Introduction
Alzheimer’s disease (AD) is an age-associated
neurodegenerative disorder characterized by progressive loss of
memory and cognition, with mortality occurring approximately a
decade following diagnosis (1).
During the pathogenesis of AD, patients exhibit a slow progression
from spatial/episodic memory dysfunction, to a complete decline of
cognitive function, at which point they are dependent on
caregivers. Presently, there is no cure for AD, and current
treatments only offer limited symptomatic benefit (2).
One pharmacological strategy for AD is the use of
acetylcholinesterase inhibitors (AChEIs), which inhibit the
breakdown of acetylcholine (ACh), and therefore increase its
concentration in neuronal synapses to compensate for the loss of
cholinergic neurons, which is characteristic of AD (2). Despite the reported benefits of
AChEIs for AD, their use is limited since they have severe
side-effects (3). Therefore, a
greater understanding of the underlying pathophysiological
mechanisms may help identify more effective and innovative
treatment strategies, and thus the development of novel drugs.
Chinese herbal medicines have been widely used for
thousands of years in China and other Asian countries. In the
clinical practice of traditional Chinese medicine (TCM), the
modification of an original formulation by the addition or
substitution of herbs according to the condition of the patient is
common to enhance the efficacy of the original formulation
(4). The majority of TCMs have not
resulted in any serious side-effects. Furthermore, since the
mechanism of action involves multiple components, pathways and
targets, TCMs may have significant advantages compared with
single-component drugs for the treatment of multifactor, complex
chronic diseases, including AD (5). Specific TCM formulations have been
shown to be effective against numerous cognitive disorders. For
example, Fructus Akebiae (Akebia fruit; FAE), the fruit of a
plant widely distributed throughout China, has been used for the
treatment of mental disorders. A number of studies have used FAE as
the major ingredient in complex prescriptions for the treatment of
mental disorders and cognitive and behavioral deficits, including
insomnia, memory loss, paraphasia, phobia and depression (6). A previous study identified that the
genus Akebia contains more than thirty types of triterpenoid
saponins, and the majority of these compounds are derivatives of
the triterpenoid hederagenin (7).
Several studies have focused on the biological activity of FAE,
particularly on its antidepressant-like activity. However, little
is known about its effects on cognition and memory in vitro
and in vivo (6).
In animal studies, the muscarinic cholinergic
receptor antagonist, scopolamine, has been widely used to induce
partial amnesia as a model for dementia (8,9).
Therefore, the present study aimed to investigate the effect of FAE
on learning and memory impairment in scopolamine-induced mouse and
rat models of dementia via tests including the Morris water maze
(MWM) task for mice and novel object recognition test for rats.
Materials and methods
Preparation of FAE
The fat was removed from FAE dry fruit powder (500
g) twice by ultrasonication in petroleum ether at room temperature,
as previously described (10).
Fructus Akebiae was obtained from Beijing Tongrentang Medicine
Facility, Beijing, China (batch #: 701001532-1). The plant was
harvested from Jiangsu Province, China. The solvent was
volatilized, and the coarse powder was extracted twice by
recirculation for 1 h with 2 l 80% ethanol (11). The extract (500 ml) was
concentrated under reduced pressure and then stored overnight at
room temperature followed by filtration. As previously described by
Wain et al (12), water was
added to the filtrate, which was then extracted three times with
800 ml ethyl acetate and four times with 800 ml water-saturated
n-butanol (n-BuOH). n-BuOH was recycled under reduced pressure and
saponins were obtained. A total of 5 g of the saponins was degraded
for 3 h with 60 ml HCl (2 mol/l) in 45% ethanol (EtOH) at 100°C.
Following filtration and further washes with water to remove the
acid, the degraded saponin was dried via vacuum, resulting in crude
crystals. These crystals were then dissolved in 300 ml hot 70% EtOH
and decolored by heating-recirculation with 1 g activated carbon
for 30 min, followed by filtration at 50°C. The filtrate was
concentrated for 12 h at 4°C under reduced pressure to obtain
clustered crystals. These crystals were then filtered, and chloride
ion was removed via washing with water, followed by drying in a
vacuum to form a white powder. The yield of the final extract was
~0.5% (w/w). The powder was stored at 4°C until use.
Animals
Sprague-Dawley rats and ICR mice were provided by
the Animal Breeding Center affiliated with Beijing Institute of
Pharmacology (Beijing, China). The animals were kept in polyacrylic
cages (6 mice per cage; 1 rat per cage) and maintained under
standard housing conditions (24–27°C and 60–65% humidity) on a 12 h
light:dark cycle, with food (dry pellets) and water available ad
libitum. However, food was not allowed during the experiment.
The use and care of animals was followed according to the guide for
laboratory animals of the National Institutes of Health (2010,
Bethesda, MD, USA).
Materials
Scopolamine hydrobromide (Sigma Aldrich, Milan,
Italy) was dissolved in 0.9% saline and administered
intraperitoneally (i.p.) to mice and rats 10 min prior to testing
at 1 or 10 mg/kg. The FAE extracts (13) were suspended in 5% EtOH
(Sigma-Aldrich) in 95% water for oral administration (p.o.) at 2.5,
5, or 10 mg/kg (n=6 animals per treatment), and administered 40 min
prior to behavioral tests. All other reagents were purchased from
Beijing Chemical Reagent Co., Ltd. (Beijing, China).
MWM task
The MWM task was performed for the assessment of
spatial learning and memory, as previously described (14), with some modifications. Briefly, a
white circular tank (130 cm diameter and 50 cm high) with a
featureless inner surface was used. The pool was filled with opaque
water (23±2°C) to a depth of 30 cm. The target platform (10 cm
diameter and 31 cm high) was submerged so that 1 cm of the platform
was above the water surface. The platform remained in a fixed
position at the midpoint of one quadrant throughout the training
phase. Training consisted of two daily sessions of four consecutive
60-sec trials, each with a 15-sec inter-trial interval, during
which mice were placed in the pool facing the wall, from various
starting points and allowed to swim freely to the escape platform.
If mice failed to find the platform within the allocated 60 sec,
they were guided to the platform by the experimenter. The trial
ended as soon as the animal climbed on the platform and remained on
it for ≥2 sec. Following each session, each mouse was allowed to
remain on the platform for 20 sec prior to being placed in a heated
chamber. To accelerate the training, an extra trial was added prior
to the first session, in which mice were placed on the hidden
platform for 60 sec. Animals were trained until they were able to
reach the escape platform in <20 sec. In the single probe trial
(performed 24 h following the last training session), the platform
was removed and each mouse was allowed to swim in the maze for 60
sec while their behavior was recorded by an automated activity
monitoring system (ANY-maze video tracking; Stoelting Co., Wood
Dale, IL, USA) and the percentage of time spent in the platform
area was calculated. FAE and scopolamine treatments (15) were administered 40 and 20 min,
respectively, prior to the first trial (T1) and the probe
trial.
Novel object recognition test
Male Sprague-Dawley rats were housed in the
experimental room and regularly handled for 1 week prior to this
experiment to test for episodic memory. The test apparatus, a black
Perspex box with sawdust bedding, (70×50×40 cm) was indirectly
illuminated with a 50 W halogen lamp. Metal triangular (8.5×5×14
cm) and rectangular prisms (5×5×14 cm) were used as objects to be
discriminated. On day 1, rats were allowed to explore the test
apparatus without objects for 150 sec. Two identical objects were
then introduced after 24 h for exploration (sample trial, T1).
Object exploration was determined when the rat sniffed and/or
touched the object <2 cm from its nose, and when the two objects
were explored for ≥10 sec, the sample trial was terminated by
moving the rat to its home cage. Retrieval was examined 24 h
following T1 (for example, choice trial, T2), when rats were
allowed to explore a novel and familiar object for 4 min. The two
objects were randomly placed to reduce the potential effect of
place or object preference. FAE or vehicle was administered 40 min
prior to T1. The behavioral characteristics and exploration time
was videotaped and measured, respectively. The total amount of time
spent exploring the novel object (N) and the familiar object (F)
was used to calculate the discrimination index (N-F/N+F) in T2.
Each acquisition trial was performed 10 min following the
administration of a single scopolamine treatment (2 mg/kg,
i.p.).
Passive avoidance task
This learning and memory test was performed in two
chambers, which were square boxes, identical size (12×10×12 cm),
juxtaposed as illuminated and dark. A lamp (50 W) was placed 1 m
above one chamber for illumination. Each test involved two separate
trials, a training trial and a test trial. For the training trial,
the mice were initially placed in the illuminated chamber. When the
mouse entered the dark chamber, an electrical shock (0.5 mA) for 3
sec was delivered through stainless steel rods. The latency times
once the mice had entered the dark compartment were measured using
a stopwatch. A test trial was performed 24 h following the training
trial, and latency times to re-enter the dark chamber were measured
up to 300 sec. FAE or vehicle were administered 40 min prior to the
acquisition trials. Each acquisition trial was performed 10 min
following a single scopolamine treatment (2 mg/kg, i.p.).
Step-down test
This test was used to measure inhibitory avoidance.
The apparatus comprised a plastic chamber (12×12×18 cm) with an
elevated rubber platform (4.8×4.8×4.5 cm) placed on the left side
wall. The floor was made of caliber stainless steel bars (0.1 cm in
length) placed in parallel, 0.5 cm apart. Mice were housed in a
dimly lit room for ≥30 min prior to the experiment. On the first
training day, mice were exposed to a 5-min learning course, during
which they were permitted to move freely throughout the chamber
prior to being placed on the platform. If the animals stepped down
from the platform (i.e. an error trial), they were exposed to an
electric foot shock (36 V, AC). After 24 h, latency was reassessed
and recorded as the learning grade (latency), which was taken as a
measure of memory retention. Each acquisition trial was performed
10 min after a single scopolamine treatment (2 mg/kg, i.p.). FAE or
vehicle were administered 30 min prior to the trials.
T-maze task
This task was used to evaluate spatial memory (via
spontaneous alternation behavior), as previously described by Amico
et al (16), with slight
modifications. The maze (Ugo Basile, Comerio, Italy) was made of a
non-reflective base plate and plastic arms (28×5×10 cm). Training
consisted of one single session, which started with one
forced-choice trial, followed by 14 free-choice trials. In the
forced-choice trial, either the left or right goal arm was blocked
by a guillotine door. Once the animal was released from the
starting arm, it was allowed to explore the maze by entering the
open goal arm and returning to the start position where it was
confined for 5 sec by the lowering of the guillotine door. During
the 14 free-choice trials, the animal was free to choose between
the left and right goal arm. Immediately upon entering one goal
arm, the other goal arm closed, and once the mouse returned to the
starting arm, the next free-choice trial started following a 5-sec
restraint in the starting arm. Animals were removed from the maze
as soon as the 14 free-choice trials were performed or 15 min had
elapsed. The series of arm entries were recorded visually, and the
alternations were calculated as a percentage of the actual
alternations/the total possible alternations. Animals that did not
complete the test within 15 min were excluded from the analyses
since they were considered to exhibit poor exploratory behavior.
The T-maze task was performed 40 and 20 min following FAE and
scopolamine injections, respectively.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean, and were analyzed using a one-way analysis of variance
followed by the Tukey’s post-hoc test. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using GraphPad Prism version 5 (Graph Pad
Software, San Diego, CA, USA).
Results
T-maze and MWM task
In the T-maze task, the percentage of alternations
was found to be positively correlated with the cognitive ability of
the animals (Fig. 1A). Scopolamine
significantly (P<0.001) reduced the percentage of alternations
compared with that of the vehicle controls. However, administration
of 5 and 10 mg/kg FAE markedly reversed scopolamine-induced memory
deficits (P<0.01). No effect was observed in the control
following FAE treatment at these two doses. In the MWM task, the
time spent in the correct quadrant was significantly decreased in
scopolamine-treated mice compared with that of the vehicle
(control) group (P<0.001), and treatment with FAE (5 and 10
mg/kg) was found to markedly reverse this effect (P<0.01).
Treatment with FAE at all three doses had no effect on the control
mice.
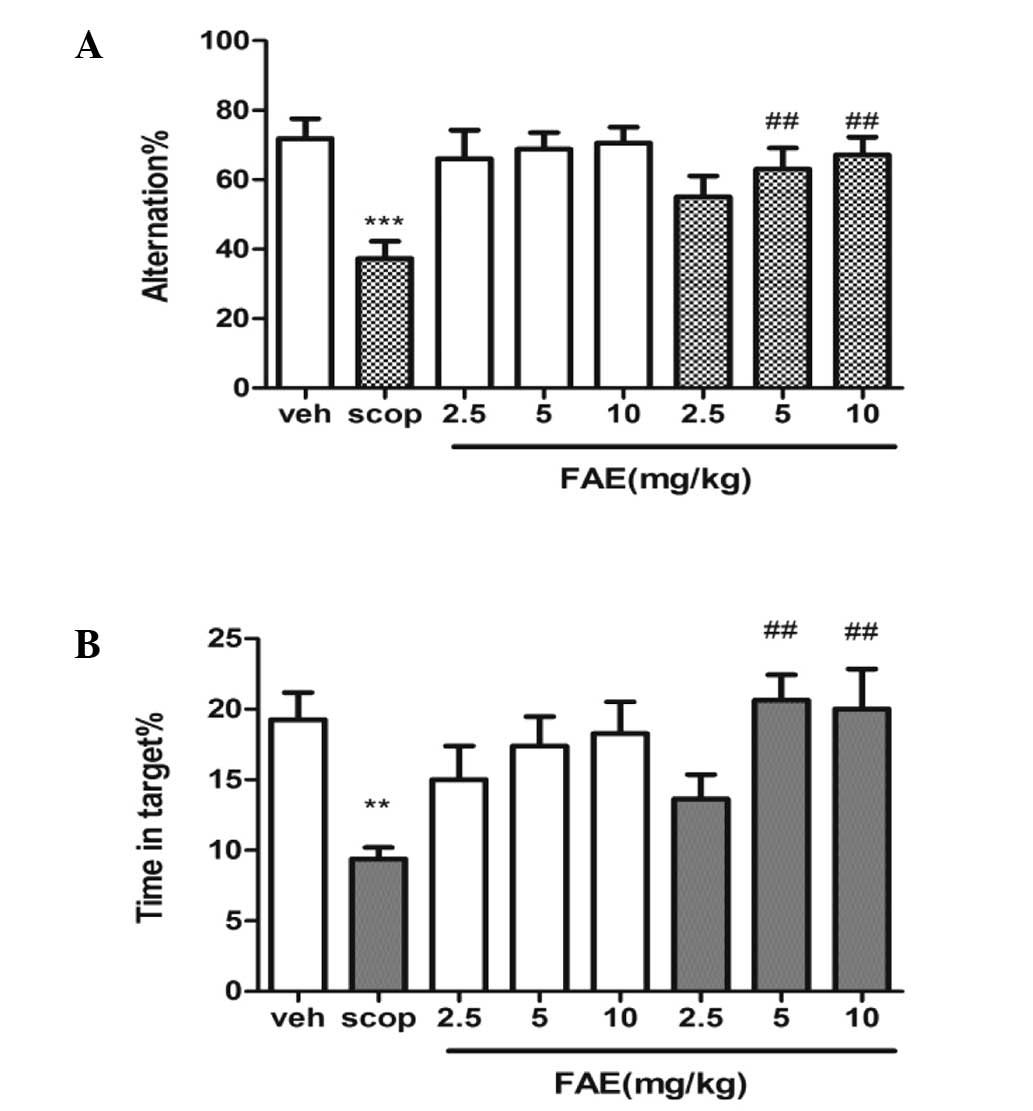 | Figure 1FAE rescues scopolamine-induced
spatial memory deficits. The effect of FAE treatment on (A)
spontaneous alternation behavior (T-maze task) and (B) MWM in a
scopolamine-induced amnesia model in mice. In the T-maze, FAE
[(2.5, 5 and 10 mg/kg, orally (p.o.)] was administered 20 min prior
to scopolamine [10 mg/kg, intraperitoneally (i.p.)]; and in the
MWM, FAE (2.5, 5 and 10 mg/kg, p.o.) was administered 20 min prior
to scopolamine (2 mg/kg, i.p.). Data are expressed as mean ±
standard error of the mean. Statistical analysis was performed
using a one-way analysis of variance, followed by post-hoc Tukey’s
test where appropriate, ***P<0.001 vs. controls
(veh); **P<0.001 vs. controls (veh);
##P<0.01 vs. scopolamine-treated animals (scop). FAE,
Fructus Akebiae; MWM, Morris water maze. |
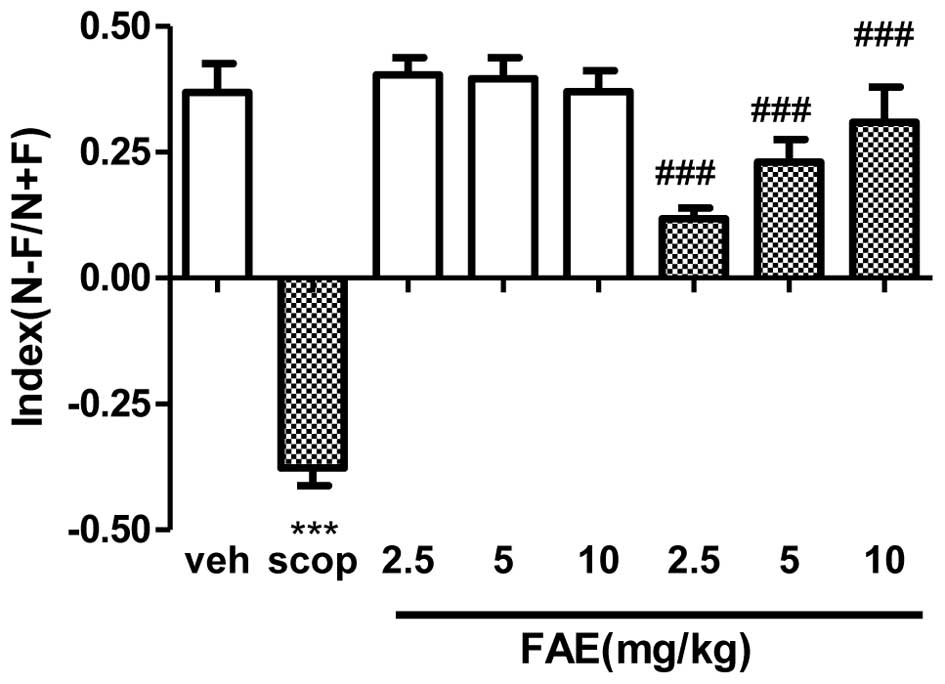
Novel object recognition test
During the adaptation phase to the open field box on
day 1, all animals exhibited similar locomotor activity and anxiety
levels. Furthermore, all rats equally explored the familiar objects
during T1. Vehicle-treated animals exhibited a positive index
(0.46±0.02) during T2, indicating a good recognition memory
(Fig. 2). However,
scopolamine-treated rats showed a significant (P<0.001) negative
index (−0.41±0.12) compared with the vehicle group, indicating a
poor exploration of the object types. This effect was fully
(P<0.001) reversed by all doses of FAE. The discrimination index
was not significantly altered in the vehicle-treated control
animals following treatment with FAE.
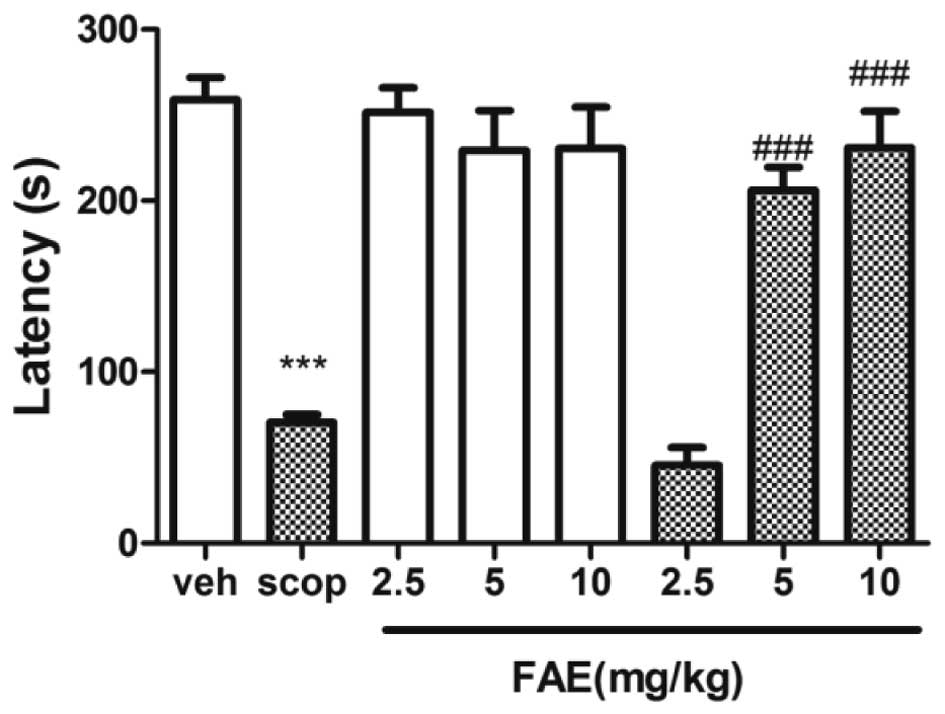
Passive avoidance task
The step-through latency of scopolamine-treated mice
was significantly (P<0.001) shorter than that of vehicle-treated
mice (Fig. 3). The retention
latency during the pre-training phase was shortened by scopolamine
treatment. This response was significantly (P<0.01) reversed by
5 and 10 mg/kg FAE.
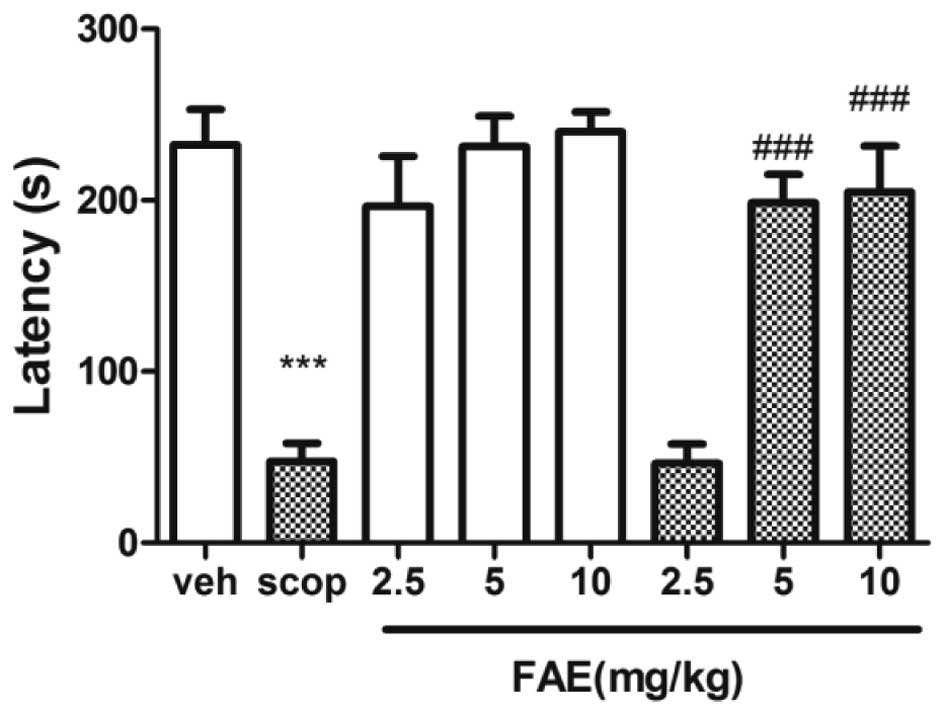
Step-down test
The step-down latency of scopolamine-treated mice
was significantly (P<0.01) shorter compared with that of the
vehicle-treated controls (Fig. 4).
This effect was significantly (P<0.001) reversed by 5 and 10
mg/kg FAE.
Discussion
Traditional Chinese herbs and other plants have been
increasingly recognized as effective natural materials for the
treatment of diseases and the alleviation of symptoms. Certain
traditional herbs may effectively treat specific cognitive
disorders, as well as manage cognitive decline during aging
(17). A number of studies have
demonstrated that FAE ameliorates aging-associated cognitive
deterioration, cerebral ischemia and chemical injury to the brain
(18,19). In the present study, the
cognitive-enhancing activity of FAE in a scopolamine model of
dementia in mice and rats was investigated.
Cholinergic neurons have a major role in cognitive
function, and their loss from the hippocampus is a characteristic
feature of AD. Scopolamine impairs learning and memory in rodents
and humans. It is used to create an experimental model of memory
impairment and has been extensively used to screen for drugs that
have potential therapeutic effect in dementia (20,21).
Scopolamine has been shown to cause reversible symptoms of senile
dementia in younger individuals (22). The passive avoidance task, novel
object recognition test, step-down test and MWM task are used to
evaluate learning and memory impairment in animals (14,23,24).
Early symptoms of AD include deficits in short-term
episodic memory, attention and spatial orientation (25,26).
Episodic memory is a type of declarative memory that depends on the
ability to remember in a determined temporal and spatial context
(27), and is particularly
vulnerable to normal aging and dementia (20). Spatial memory is a subtype of
episodic memory, storing past information of events within the
spatiotemporal frame (14). To
evaluate the effect of FAE on short- and long-term spatial memory,
the T-maze and the MWM task were used, as previously described by
Gacar et al (14). In the
two behavioral tests, scopolamine induced a marked decline in
cognitive performance, and FAE (5 and 10 mg/kg) was found to
significantly attenuate this effect.
To further investigate the effect of FAE on other
types of memory, the novel object recognition test and the passive
avoidance task were performed. The novel object recognition test
evaluates recognition memory (28), whilst the passive avoidance task is
dependent on the amygdala and evaluates emotional memory. Passive
avoidance has been associated with long-term or reference memory
and has been used to study learning and memory following a
stressful stimulus (29). In these
behavioral tests, treatment with FAE (5 and 10 mg/kg) was shown to
prevent scopolamine-mediated cognitive impairment.
The present study clearly demonstrates that FAE
significantly attenuates scopolamine-mediated cognitive impairment.
In conclusion, the results suggest that FAE may be a potential
novel therapeutic strategy for the treatment of dementia.
References
|
1
|
Schaeffer EL, Figueiro M and Gattaz WF:
Insights into Alzheimer disease pathogenesis from studies in
transgenic animal models. Clinics (Sao Paulo). 66(Suppl 1): 45–54.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jakob-Roetne R and Jacobsen H: Alzheimer’s
disease: from pathology to therapeutic approaches. Angew Chem Int
Ed Engl. 48:3030–3059. 2009.
|
|
3
|
Casey DA, Antimisiaris D and O’Brien J:
Drugs for Alzheimer’s disease: are they effective? PT. 35:208–211.
2011.
|
|
4
|
Zhu L, Zhang L, Zhan L, et al: The effects
of Zibu Piyin Recipe components on scopolamine-induced learning and
memory impairment in the mouse. J Ethnopharmacol. 151:576–582.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi X, Lu XG, Zhan LB, et al: The effects
of the Chinese medicine ZiBu PiYin recipe on the hippocampus in a
rat model of diabetes-associated cognitive decline: a proteomic
analysis. Diabetologia. 54:1888–1899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin ZL, Gao N, Zhou D, Chi MG, Yang XM and
Xu JP: The extracts of Fructus Akebiae, a preparation containing
90% of the active ingredient hederagenin: serotonin, norepinephrine
and dopamine reuptake inhibitor. Pharmacol Biochem Behav.
100:431–439. 2012.PubMed/NCBI
|
|
7
|
Zhou D, Jin H, Lin HB, et al:
Antidepressant effect of the extracts from Fructus Akebiae.
Pharmacol Biochem Behav. 94:488–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, Iwasaki K, Suzuki T, et al:
Potentiation of brain acetylcholine neurons by Kami-Untan-To (KUT)
in aged mice: implications for a possible antidementia drug.
Phytomedicine. 7:253–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barr AM, Powell SB, Markou A and Geyer MA:
Iloperidone reduces sensorimotor gating deficits in pharmacological
models, but not a developmental model, of disrupted prepulse
inhibition in rats. Neuropharmacology. 51:457–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen CJ, Kubota M, Brachman PS, et al;
Abacavir-Lamivudine Once-Daily HIV Assessment (ALOHA) Study Group.
Short-term safety and tolerability of a once-daily fixed-dose
abacavir-lamivudine combination versus twice-daily dosing of
abacavir and lamivudine as separate components: findings from the
ALOHA study. Pharmacotherapy. 28:314–322. 2008. View Article : Google Scholar
|
|
11
|
Wen RT, Zhang M, Qin WJ, et al: The
phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol
seeking and consumption in alcohol-preferring Fawn-Hooded rats.
Alcohol Clin Exp Res. 36:2157–2167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wain LV, Verwoert GC, O’Reilly PF, et al:
Genome-wide association study identifies six new loci influencing
pulse pressure and mean arterial pressure. Nat Genet. 43:1005–1011.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pradhan M, Raffaelli RM, Lind C, et al:
Successful deceased donor renal transplant in a sensitized
pediatric recipient with the use of plasmapheresis. Pediatr
Transplant. 12:711–716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gacar N, Mutlu O, Utkan T, Komsuoglu CI,
Gocmez SS and Ulak G: Beneficial effects of resveratrol on
scopolamine but not mecamylamine induced memory impairment in the
passive avoidance and Morris water maze tests in rats. Pharmacol
Biochem Behav. 99:316–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Figueiro M, Ilha J, Linck VM, et al: The
Amazonian herbal Marapuama attenuates cognitive impairment and
neuroglial degeneration in a mouse Alzheimer model. Phytomedicine.
18:327–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amico F1, Spowart-Manning L, Anwyl R and
Rowan MJ: Performance- and task-dependent effects of the dopamine
D1/D5 receptor agonist SKF 38393 on learning and memory in the rat.
Eur J Pharmocol. 577:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Howes MJ and Houghton PJ: Plants used in
Chinese and Indian traditional medicine for improvement of memory
and cognitive function. Pharmacol Biochem Behav. 75:513–527. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hatip-Al-Khatib I, Egashira N, Mishima K,
et al: Determination of the effectiveness of components of the
herbal medicine Toki-Shakuyaku-San and fractions of Angelica
acutiloba in improving the scopolamine-induced impairment of
rat’s spatial cognition in eight-armed radial maze test. J
Pharmacol Sci. 96:33–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizushima Y, Kan S, Yoshida S, Irie Y and
Urata Y: Effect of Choto-san, a Kampo medicine, on impairment of
passive avoidance performance in senescence accelerated mouse
(SAM). Phytother Res. 17:542–545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma S, Rakoczy S and Brown-Borg H:
Assessment of spatial memory in mice. Life Sci. 87:521–536. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saxena G, Singh SP, Agrawal R and Nath C:
Effect of donepezil and tacrine on oxidative stress in
intracerebral streptozotocin-induced model of dementia in mice. Eur
J Pharmacol. 581:283–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prohovnik I, Arnold SE, Smith G and Lucas
LR: Physostigmine reversal of scopolamine-induced hypofrontality. J
Cereb Blood Flow Metab. 17:220–228. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JH, Hahm DH, Lee HJ, Pyun KH and Shim
I: Acori graminei rhizoma ameliorated ibotenic acid-induced amnesia
in rats. Evid Based Complement Alternat Med. 6:457–464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Utkan T, Gocmez SS, Regunathan S and
Aricioglu F: Agmatine, a metabolite of L-arginine, reverses
scopolamine-induced learning and memory impairment in rats.
Pharmacol Biochem Behav. 102:578–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Capurro V, Busquet P, Lopes JP, et al:
Pharmacological characterization of memoquin, a multi-target
compound for the treatment of Alzheimer’s disease. PLoS One.
8:e568702013.PubMed/NCBI
|
|
26
|
Snowden JS, Thompson JC, Stopford CL, et
al: The clinical diagnosis of early-onset dementias: diagnostic
accuracy and clinicopathological relationships. Brain.
134:2478–2492. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaminsky CA and DeRosa DV: Influence of
retrieval cues and set organization on short-term recognition
memory. J Exp Psychol. 96:449–454. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dere E, Huston JP and De Souza SMA: The
pharmacology, neuroanatomy and neurogenetics of one-trial object
recognition in rodents. Neurosci Biobehav Rev. 31:673–704. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuji M, Takeda H and Matsumiya T:
Modulation of passive avoidance in mice by the 5-HT1A receptor
agonist flesinoxan: comparison with the benzodiazepine receptor
agonist diazepam. Neuropsychopharmacology. 28:664–674. 2003.
View Article : Google Scholar : PubMed/NCBI
|