Introduction
Post-operative cognitive dysfunction (POCD), a
severe central nervous system complication, is an acute cognitive
deficit following anesthesia and surgery (1,2).
POCD may be self-limiting in the majority of patients; however, it
may affect the prognosis and life quality of certain individuals
(3,4). Therefore, reducing the incidence of
POCD is of social importance (5).
brain-derived neurotrophic factor number of studies
have suggested that there is a strong link between volatile
anesthetics, for example isoflurane, and cognitive impairment
(6–8). Emulsified isoflurane (EI) is a novel
type of anesthetic that subverts the requirement for specific
ventilatory circuits, induces rapid anesthesia and is less
environmentally polluting than inhaled isofluorane (9,10).
However, very little is known about the effects of EI on the
cognitive function of adult rats. Therefore, in the present study
the EI-induced alterations and possible mechanisms were
investigated.
In the present study, the Morris water maze was used
to test spatial learning and memory. Enzyme-linked immunosorbent
assays (ELISAs) were performed to measure the levels of plasma
corticosterone, brain-derived neurotrophic factor (BDNF) and nerve
growth factor (NGF), whilst immunohistochemistry was used to
measure BDNF and NGF expression in the hippocampus.
Materials and methods
Experimental approval
The animal protocol was approved by the
institutional Animal Care and Use Committee of Zunyi Medical
College (Zunyi, China). All animal experiments were performed in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (11).
Animal groups and anesthetic
exposure
Eight-month-old adult male Sprague Dawley (SD) rats,
weighing between 250 and 300 g, were obtained from the Laboratory
Animal Center of the Third Military Medical University (Chongqing,
China). The rats were randomly divided into six groups (12 rats in
each group): a control group, a 30% intralipid group (group E) and
four EI groups (2 h, 1 day, 7 days, 14 days following recovery from
the anesthesia induced by intravenous EI injection; 2h, 1d, 7d and
14d groups, respectively). Rats in the control group did not
receive any injection, whilst animals in the 30% intralipid group
(group E) received a single intravenous injection of 1.5 ml/kg 30%
intralipid (Xian Pharmaceutical, Ltd., Xian, China) via the vena
caudalis. Animals in the EI groups were given a single injection of
1.5 ml/kg 8% EI. As previously described (12), the loss of the tail-clamped
response and righting reflex were used as the criteria for the
anesthesia taking effect, whilst the recovery of the righting
reflex was used as the criteria for anesthesia recovery. This
method of EI application has been used in previous studies
(13,14). An 8% EI (V/V) solution was provided
by the New Drug Research Center of Sichuan University (Chengdu,
China). Briefly, 1.6 ml liquid isoflurane and 18.4 ml 30%
intralipid were mixed in a 20 ml glass ampoule. The EI ampoule was
opened immediately prior to use, and any residual drug was
discarded.
Morris water maze
Rats in the EI groups were all tested using the
Morris water maze (Chengdu Taimeng Technology Ltd., Chengdu, China)
equipped with WMT-100 maze video tracking system (Chengdu Taimeng
Software, Chengdu, China) at 2 h, 1 day, 7 days and 14 days
following EI injection, respectively. Rats in the 30% intralipid
group were subjected to water maze testing 2 h following drug
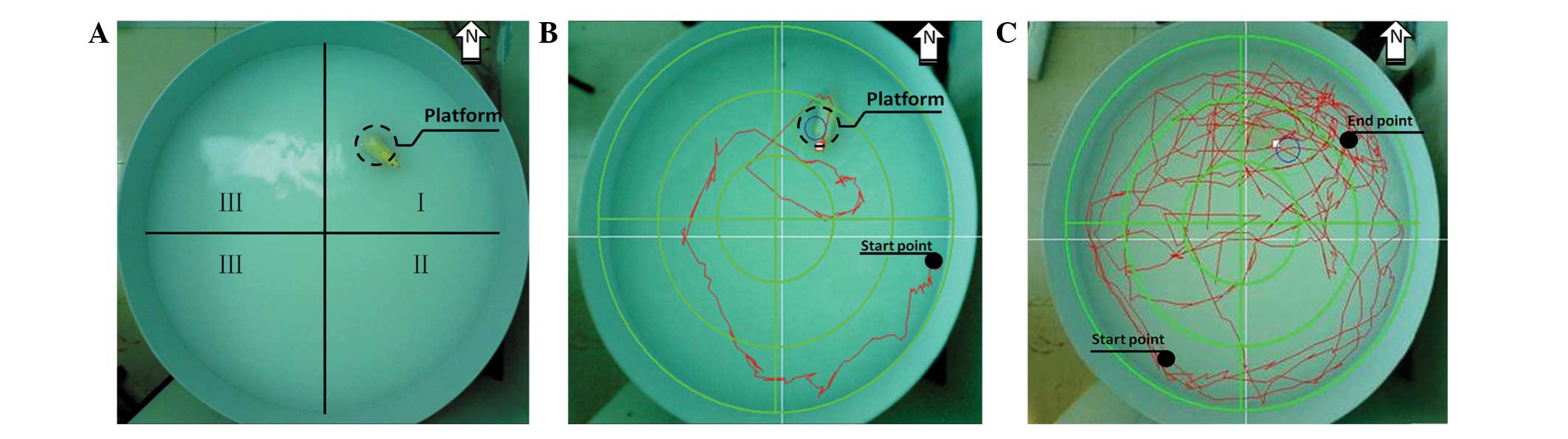
injection together with the control group. The water maze (Fig. 1A) consisted of a circular pool (120
cm in diameter and 60 cm high) and a round platform (15 cm in
diameter and 30cm high). The pool was divided into four quadrants:
north (where the platform was located), south, east and west. Water
in the pool was colored opaque with milk powder (Full Cream Milk
Powder; Nestle Shuangcheng Ltd., China) prior to each test to avoid
visual cues for the rats.
The test was performed as previously described
(15,16) with minor modifications. Each animal
was subjected to two tests: a place navigation test (Fig. 1B) and a spatial probe test
(Fig. 1C). In the place navigation
test, animals were encouraged to find the hidden platform. At the
beginning of each trial, the rats were placed into the water facing
the wall of the pool in one of the four quadrants. Each rat was
allowed 120 sec to find and mount the platform. The amount of time
spent finding and mounting the platform (escape latency) and total
swimming distance (path length) were calculated using the digital
tracking system. If the rats failed to find and mount the platform
within 120 sec, the escape latency was recorded as 120 sec. The
mean value of the results from four quadrant starting points from
12 rats in each group was used as the final result for the group.
The spatial memory of the rats was then analyzed using the spatial
probe test. The platform was removed from the pool and the starting
point was randomly selected. The swimming time in the former
platform quadrant, the percentage of swimming distance in the
target quadrant, the average swimming speed and the former platform
location passing times within 120 sec were recorded.
Brain tissue and blood sampling
Immediately following the Morris water maze
behavioral tests, rats were anesthetized with 4 mg/100 g 0.1%
sodium pentobarbital (Tianjin Damao Chemical Reagent Factory,
Tianjin, China) via intraperitoneal injection. Blood (2–4 ml) was
collected from the eye orbit of each rat and centrifuged at 300 × g
at 6°C for 15 min (Multifuge X1R; Thermo Fisher Scientific,
Waltham, MA, USA). Plasma was then collected in order to analyze
the corticosterone content by ELISA. Rats were then sacrificed
following blood sample collection. Six rats in each group were
randomly selected and hippocampi were dissected out and homogenized
(T10 basic Ultra-Turrax; IKA, Staufen, Germany). The homogenates
were then centrifuged at 900×g (0–4°C) for 15 min. The supernatant
was collected and an ELISA was used to measure the expression of
BDNF and NGF. The thoracic cavities of the remaining six rats in
each group were opened and their aortas were cannulated. The
animals were firstly perfused with 200 ml normal saline, then with
300 ml 4% paraformaldehyde (Tianjin Damao Chemical Reagent Factory)
until the extremities were rigid. The brains were then removed from
the cranial cavity and the tissues were embedded in paraffin.
Coronal sections (3 μm thick) were prepared using a freezing
microtome (Leica RM 223; Leica Instruments, Nussloch, Germany). A
total of 24 sections were obtained from each group, 12 of which
were used to determine the expression of BDNF and 12 of which were
used for analysis of the expression of NGF by
immunohistochemistry.
Analysis of plasma corticosterone, BDNF
and NGF expression using ELISA
Plasma corticosterone, hippocampal BDNF and NGF
levels were measured using corticosterone, BDNF and NGF ELISA kits
(R&D Systems, Minneapolis, MN, USA) in accordance with the
manufacturer’s instructions. Samples were immediately extracted
using the methods described above. Briefly, a double-antibody
sandwich ELISA was performed. The amount of plasma corticosterone,
BDNF and NGF was determined by measuring the absorbance at 450 nm
(ELx800; BioTek Instruments, Inc., Winooski, VT, USA). The optical
density values from the samples were then used to calculate the
concentration based on the standard curve.
Analysis of BDNF and NGF expression using
immunohistochemistry
Immunohistochemistry was conducted as previously
described (18–20) with minor modifications. Sections
were incubated overnight at 4°C with mouse anti-BDNF antibody
immunoglobulin G (IgG; 1:200; Beijing Bioss Biotechnology, Beijing,
China) or with mouse anti-NGF antibody IgG (1:100; Beijing Bioss
Biotechnology), followed by incubation with biotinylated mouse
secondary antibody (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China). The secondary antibody was amplified using an
streptavidin-biotin complex (SABC) kit (Wuhan Boster Biological
Technology, Ltd.). The complexes were then visualized using 0.03%
diaminobenzidine (Wuhan Boster Biological Technology, Ltd.), and
the sections were mounted onto gelatin-coated slides. The slides
were air dried overnight at room temperature. Coverslips were
mounted using Permount™ Mounting Medium (Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). The area in the selected
region of the hippocampus was measured using Image-Pro Plus
software (Leica CW 4000; Leica Instruments). The mean density (MD)
of BDNF-positive and NGF-positive cells in the hippocampi was
counted. Four distinct views were chosen from each region of the
hippocampal CA1, CA2, CA3 and DG regions, and the mean value from
the four views was used as the result for the corresponding region.
The mean value from 12 sections from each group was used as the
final result for that group.
Statistical analysis
The data in the present study, including the results
from the physiological tests, behavioral tests, ELISA and
immunohistochemistry, were parametric. They are presented as the
mean ± standard deviation. Data from the different groups of
animals were analyzed using a one way analysis of variance followed
by the Student-Newman-Keuls test following confirmation of normal
distribution of the data using SPSS software version 17.0 (SPSS
Inc., Chicago, IL, USA). One way repeated measures analysis of
variance was used for the comparisons of the values from the same
animals at different time-points. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of a single injection of EI on the
cognitive function of rats
The adult SD rats started to manifest restlessness
following the EI injection (0.1–0.2 ml) and all the rats were
clearly anesthetized. The anesthesia recovery time of the SD rats
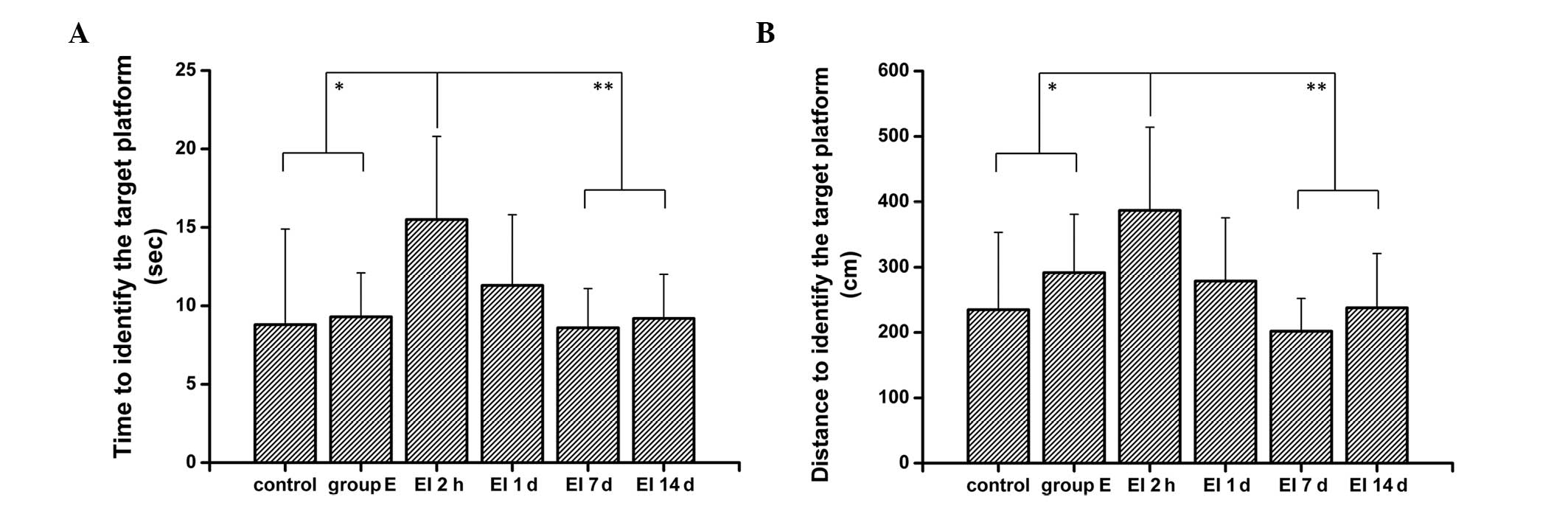
was 46.5±12.3 sec (P<0.05) in the present study. As shown in
Fig. 2, in the place navigation
test, the rats in the 2h group spent significantly more time and
had a longer path length to find the platform compared with rats in
the control and the E groups (P<0.01). These results suggest
that the rats developed cognitive dysfunction following the EI
injection. There was also a significant increase in the escape
latency and path length of the 2h group compared with the 1d, 7d
and 14 groups (P<0.05). However, there was no significant
difference in the escape latency and path length among the control,
the E, and the 1d, 7d and 14 groups (P>0.05). Therefore, these
results suggest that the EI-induced cognitive dysfunction may be
reversed in a relatively short period of time and will not cause
long-term damage to the cognitive function of animals.
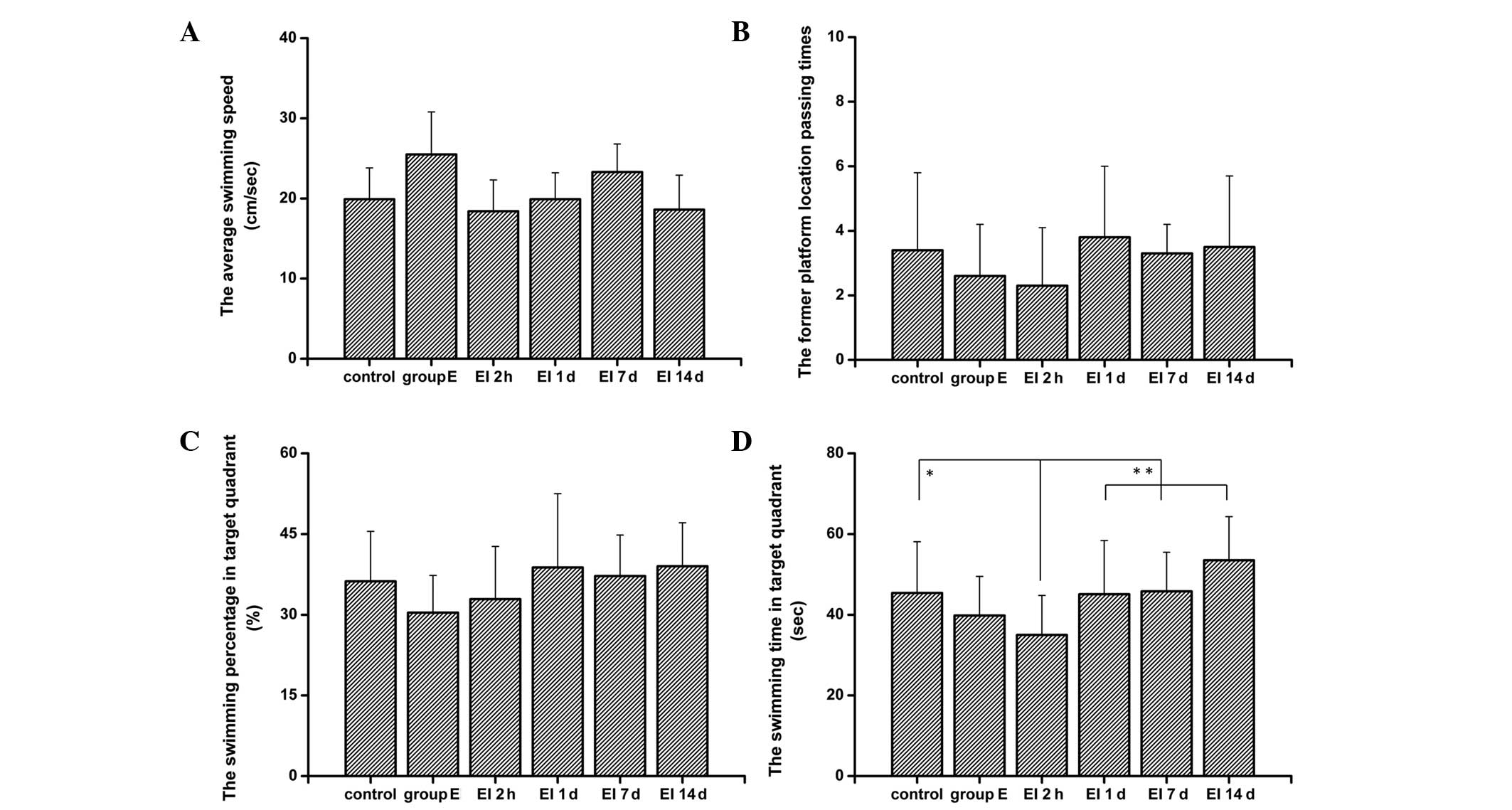
The results from the spatial probe test (Fig. 3) revealed that there were no
significant differences between rats in each test group in the
platform quadrant swimming time, the average swimming speed and the
platform passing times. Similar to the results from the place
navigation test, rats in the 2h group spent less time in the target
quadrant than the control group and the 1d, 7d and 14d groups did
(P<0.01, compared with control group; P<0.05 compared with
the 1d, 7d and 14 groups).
Cognitive dysfunction may not be due to
plasma corticosterone levels
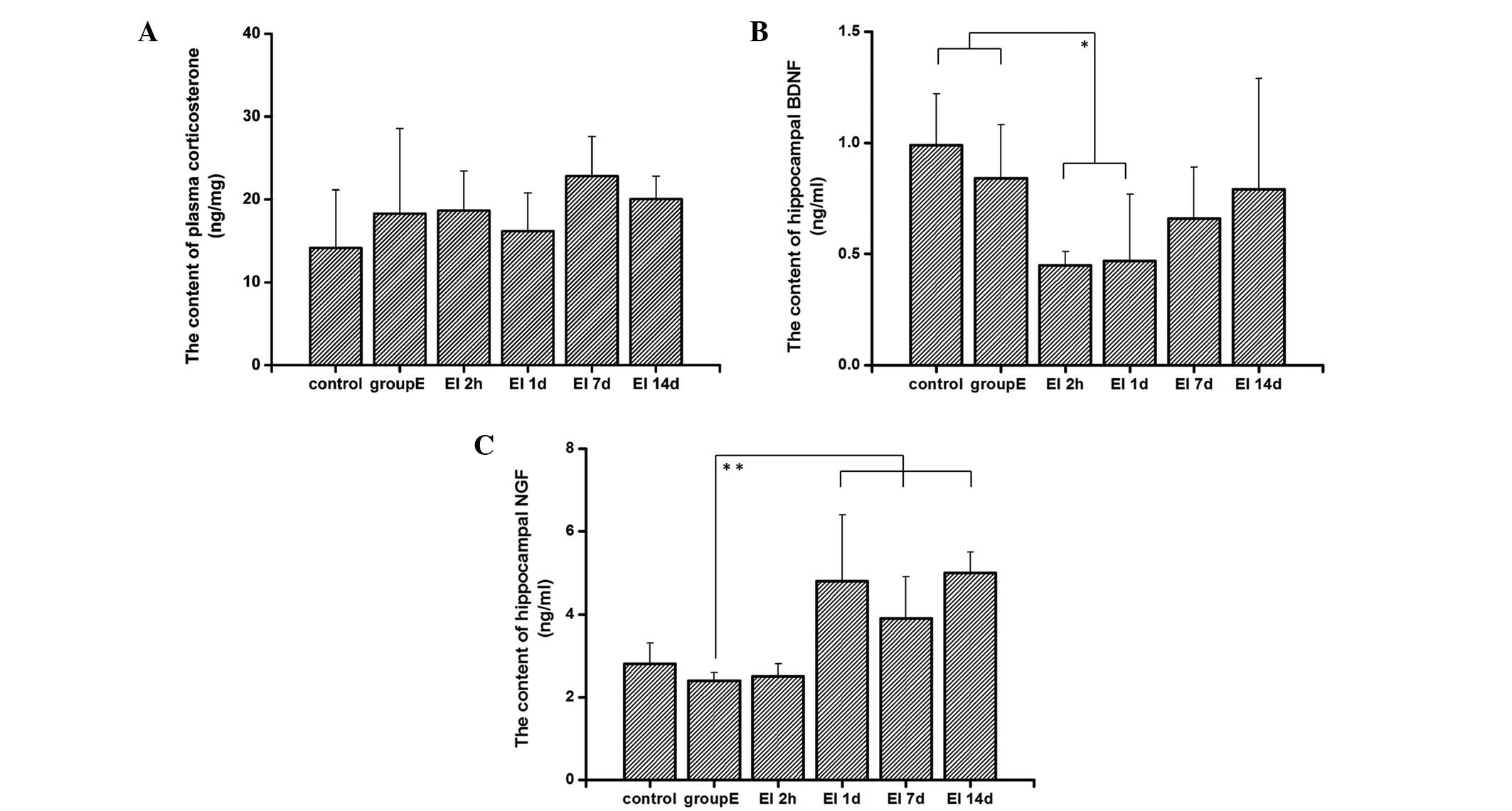
The results from the present study showed that rats
in the control group had lower levels of plasma corticosterone
compared with the other groups. This may be due to the stress
reaction as a result of the drug injection. However, there was no
significant difference between the plasma corticosterone levels of
rats in different groups (Fig.
4A). This suggests that plasma corticosterone levels were not
affected by EI anesthesia, indicating that corticosterone levels do
not have a role in EI-induced cognitive dysfunction.
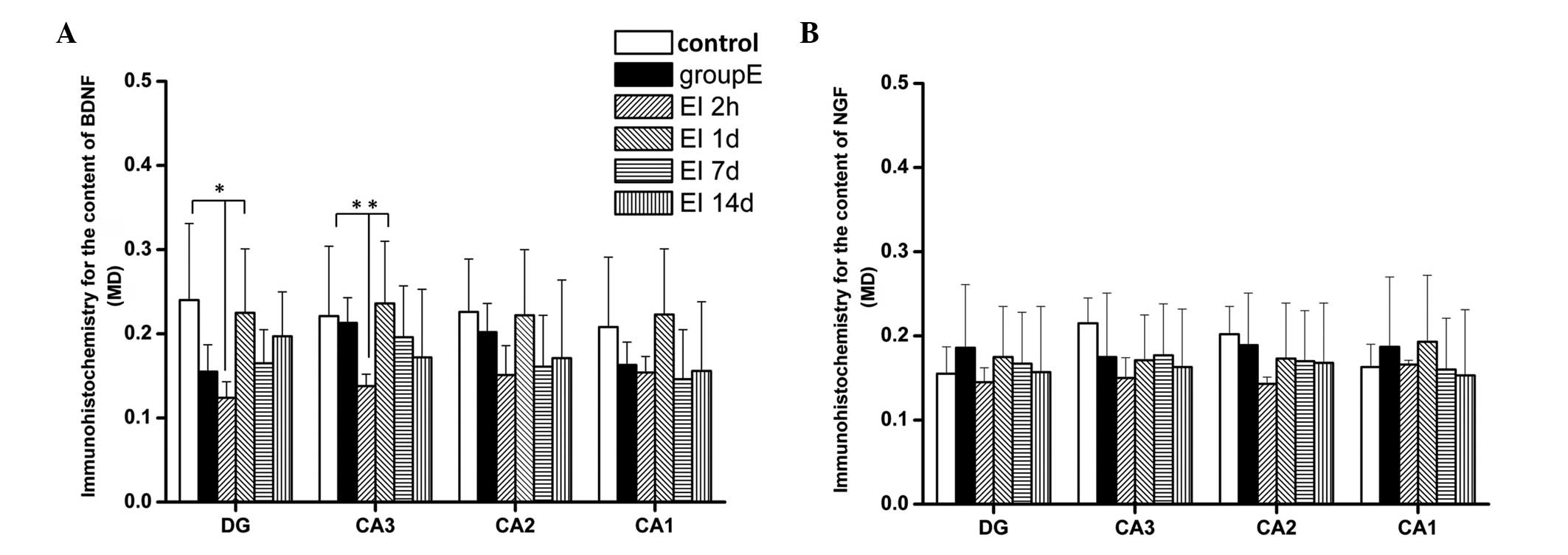
Levels of BDNF were decreased in the
hippocampi of rats
As shown in Fig.
4B, the BDNF levels in the hippocampi of rats in the 2h and 1d
groups were significantly lower compared with those of the control
group (P<0.05). There were increases of BDNF levels in the 7d
and 14d groups compared with those in the 2h and in the 1d group.
The BDNF levels in the 7d and 14d groups showed no difference from
those in the control and group E (P>0.05). These results suggest
that EI injection causes a reduction of BDNF expression in the
hippocampus, which may be responsible for the cognitive impairment
observed in SD rats following EI anesthesia. The lowest levels of
BDNF expression were observed 2 h following recovery from
anesthesia; however, the BDNF levels then returned to previous
levels.
Expression levels of NGF were increased
in the hippocampi of rats
As shown in Fig.
4C, the levels of NGF were increased 1 day following recovery
from the EI injection and the increase was sustained; the levels of
NGF in groups 1d, 7d and 14d all showed an increase compared with
those in the control, E and 2h groups (P<0.05). These results
indicate that NGF, as a nerve protective factor, starts to restore
nerve functions following EI injection and possibly attenuates the
reduction of BDNF expression, consequently promoting the recovery
progress of EI-induced cognitive impairment.
Effect of EI on the expression of BDNF
and NGF detected using immunohistochemistry
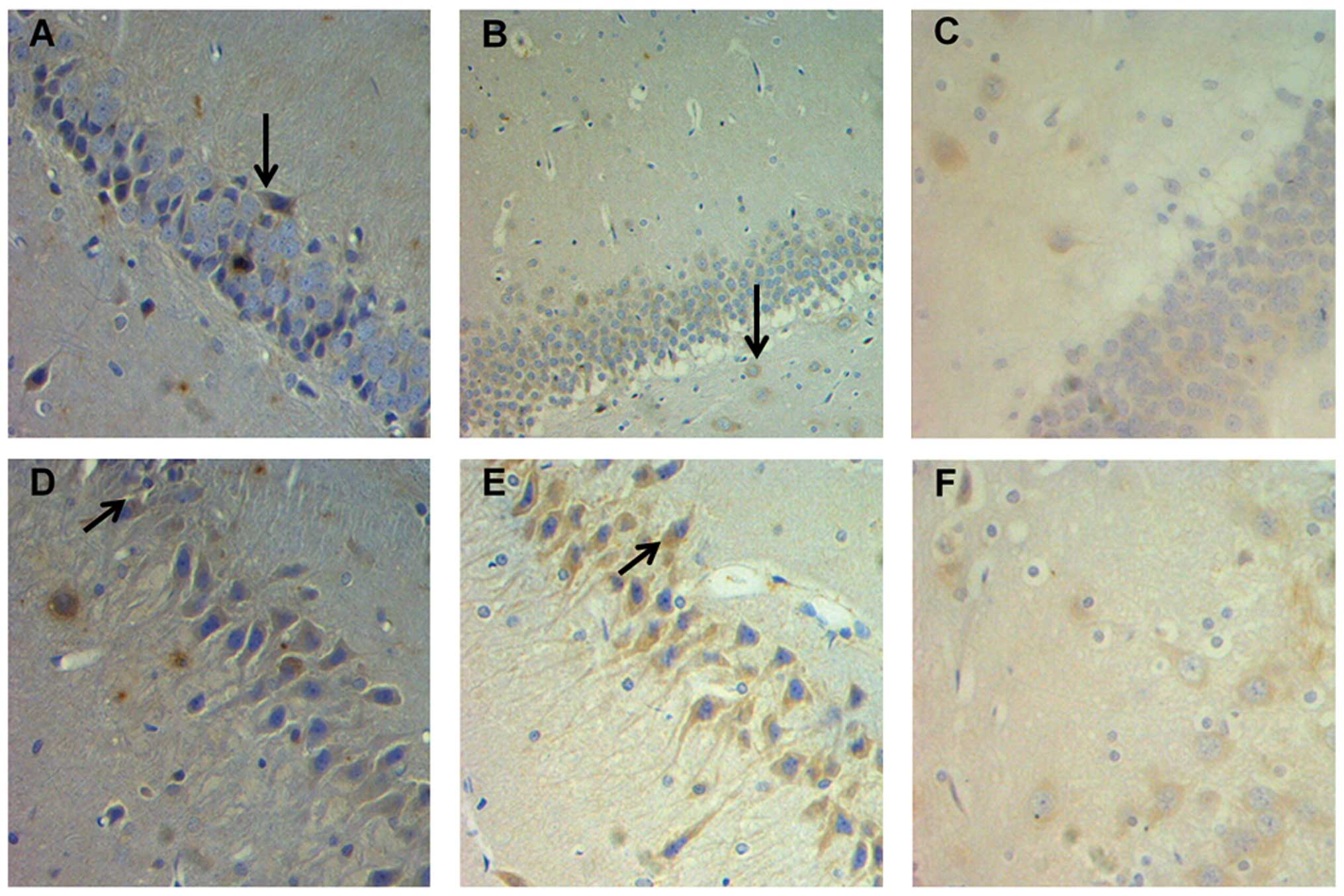
The results from the immunohistochemical analysis
revealed (Fig. 5 and 6) that the MDs of BDNF-positive cells in
the DG and CA3 region in the 2h group were significantly lower
compared with those in the control and 1d groups (P<0.05). There
were no significant differences in BDNF expression in the CA2 and
CA1 regions among the groups (P>0.05). There was also no
significant difference between the BDNF levels in different brain
areas in the 1d, 7d and 14d groups (P>0.05). Similar to the
results observed from the ELISA test, EI exposure markedly
decreased the levels of BDNF in the hippocampal DG and CA3 region 2
h following EI anesthesia recovery. There were no significant
differences among all experimental groups with regards to NGF
expression in the different hippocampal regions (P>0.05).
Discussion
The Morris water maze is a test developed by the
British psychologist Richard G. Morris in the 1980s to assess
spatial memory and learning of rodents, which has become one of the
‘gold standards’ of behavioral neuroscience (20). The Morris water maze usually
consists of two parts: the place navigation test and the spatial
probe test. The place navigation test reflects the spatial learning
ability of animals; escape latency and path length have been shown
to be negatively correlated with spatial learning ability. The
spatial probe test is used to determine the spatial association and
reference memory. Swimming time in the former platform quadrant,
the percentage of swimming distance in target quadrant, the average
swimming speed and the former platform location passing times
within 120 sec are positively correlated with reference memory
ability (21).
The results from this experiment in the present
study demonstrated that EI exposure may cause transient deficits in
water maze performance. Compared with those in the control group
and the E group, the escape latency and path length to the target
quadrant was prolonged 2 h after the EI injection. In addition, the
time that rats were in the quadrant where the platform had been
located was shortened. These results indicate that the learning and
memory ability decline may be due to the effect of the EI
injection. However, 1 day following EI injection, the Morris water
maze results showed no significant difference compared with those
in the E and the control groups. Several previous studies have
shown impairments of learning and memory following exposure to
isoflurane inhalation anesthesia and also found that these
isoflurane-induced cognitive deficits are reversible (22–25).
The results from the present study are in accordance with these
previous studies and they suggest that the cognitive function of
rats may completely recover to pre-anesthesia levels. It was shown
in the present study that EI-induced cognitive impairment is not a
persistent and irreversible process. No difference was observed
between the results from the Morris water maze from the 7d and 14d
groups compared with the control and E groups, suggesting that
after two weeks of recovery, the cognitive function of rats has
already recovered to a stable stage.
The central nervous system mainly contains
mineralocorticoid receptors and glucocorticoid receptors (GRs). The
hippocampus is a brain area crucial for memory storage and is very
vulnerable to the effects of glucocorticoids due to its high levels
of GRs. The high levels of corticosterone generated as a stress
response bind to GRs. The activated GRs then induce the
downregulation of nerve survival genes and the upregulation of
nerve cell apoptosis genes, therefore leading to the reduction of
hippocampal nerve cell synapses and the induction of the apoptosis
process of hippocampal nerve cells and cognitive dysfunction
(26). Previous studies have shown
that external or internal corticosterone reduces BDNF expression.
Exogenous dexamethasone downregulates the expression of the
tyrosine kinase receptor, reduces phospholipase and Ca2+
channel activation and causes learning and memory dysfunction
(27,28). However, the results from the
present study demonstrate that there were no significant
differences in plasma corticosterone levels among all groups
(P>0.05), suggesting that the EI-induced cognitive dysfunction
was not due to an increase in plasma corticosterone levels.
BDNF and NGF are important in the development,
survival and maintenance of neurons in the central nervous system
(29). The hippocampus has a key
role in memory and spatial location since it is an important area
of the brain required for learning and memory. Previous studies
have demonstrated that the downregulation of BDNF and NGF in the
brain may result in memory and learning deficits (30,31).
The results from the present study showed that the
MD of BDNF-positive cells decreased in the DG and CA3 regions of
the hippocampus 2 h following EI injection, which is in accordance
with the results obtained from the ELISA, indicating that the
reduction in BDNF caused by anesthesia toxicity is a cause of the
EI-induced cognitive dysfunction. The results also demonstrated
that there was a marked increase and recovery of BDNF expression
levels from 1 day following anesthesia, which eventually reached
normal levels. There was no significant difference observed between
BDNF concentrations in the 7d and 14d groups compared with those in
the control and E groups, indicating that the EI-induced cognitive
dysfunction was reversible.
In the present study, the effect of emulsified
isoflurane on the cognitive function of rats was investigated for
the first time, to the best of our knowledge. It was found that a
single injection of EI caused transient cognitive impairment in
rats, which may be due to the downregulation BDNF expression, which
is similar to the results observed with isoflurane. However, the
effect of repeated intravenous EI administration on cognitive
function requires further investigation.
Acknowledgements
This study was supported by a grant ([2007]2132) to
Professor Zhao-Qiong Zhu from the Guizhou Science and Technology
Department, (Guizhou, China) and a grant (2010001) to Professor
Zhao-Qiong Zhu from the Joint Research Program, (Shandong,
China).
References
|
1
|
Li X, Wen DX, Zhao YH, Hang YN and Mandell
MS: Increase of beta-amyloid and C-reactive protein in liver
transplant recipients with postoperative cognitive dysfunction.
Hepatobiliary Pancreat Dis Int. 12:370–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudolph JL and Marcantonio ER: Review
articles: postoperative delirium: acute change with long-term
implications. Anesth Analg. 112:1202–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rudolph JL, Marcantonio ER, Culley DJ, et
al: Delirium is associated with early postoperative cognitive
dysfunction. Anesthesia. 63:941–947. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steinmetz J, Christensen KB, Lund T, Lohse
N and Rasmussen LS; ISPOCD Group. Long term consequences of
postoperative cognitive dysfunction. Anesthesiology. 110:548–555.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang G, Ward C, Peng J, Zhao Y, Huang B
and Wei H: Isoflurane causes greater neurodegeneration than an
equivalent exposure of sevoflurane in the developing brain of
neonatal mice. Anesthesiology. 112:1325–1334. 2010. View Article : Google Scholar
|
|
6
|
Braunecker S and Hinkelbein J: Isoflurane
is not necessarily the only cause of cognitive deficits. Eur J
Anaesthesiol. 30:432013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Callaway Jk, Jones NC and Royse CF:
Isoflurane induces cognitive deficits in the Morris water maze task
in rats. Eur J Anaesthesiol. 29:239–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin D and Zuo Z: Isoflurane induces
hippocampal cell injury and cognitive impairments in adult rats.
Neuropharmacology. 61:1354–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu ZY, Luo NF and Liu J: The protective
effects of emulsified isoflurane on myocardial ischemia and
reperfusion injury in rats. Can J Anaesth. 56:115–125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lucchinetti E, Schaub MC and Zaugg M:
Emulsified intravenous versus evaporated inhaled isoflurane for
heart protection: old wine in a new bottle or true innovation?
Anesth Analg. 106:1346–1349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Institutes of Health. Guide for
the Care and Use of Laboratory Animals. 8th edition. Bethesda, MD,
USA: 2011
|
|
12
|
Miller RD, Zeng YM and Deng XM: Miller’s
Anesthesia. Inhaled anesthesia. 6th edition. Peking University
Medical Press; Beijing: pp. 109–110. 2006, (In Chinese).
|
|
13
|
Lv X, Wang ZM, Huang SD, Song SH, Wu FX
and Yu WF: Emulsified isoflurane preconditioning reduces lung
injury induced by hepatic ischemia/reperfusion in rats. Int J Med
Sci. 8:353–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XL, Ma HX, Yang ZB, et al: Comparison
of minimum alveolar concentration between intravenous isoflurane
lipid emulsion and inhaled isoflurane in dogs. Anesthesiology.
104:482–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clausen F, Lewén A, Marklund N, Olsson Y,
McAuthor DL and Hillered L: Correlation of hippocampal
morphological changes and Morris water maze performance after
cortical contusion injury in rats. Neurosurgery. 57:154–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar
|
|
17
|
Abelson KS, Adem B, Royo F, Carlsson HE
and Hau J: High plasma corticosterone levels persist during
frequent automatic blood sampling in rats. In Vivo. 19:815–819.
2005.PubMed/NCBI
|
|
18
|
Kashyap M, Kawamorita N, Tyaqi V, et al:
Down-regulation of nerve growth factor expression in the bladder by
antisense oligonucleotides as new treatment for overactive bladder.
J Urol. 190:757–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, DU X, Xu Y, Dang L, Xiang L and
Zhang J: The effects of Gouqi extracts on Morris maze learning in
the APP/PS1 double transgenic mouse model of Alzheimer’s disease.
Exp Ther Med. 5:1528–1530. 2013.PubMed/NCBI
|
|
20
|
Bromley-Brits K, Deng Y and Song W: Morris
water maze test for learning and memory deficits in Alzheimer’s
disease model mice. J Vis Exp. 53:e29202011.
|
|
21
|
Vorhees CV and Williams TM: Morris water
maze: procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Butterfield NN, Graf P, Ries CR and
MacLeod BA: The effect of repeated isoflurane anesthesia on spatial
and psychomotor performance in young and aged mice. Anesth Analg.
98:1305–1311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crosby C, Culley DJ, Baxter MG, Yukhananov
R and Crosby G: Spatial memory performance 2 weeks after general
anesthesia in adult rats. Anesth Analg. 101:1389–1392. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee S, Park SH and Zuo Z: Effects of
isoflurane on learning and memory functions of wild-type and
glutamate transporter type 3 knockout mice. J Pharm Pharmacol.
64:302–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin D, Cao L, Wang Z, Li J, Washington JM
and Zuo Z: Lidocaine attenuates cognitive impairment after
isoflurane anesthesia in old rats. Behav Brain Res. 228:319–327.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yau JL, Noble J and Seckl JR:
11beta-hydroxysteroid dehydrogenase type 1 deficiency prevents
memory deficits with aging by switching from glucocorticoid
receptor to mineralocorticoid receptor-mediated cognitive control.
J Neurosci. 31:4188–4193. 2011. View Article : Google Scholar
|
|
27
|
Choy KH, de Visser Y, Nichols NR and van
den Buuse M: Combined neonatal stress and young-adult
glucocorticoid stimulation in rats reduce BDNF expression in
hippocampus: effects on learning and memory. Hippocampus.
18:655–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Numakawa T, Kumamaru E, Adachi N, Yagasaki
Y, Izumi A and Kunuqi H: Glucocorticoid receptor interaction with
TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate
release via a glutamate transporter. Proc Natl Acad Sci USA.
106:647–652. 2009. View Article : Google Scholar
|
|
29
|
Henriksson BG, Söderström S, Gower AJ,
Ebendal T, Winblad B and Mohammed AH: Hippocampal nerve growth
factor levels are related to spatial learning ability in aged rats.
Behav Brain Res. 48:15–20. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Conner JM, Franks KM, Titterness AK, et
al: NGF is essential for hippocampal plasticity and learning. J
Neurosci. 29:10883–10889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li B, Arime Y, Hall FS, Uhi GR and Sora I:
Impaired spatial working memory and decreased frontal cortex BDNF
protein level in dopamine transporter knockout mice. Eur J
Pharmacol. 628:104–107. 2010. View Article : Google Scholar : PubMed/NCBI
|