Introduction
In sarcoidosis (SARC), a Th1 immune reaction
predominates (1), while in
extrinsic allergic alveolitis (EAA), Th2 immunity, associated with
an allergen exposure, is primarily involved (2,3).
Despite having a different etiopathogenesis, morphologically, the
two etiologies share certain markers, including granulomas,
interstitial lymphocyte infiltration and fibrosis, making a
differential diagnosis difficult even with histopathological
investigation. While SARC can be diagnosed by an endoscopical
transbronchial biopsy of the lung parenchyma and an endobronchial
ultrasound-guided transbronchial needle aspiration of the
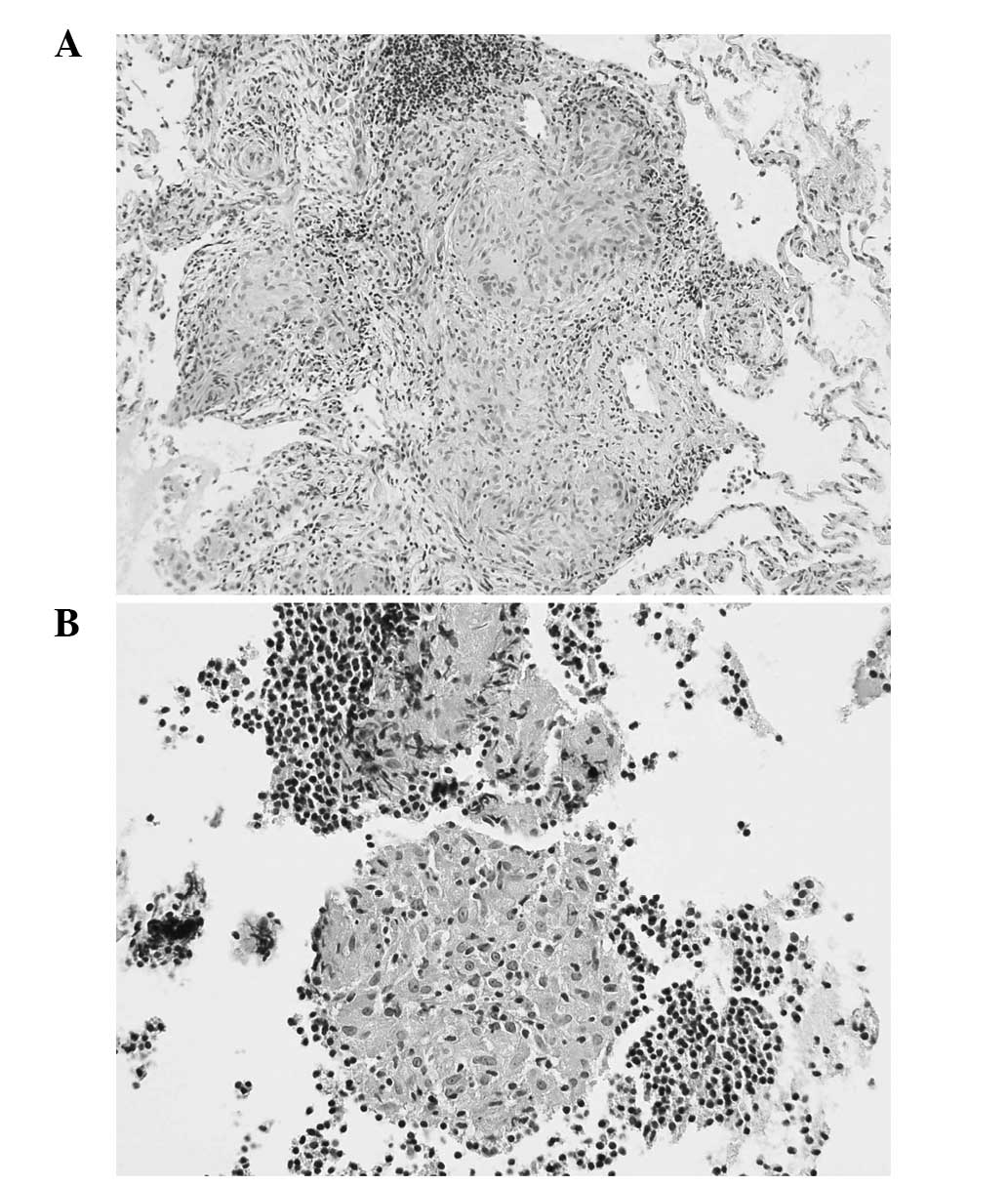
mediastinal lymphatic nodes (Fig.
1; sample from subject belonging to target SARC cohort),
histopathological confirmation of EAA often requires a surgical
biopsy.
Proteinase-activated receptors (PARs) are ubiquitous
surface molecules directly interconnecting immunity and coagulation
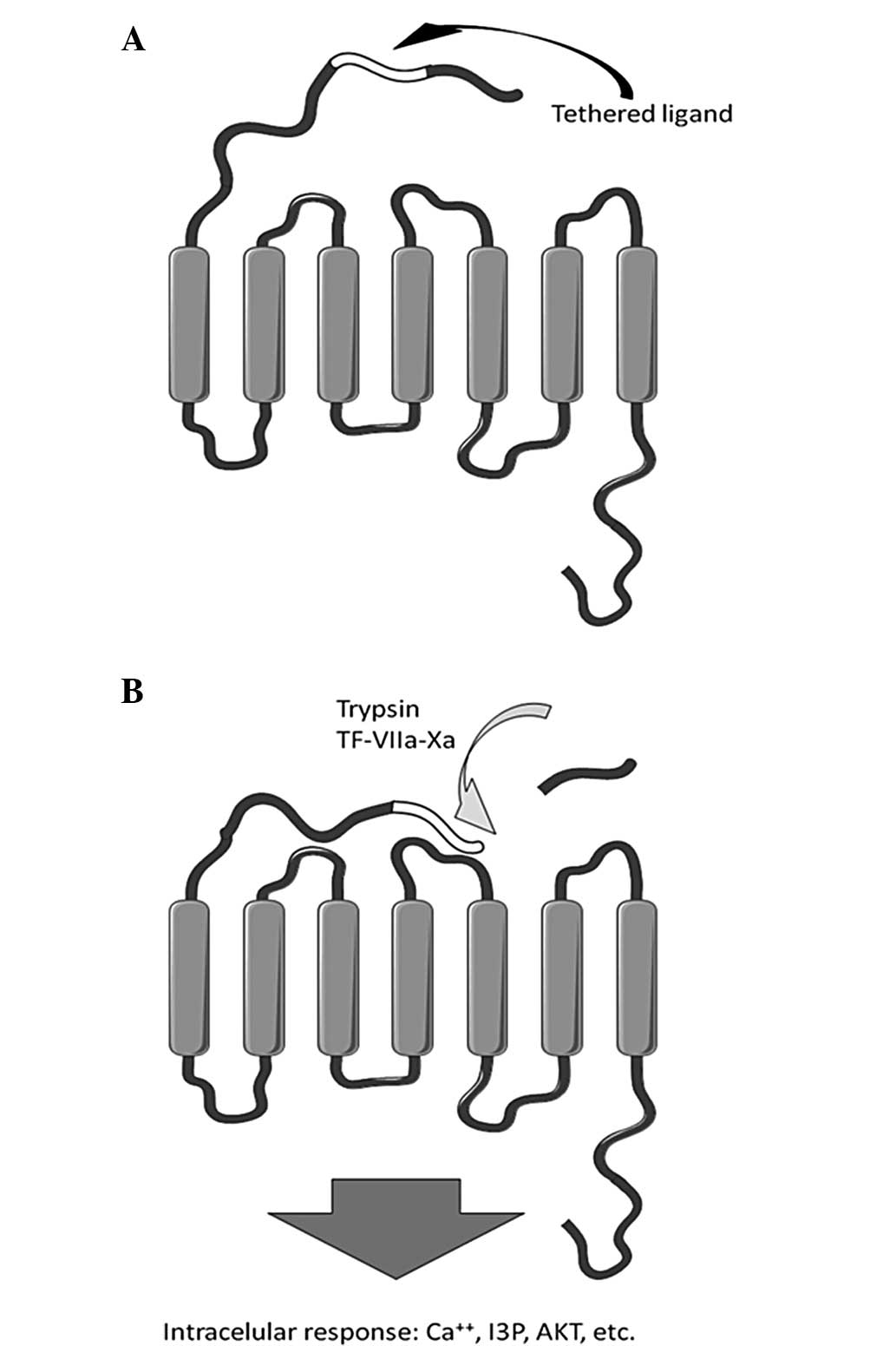
(4,5). PARs belong to a family of G
protein-coupled receptors, activated by tethered ligand sequences
within the N-terminal, that are accessible following site specific
cleavage of the protein (6,7), as
demonstrated in Fig. 2. PAR-1,
PAR-3 and PAR-4, among others, are activated by the plasma
coagulation factor, thrombin, while PAR-2 is activated by trypsin,
tryptase and a complex of coagulation factors, including tissue
factor/VIIa/Xa (8).
In lung tissue, PAR-2 is predominantly investigated
with regard to epithelial and inflammatory perspectives. In the
lungs, PAR-2 is also a target for mast cell tryptase, Alternaria
alternata serine proteinases and fungal asthmagens (9).
Associations among PAR-2, interleukin-4 receptor
(IL-4R), transforming growth factor (TGF)-β and thymic stromal
lymphoprotein (TSLP) have already been investigated in bronchial
asthma, chronic obstructive pulmonary disease (COPD) (10) and idiopathic lung fibrosis
(11). PAR-2 has been shown to
induce (12) TSLP, which is a
known inducer of Th2 naive T cell differentiation via dendritic
cell maturation (13,14). In addition, TSLP and Alternaria
alternata-induced production in bronchial epithelial cells via
PAR-2 activation, is synergically enhanced by IL-4 (12). There are no available data directly
connecting PAR-2, IL-4 and TSLP in the involvement of alveolar
epithelium in EAA or SARC, however, there is a high probability
that this autocrine/paracrine loop may contribute to the
upregulation of IL-4 in these nosologies (15). There is marked evidence that a
number of additional enzymes and their receptors are involved in
these complex processes; which raises the question of whether their
role is primary or unspecific and secondarily-induced. However, it
has recently been reported that TGF-β stimulates PAR-2 production
in human lung fibroblasts (16),
which demonstrates its role in the pathophysiology of idiopathic
pulmonary fibrosis. The evidence indicates that TGF-β generally
induces PAR-2 overexpression, regulating fibrosis and scar
formation (17). However, higher
levels of TGF-β have been observed in bronchial asthma and COPD
patients (18), as well as in SARC
(19) and EAA (20). In the two diseases, tumor necrosis
factor (TNF)-α also plays an important proinflammatory role;
alveolar macrophages are the main source of this cytokine (21). In an in vitro model of
alternatively activated macrophages, lipopolysacharide-induced
PAR-2 activation suppressed the mRNA expression of proinflammatory
cytokines, including TNF-α (22),
with a feedback loop to the previously mentioned IL-4. By contrast,
PAR-2 activation together with parallel ovalbumin exposure leads to
TNF-dependent allergic sensitization (23).
Materials and methods
Subjects
A total of 20 patients were enrolled in the study.
All the individuals were outpatients of the Department of
Respiratory Medicine at Thomayer Hospital (Prague, Czech Republic).
The patients underwent a bronchoscopic investigation as part of a
differential diagnosis for interstitial lung disease with
bronchoalveolar lavage fluid (BALF) analysis.
In total, six patients (mean age, 44.7 years; male,
4; female, 2) were diagnosed with SARC, according to the American
Thoracic Society/European Respiratory Society/World Association of
Sarcoidosis and Granulomatous Disorders statement on SARC (24). The diagnosis was based on patient
history, clinical symptoms, standard chest radiography, high
resolution computed tomography (HRCT) and laboratory tests (serum
angiotensin converting enzyme, calcemia and calciuria). All the
patients underwent a transbronchial biopsy, transbronchial lymph
node puncture or video-thoracoscopic lung biopsy with
histopathological evidence supporting the diagnosis of SARC. For
histopatology, 10% formalin-fixed, paraffin-emebeded biopsy samples
were cut to microscopic tissue slices, 5 μm thick,
xylene-deparaffined, ethanol-rehydrated and according to standard
protocol stained with hematoxylin and eosin (HE).
The diagnosis of EAA in 14 patients (mean age, 56.2
years; male, 7; female, 7) was based on the history of exposure to
a suspect antigen, the typical clinical course, HRCT radiological
observations compatible with EAA, the BALF cell count and levels of
specific IgG to the suspect antigen.
All the patients signed an informed consent form
prior to the start of the study. The study design and the informed
consent form were approved by the Central Ethical Committee of the
Thomayer Hospital and the Institute for Clinical and Experimental
Medicine (Prague, Czech Republic). Additionally, all data were
analyzed with respect for patient privacy.
BALF collection
BALF collection was performed during the fiber-optic
bronchoscopy under local anesthesia. Five 50-ml fractions of
lukewarm saline were instilled into the segmental section of the
middle lobe where the bronchoscope was wedged. The fluid was
retrieved using syringe suction and mixed in a container prior to
being divided for further investigation. The sample was determined
to be valid if the recovery was >20 ml per fraction and a
significant mixture of polymorphic bronchial epithelial cells was
not identified.
ELISA
Concentrations of particular analytes in the BALF
were determined using the ELISA method. The kits were purchased
from Uscn Life Science, Inc. (Wuhan, China). Reactions were
conducted in microtiter plate wells that had been precoated with
monoclonal antibodies (mAb) specific for the examined analyte
(IL-4R, E92031Hu; PAR-2, E90852Hu; TGF-β1, E90124Hu; TNF-α,
E90133Hu). A labeled polyclonal antibody was intended to bind to
the mAb-analyte complex. Following the reaction with the substrate
solution, the process was terminated. The colored products that
were formed were measured with a vertical colorimeter (EL800;
Bio-Tek Instruments, Inc., Winooski, VT, USA) and the concentration
of the analyte in the samples was determined using a standard
curve.
Statistical analysis
Data were collected from the two groups consisting
of 14 EAA patients and six SARC patients. The differences in 25
basic and derived characteristics were analyzed with a standard
two-sample, two-sided t-test, where P<0.05 was considered to
indicate a statistically significant difference. In cases of
multiple testing, the Bonferroni correction was used as required.
In addition, the false discovery rate (FDR) methodology was used
for the 25 independent tests and a corrected critical level of
0.004 [2 × (0.05/25)] was calculated, which resulted in only two
significant differences. All the calculations were performed using
MATLAB 7.8.0 Statistical Toolbox (Mathworks Inc., Natick, MA, USA,
2009).
Results
Higher parameters in EAA
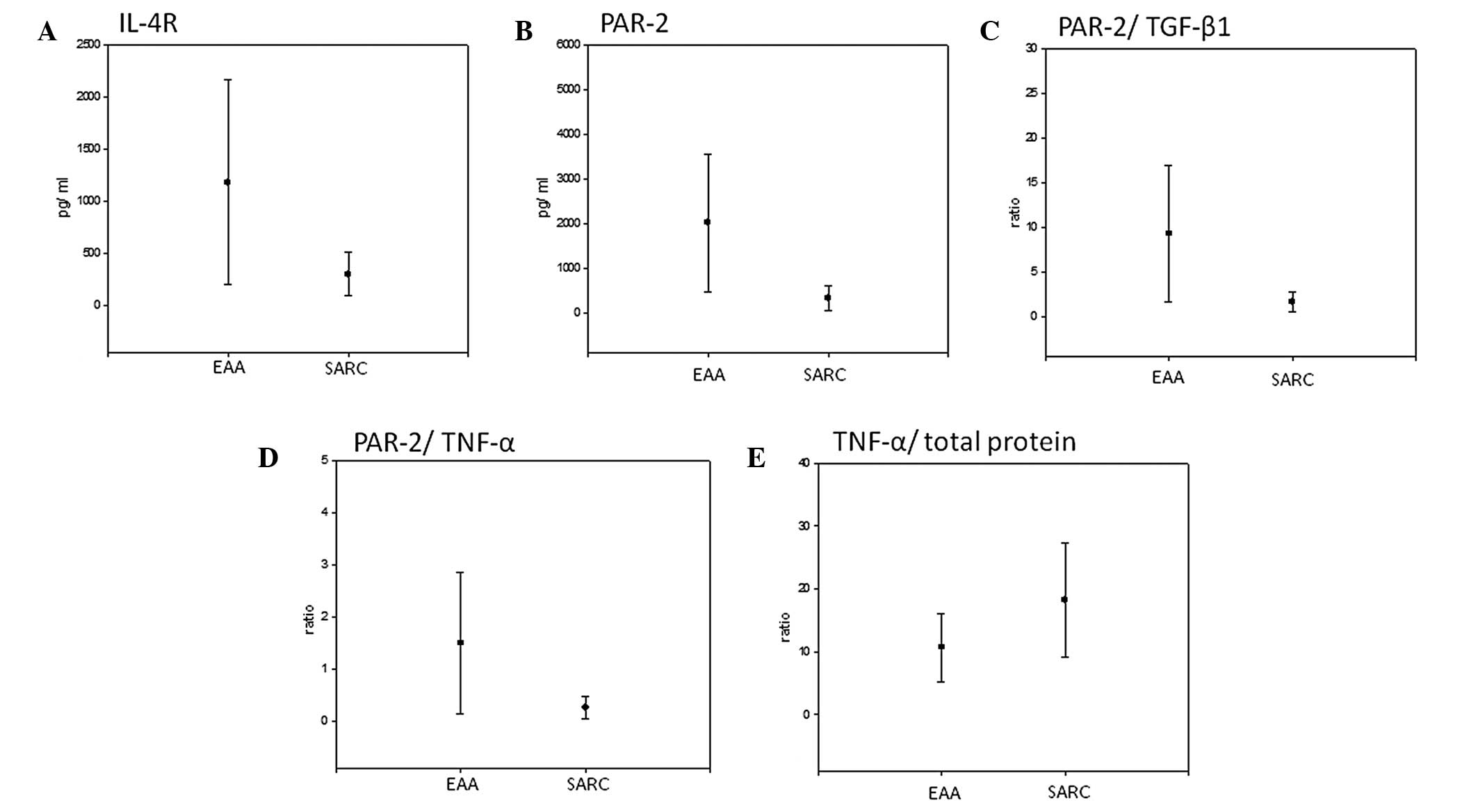
Statistically significant higher levels of IL-4R
(1182.7 pg/ml vs. 302.7 pg/ml; P=0.046; Fig. 3A), PAR-2 (2009.4 pg/ml vs. 329.5
pg/ml; P=0.018; Fig. 3B) and the
PAR-2/TGF-β1 (9.29 vs. 1.61; P=0.026, Fig. 3C) and PAR-2/TNF-α ratios (1.5 vs.
0.26; P=0.042; Fig. 3D) were
identified in EAA patients as compared with SARC patients. All the
tested characteristics, average values per group and respective
P-values are shown in Table I.
 | Table IStatistical analysis of the basic and
derived characteristics (n=25). |
Table I
Statistical analysis of the basic and
derived characteristics (n=25).
| Characteristic | Average EAA value
(pg/ml) | Average SARC value
(pg/ml) | P-value |
|---|
| Total protein | 209.4286 | 80.5667 | 0.16239 |
| IL-4R | 1182.7071 | 302.7167 | 0.045507a |
| PAR-2 | 2009.4143 | 329.5333 | 0.017622a |
| TGF-β | 227.8143 | 194.0333 | 0.40364 |
| TNF-α | 1381.9357 | 1292.2333 | 0.6782 |
| Total
protein/IL-4R | 1.2195 | 0.70294 | 0.57665 |
| Total
protein/PAR-2 | 0.30052 | 0.32913 | 0.92454 |
| Total
protein/TGF-β | 1.0184 | 0.40749 | 0.18103 |
| Total
protein/TNF-α | 0.15603 | 0.065155 | 0.22735 |
| IL-4R/total
protein | 8.1408 | 4.6233 | 0.26063 |
| IL-4R/PAR-2 | 1.2863 | 1.6243 | 0.73537 |
| IL-4R/TGF-β | 5.9655 | 1.7875 | 0.080645 |
| IL-4R/TNF-A | 0.92805 | 0.30629 | 0.092826 |
| PAR-2/total
protein | 17.8294 | 4.0955 | 0.14669 |
| PAR-2/IL-4R | 8.1433 | 2.7456 | 0.25928 |
| PAR-2/TGF-β | 9.292 | 1.6143 | 0.025914a |
| PAR-2/TNF-α | 1.5019 | 0.25849 | 0.041555a |
| TGF-β/total
protein | 1.9687 | 2.5616 | 0.38217 |
| TGF-β/IL-4R | 1.7496 | 1.7226 | 0.98542 |
| TGF-β/PAR-2 | 0.217 | 0.79093 | 0.00009231b |
| TGF-β/TNF-α | 0.17341 | 0.15672 | 0.56945 |
| TNF-α/total
protein | 10.6382 | 18.2398 | 0.032419a |
| TNF-α/IL-4R | 10.0243 | 12.3861 | 0.77667 |
| TNF-α/PAR-2 | 1.2594 | 5.4468 | 0.000050292b |
| TNF-α/TGF-β | 6.5795 | 6.947 | 0.76699 |
Higher parameters in SARC
By contrast, the ratio of TNF-α/total protein was
significantly lower in EAA patients than in SARC patients (10.64
vs. 18.24; P=0.032; Fig. 3E).
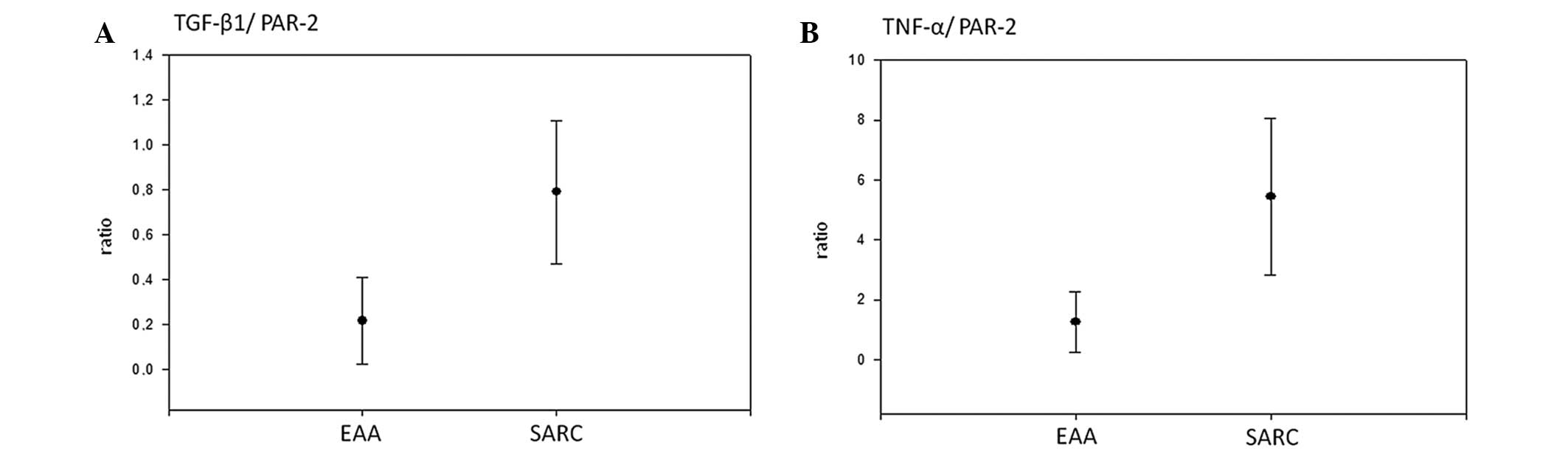
Furthermore, the ratios of TGF-β1/PAR-2 (0.217 vs. 0.791;
P=0.0000923) and TNF-α/PAR-2 (1.26 vs. 5.45; P=0.0000503) were
significantly lower in EAA cases with regard to the FDR
methodology, as shown in Fig.
4.
Discussion
In a pilot immunoassay study, statistically
significant higher levels of PAR-2, IL-4R and PAR-2/TGF-β and
PAR-2/TNF-α ratios were identified in BALF samples from EAA
patients. In addition, following FDR adjustment, statistically
significant higher ratios of TGF-β/PAR-2 and TNF-α/PAR-2 were
observed in BALF samples from SARC patients.
The immunoassay differences in the level of PAR-2
and the associated TGF-β and TNF-α ratios in BALF may have resulted
from a different ratio between specific (activation) and
non-specific (shedding induced inactivation) cleavage of the
receptor. A variety of enzymes, including tryptase, elastase, human
airway trypsin-like protease, cathepsin G and matrix
metaloproteinases (MMPs), are released from neutrophils, mast
cells, alveolar macrophages and airway epithelium, which results in
rate dependent activation/inactivation effects on PAR-2 (25). In addition, membrane-bound
proteinases, such as proteinase 3 that is involved in activation
and inactivation modes of PAR-2, was also expressed in neutrophils
and alveolar macrophages clearing TNF-α, which is more common in
interstitial pneumonitis than in SARC (26). BALF and membrane proteinases may,
in a rate dependent manner, influence (in parallel) membrane
receptors, such as PARs, and soluble cytokines, including TGF-β and
TNF-α. By contrast, PAR-2 induces the mRNA expression of MMP-9
(27), and MMP-9 induces TGF-β
production in airway epithelial cells (28). Higher levels of TGF-β were detected
in BALF samples from lung regions, indicating increased EAA and
SARC activity, as estimated by the HRCT score (19).
To date, it is not clear whether a specific or a
non-specific chain from the N-terminal has been detected, however,
the results of the present study indicate that in EAA,
substantially more PAR-2 terminals are released. The results
demonstrate a higher detection of PAR-2 in EAA samples, which is in
association with levels of TNF-α and TGF-β. As EAA and PAR-2, in
parallel, belong to the Th2-mediated pathway (29), the results strongly indicate an
association between this receptor and etiology. The results of the
current study also indicate that SARC is predominantly a
granulomatous inflammatory disease, thus, higher levels of TNF-α
are observed (30). The EAA
subjects in the present study were predominantly elderly, with a
sub-acute or chronic course of the disease. Thus, inclination
toward fibrosis and correlation with higher PAR-2 levels is
expected in association with repeated, long term exposure to
different proteolytic enzymes (31) despite to its specific and
non-specific cleavage. A previous study investigated the
dissociated gene and protein expression levels of PAR-2 in cultured
alveolar macrophages from smokers and healthy subjects (15), and raised the question of whether
the presence of surface protein in the BALF may also be
investigated as a possible biomarker for the transformation of EAA,
or an additional interstitial process, into a more chronic
fibrosing course.
In conclusion, the detection of PAR-2 and specific
chemokines in the BALF may serve as a useful tool in the
differential diagnosis between EAA and SARC during routinely used
bronchoscopical investigation. This method can prevent more
invasive surgical pulmonary biopsy verification, particularly in
cases of EAA.
Acknowledgements
The authors thank Thomas Secrest for revisions on
the English version of the article. The study was supported by a
grant from the Grant Agency of the Ministry of Health of the Czech
Republic (no. NT/13433/2012).
References
|
1
|
Facco M, Cabrelle A, Teramo A, et al:
Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 66:144–150.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitaka K, Miyazaki Y, Yasui M, et al:
Th2-biased immune responses are important in a murine model of
chronic hypersensitivity pneumonitis. Int Arch Allergy Immunol.
154:264–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barrera L, Mendoza F, Zuñiga J, et al:
Functional diversity of T-cell subpopulations in subacute and
chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med.
177:44–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Poll T, de Boer JD and Levi M: The
effect of inflammation on coagulation and vice versa. Curr Opin
Infect Dis. 24:273–278. 2011.PubMed/NCBI
|
|
5
|
Petäjä J: Inflammation and coagulation. An
overview Thromb Res. 127(Suppl 2): S34–S37. 2011.
|
|
6
|
Déry O, Corvera CU, Steinhoff M and
Bunnett NW: Proteinase-activated receptors: novel mechanisms of
signaling by serine proteases. Am J Physiol. 274:C1429–C1452.
1998.PubMed/NCBI
|
|
7
|
Macfarlane SR, Seatter MJ, Kanke T, et al:
Proteinase-activated receptors. Pharmacol Rev. 53:245–282.
2001.PubMed/NCBI
|
|
8
|
Leger AJ, Covic L and Kuliopulos A:
Protease-activated receptors in cardiovascular diseases.
Circulation. 114:1070–1077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boitano S, Flynn AN, Sherwood CL, et al:
Alternaria alternata serine proteases induce lung
inflammation and airway epithelial cell activation via PAR2. Am J
Physiol Lung Cell Mol Physiol. 300:L605–L614. 2011. View Article : Google Scholar
|
|
10
|
Matěj R, Vašáková M, Kukal J, Sterclová M
and Olejár T: Higher TGF-β with lower CD124 and TSLP, but no
difference in PAR-2 expression in bronchial biopsy of bronchial
asthma patients in comparison with COPD patients. Appl
Immunohistochem Mol Morphol. Oct 31–2013.(Epub ahead of print).
|
|
11
|
Vasakova M, Sterclova M, Matej R, et al:
IL-4 polymorphisms, HRCT score and lung tissue markers in
idiopathic pulmonary fibrosis. Hum Immunol. 74:1346–1351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kouzaki H, O‘Grady SM, Lawrence CB and
Kita H: Proteases induce production of thymic stromal lymphopoietin
by airway epithelial cells through protease-activated receptor-2. J
Immunol. 183:1427–1434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito T, Wang YH, Duramad O, et al:
TSLP-activated dendritic cells induce an inflammatory T helper type
2 cell response through OX40 ligand. J Exp Med. 202:1213–1223.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YH, Ito T, Wang YH, et al:
Maintenance and polarization of human TH2 central memory T cells by
thymic stromal lymphopoietin-activated dendritic cells. Immunity.
24:827–838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wallace WA and Howie SE: Immunoreactive
interleukin 4 and interferon-gamma expression by type II alveolar
epithelial cells in interstitial lung disease. J Pathol.
187:475–480. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wygrecka M, Kwapiszewska G, Jablonska E,
et al: Role of protease-activated receptor-2 in idiopathic
pulmonary fibrosis. Am J Respir Crit Care Med. 183:1703–1714. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Materazzi S, Pellerito S, Di Serio C, et
al: Analysis of protease-activated receptor-1 and -2 in human scar
formation. J Pathol. 212:440–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higashimoto Y, Yamagata Y, Taya S, et al:
Systemic inflammation in chronic obstructive pulmonary disease and
asthma: Similarities and differences. Respirology. 13:128–133.
2008.PubMed/NCBI
|
|
19
|
Szlubowski A, Soja J, Grzanka P, et al:
TGF-beta1 in bronchoalveolar lavage fluid in diffuse parenchymal
lung diseases and high-resolution computed tomography score. Pol
Arch Med Wewn. 120:270–275. 2010.PubMed/NCBI
|
|
20
|
Mohr LC: Hypersensitivity pneumonitis.
Curr Opin Pulm Med. 10:401–411. 2004. View Article : Google Scholar
|
|
21
|
Dai H, Guzman J, Chen B and Costabel U:
Production of soluble tumor necrosis factor receptors and tumor
necrosis factor-alpha by alveolar macrophages in sarcoidosis and
extrinsic allergic alveolitis. Chest. 127:251–256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nhu QM, Shirey KA, Pennini M, et al:
Protease-activated receptor 2 activation promotes an
anti-inflammatory and alternatively activated phenotype in
LPS-stimulated murine macrophages. Innate Immun. 18:193–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ebeling C, Lam T, Gordon JR, et al:
Proteinase-activated receptor-2 promotes allergic sensitization to
an inhaled antigen through a TNF-mediated pathway. J Immunol.
179:2910–2917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
No authors listed. Statement on
sarcoidosis. In: Joint Statement of the American Thoracic Society
(ATS), the European Respiratory Society (ERS) and the World
Association of Sarcoidosis and Other Granulomatous Disorders
(WASOG) adopted by the ATS Board of Directors and by the ERS
Executive Committee; February 1999; Am J Respir Crit Care Med. 160.
pp. 736–755. 1999, View Article : Google Scholar
|
|
25
|
Chignard M and Pidard D: Neutrophil and
pathogen proteinases versus proteinase-activated receptor-2 lung
epithelial cells: more terminators than activators. Am J Respir
Cell Mol Biol. 34:394–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Armstrong L, Godinho SI, Uppington KM, et
al: Tumour necrosis factor-alpha processing in interstitial lung
disease: a potential role for exogenous proteinase-3. Clin Exp
Immunol. 156:336–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vliagoftis H, Schwingshackl A, Milne CD,
et al: Proteinase-activated receptor-2-mediated matrix
metalloproteinase-9 release from airway epithelial cells. J Allergy
Clin Immunol. 106:537–545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perng DW, Chang KT, Su KC, et al: Matrix
metalloprotease-9 induces transforming growth factor-β(1)
production in airway epithelium via activation of epidermal growth
factor receptors. Life Sci. 89:204–212. 2011.
|
|
29
|
Lewkowich IP, Day SB, Ledford JR, et al:
Protease-activated receptor 2 activation of myeloid dendritic cells
regulates allergic airway inflammation. Respir Res. 12:1222011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Antoniu SA: Targeting the TNF-alpha
pathway in sarcoidosis. Expert Opin Ther Targets. 14:21–29. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Costabel U: The alveolitis of
hypersensitivity pneumonitis. Eur Respir J. 1:5–9. 1988.
|