combination had excellent decellularization effects, however, it
also clearly damaged the glycosaminoglycan and increased the
collagen tensile strength. The current study also demonstrated that
the combination of Triton-X 100 and SDS caused collagen
disorganization, local rupture and decreased biomechanical
strength, but this decrease was not significant when compared with
the other treatment groups. Histological examinations performed in
the current study revealed that Triton-X with SDS effectively
removed cells from the ST muscles, but not from the robust flexor
DT muscles, which had cells remaining. This observation indicated
that more time was required to completely remove the cells from the
flexor DT muscles, and that the removal of these cells may increase
the collagen damage. Thus, joint application of different
decellularization reagents is recommended. For example, the present
study found that the combination of TnBP and SDS completely removed
cells from the rabbit ST and flexor DT muscles, while leaving the
collagen structure undamaged. A minimal decrease in biomechanical
strength was observed and no statistically significant difference
was identified when compared with the normal tendon tissue. When
two reagents are used in combination, they compliment each other
and produce improved decellularization effects, while leaving the
tendon integrity and biomechanical strength undamaged.
References
|
1
|
Potter HG, Jain SK, Ma Y, et al: Cartilage
injury after acute, isolated anterior cruciate ligament tear:
immediate and longitudinal effect with clinical/MRI follow-up. Am J
Sports Med. 40:276–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Omae H, Sun YL, An KN, Amadio PC and Zhao
C: Engineered tendon with decellularized xenotendon slices and bone
marrow stromal cells: an in vivo animal study. J Tissue Eng Regen
Med. 6:238–244. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leys T, Salmon L, Waller A, Linklater J
and Pinczewski L: Clinical results and risk factors for reinjury 15
years after anterior cruciate ligament reconstruction: a
prospective study of hamstring and patellar tendon grafts. Am J
Sports Med. 40:595–605. 2012.PubMed/NCBI
|
|
4
|
Harrison RD and Gratzer PF: Effect of
extraction protocols and epidermal growth factor on the cellular
repopulation of decellularized anterior cruciate ligament
allografts. J Biomed Mater Res A. 75:841–854. 2005. View Article : Google Scholar
|
|
5
|
Wilshaw SP, Kearney JN, Fisher J and
Ingham E: Production of an acellular amniotic membrane matrix for
use in tissue engineering. Tissue Eng. 12:2117–2129. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuo CK, Marturano JE and Tuan RS: Novel
strategies in tendon and ligament tissue engineering: Advanced
biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil
Ther Technol. 2:202010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longo UG, Lamberti A, Maffulli N and
Denaro V: Tissue engineered biological augmentation for tendon
healing: a systematic review. Br Med Bull. 98:31–59. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Zou XH, Yin GL and Ouyang HW:
Tendon tissue engineering with mesenchymal stem cells and
biografts: an option for large tendon defects? Front Biosci (Schol
Ed). 1:23–32. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Butler DL, Juncosa-Melvin N, Boivin GP, et
al: Functional tissue engineering for tendon repair: A
multidisciplinary strategy using mesenchymal stem cells,
bioscaffolds, and mechanical stimulation. J Orthop Res. 26:1–9.
2008. View Article : Google Scholar
|
|
10
|
Karaoglu S, Fisher BM, Woo SL, et al: Use
of a bioscaffold to improve healing of patellar tendon defect after
graft harvest for ACL reconstruction: A study in rabbits. J Orthop
Res. 26:255–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Worth DC, Hodivala-Dilke K, Robinson SD,
et al: Alpha v beta3 integrin spatially regulates VASP and RIAM to
control adhesion dynamics and migration. J Cell Biol. 189:369–383.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tischer T, Vogt S, Aryee S, et al: Tissue
engineering of the anterior cruciate ligament: a new method using
acellularized tendon allografts and autologous fibroblasts. Arch
Orthop Trauma Surg. 127:735–741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deeken CR, White AK, Bachman SL, et al:
Method of preparing a decellularized porcine tendon using tributyl
phosphate. J Biomed Mater Res B Appl Biomater. 96:199–206. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lidén M, Sernert N, Rostgård-Christensen
L, Kartus C and Ejerhed L: Osteoarthritic changes after anterior
cruciate ligament reconstruction using bone-patellar tendon-bone or
hamstring tendon autografts: a retrospective, 7-year radiographic
and clinical follow-up study. Arthroscopy. 24:899–908. 2008.
|
|
15
|
Butler DL, Juncosa-Melvin N, Boivin GP, et
al: Functional tissue engineering for tendon repair: A
multidisciplinary strategy using mesenchymal stem cells,
bioscaffolds, and mechanical stimulation. J Orthop Res. 26:1–9.
2008. View Article : Google Scholar
|
|
16
|
Carey JL, Dunn WR, Dahm DL, Zeger SL and
Spindler KP: A systematic review of anterior cruciate ligament
reconstruction with autograft compared with allograft. J Bone Joint
Surg Am. 91:2242–2250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gilbert TW, Sellaro TL and Badylak SF:
Decellularization of tissues and organs. Biomaterials.
27:3675–3683. 2006.PubMed/NCBI
|
|
18
|
Stone KR, Abdel-Motal UM, Walgenbach AW,
Turek TJ and Galili U: Replacement of human anterior cruciate
ligaments with pig ligaments: a model for anti-non-gal antibody
response in long-term xenotransplantation. Transplantation.
83:211–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomford WW: Transmission of disease
through transplantation of musculoskeletal allografts. J Bone Joint
Surg Am. 77:1742–1754. 1995.PubMed/NCBI
|
|
20
|
Malcarney HL, Bonar F and Murrell GA:
Early inflammatory reaction after rotator cuff repair with a
porcine small intestine submucosal implant: a report of 4 cases. Am
J Sports Med. 33:907–911. 2005. View Article : Google Scholar
|
|
21
|
Elder BD, Eleswarapu SV and Athanasiou KA:
Extraction techniques for the decellularization of tissue
engineered articular cartilage constructs. Biomaterials.
30:3749–3756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhrany AD, Beckstead BL, Lang TC, Farwell
DG, Giachelli CM and Ratner BD: Development of an esophagus
acellular matrix tissue scaffold. Tissue Eng. 12:319–330. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cartmell JS and Dunn MG: Effect of
chemical treatments on tendon cellularity and mechanical
properties. J Biomed Mater Res. 49:134–140. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cartmell JS and Dunn MG: Development of
cell-seeded patellar tendon allografts for anterior cruciate
ligament reconstruction. Tissue Eng. 10:1065–1075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Courtman DW, Errett BF and Wilson GJ: The
role of crosslinking in modification of the immune response
elicited against xenogenic vascular acellular matrices. J Biomed
Mater Res. 55:576–586. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Woods T and Gratzer PF: Effectiveness of
three extraction techniques in the development of a decellularized
bone-anterior cruciate ligament-bone graft. Biomaterials.
26:7339–7349. 2005. View Article : Google Scholar : PubMed/NCBI
|