Introduction
McCune-Albright syndrome (MAS) is a rare, sporadic
condition with an estimated prevalence between 1/100,000 and
1/1,000,000 (1), which is
characterized by the following three features: Fibrous dysplasia
(usually polyostotic), café-au-lait macules and endocrine
hyperfunction (2).
Endocrinopathies often include sexual precocity, as well as
hyperthyroidism, hypercortisolism, growth hormone (GH) excess and
hyperprolactinemia. MAS is caused by post-zygotic mutations of
G-proteins, such as GNAS (1,3–5).
Successful diagnosis using GNAS mutation analysis has been reported
to be associated with disease severity, yet <50% yield positive
results in patients with the classic triad. However, atypical
presentations of MAS, with only one or two of the classic symptoms,
have been reported in literature and remain particularly
challenging due to the lack of a diagnostic phenotype. Therefore,
the utility of GNAS mutation analysis in these patients is limited
and diagnosis is often based on clinical judgment (6).
In classic and atypical forms, hypersecretion of GHs
is not often associated with MAS (7). Symptoms are caused by excessive
GH-secreting pituitary adenoma and craniofacial fibrous dysplasia;
thus, the overall aim of treatment is to inhibit excessive GH
secretion, with optimal facial esthetics following treatment. The
most effective treatment of atypical MAS with GH-secreting
pituitary adenoma is surgical resection, however, the treatment
protocols are varied depending on the complexity of the symptoms.
Therefore, treatment of MAS remains a challenge for clinicians. In
the present study, a case of atypical MAS associated with
GH-secreting pituitary adenoma was reported, with effective
management using radiotherapy and surgery.
Case report
A 27-year-old male patient was admitted to the
Department of Maxillofacial Surgery (Peking Union Medical College,
Beijing, China) with frontal deformity. Written informed consent
was obtained from the patient. At the age of six years, the patient
started to present with abnormal progressive growth of the cranial
bones, primarily in the left frontal bone, and a subsequent
progressive decline in vision in the left eye. At 12-years of age,
the patient lost vision in the left eye and was diagnosed at a
different hospital with ‘skull structural abnormalities’, but
received no treatment. The patient’s feet then began to grow
rapidly and at the age of 13 years, the height of the patient
increased to 190 cm. At 21-years of age (height, 198.3 cm), the
patient was admitted to Peking Union Medical Hospital and was
diagnosed with a pituitary adenoma, for which the patient received
radiotherapy for 26 days. The patient stopped growing and had no
recurrence for five years. To improve appearance, the patient
sought treatment at the Peking Union Medical Hospital. The patient
had no family history of this disorder.
On examination, the patient appeared anxious and had
a pulse rate of 100/min, blood pressure of 140/90 mmHg and a height
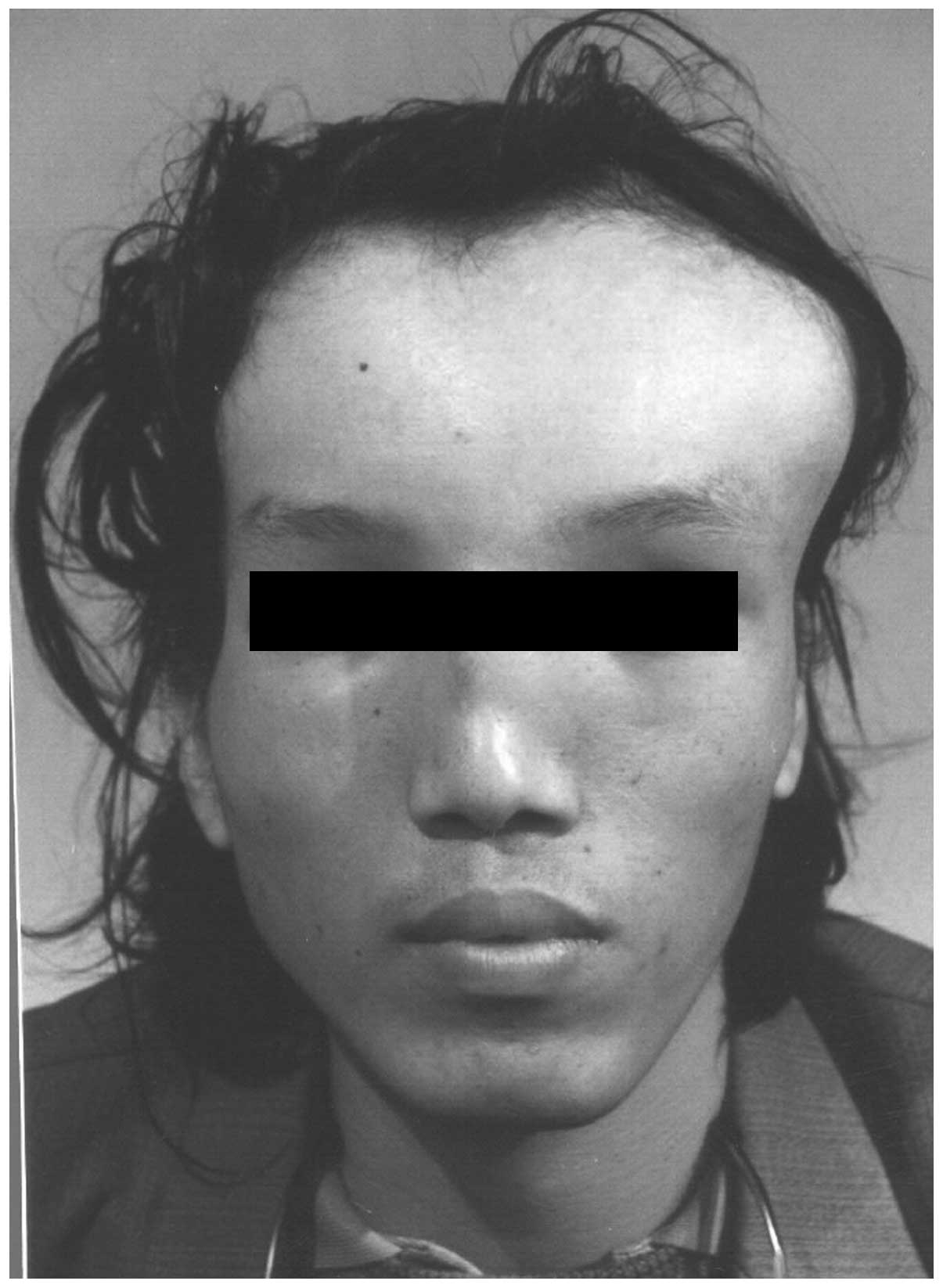
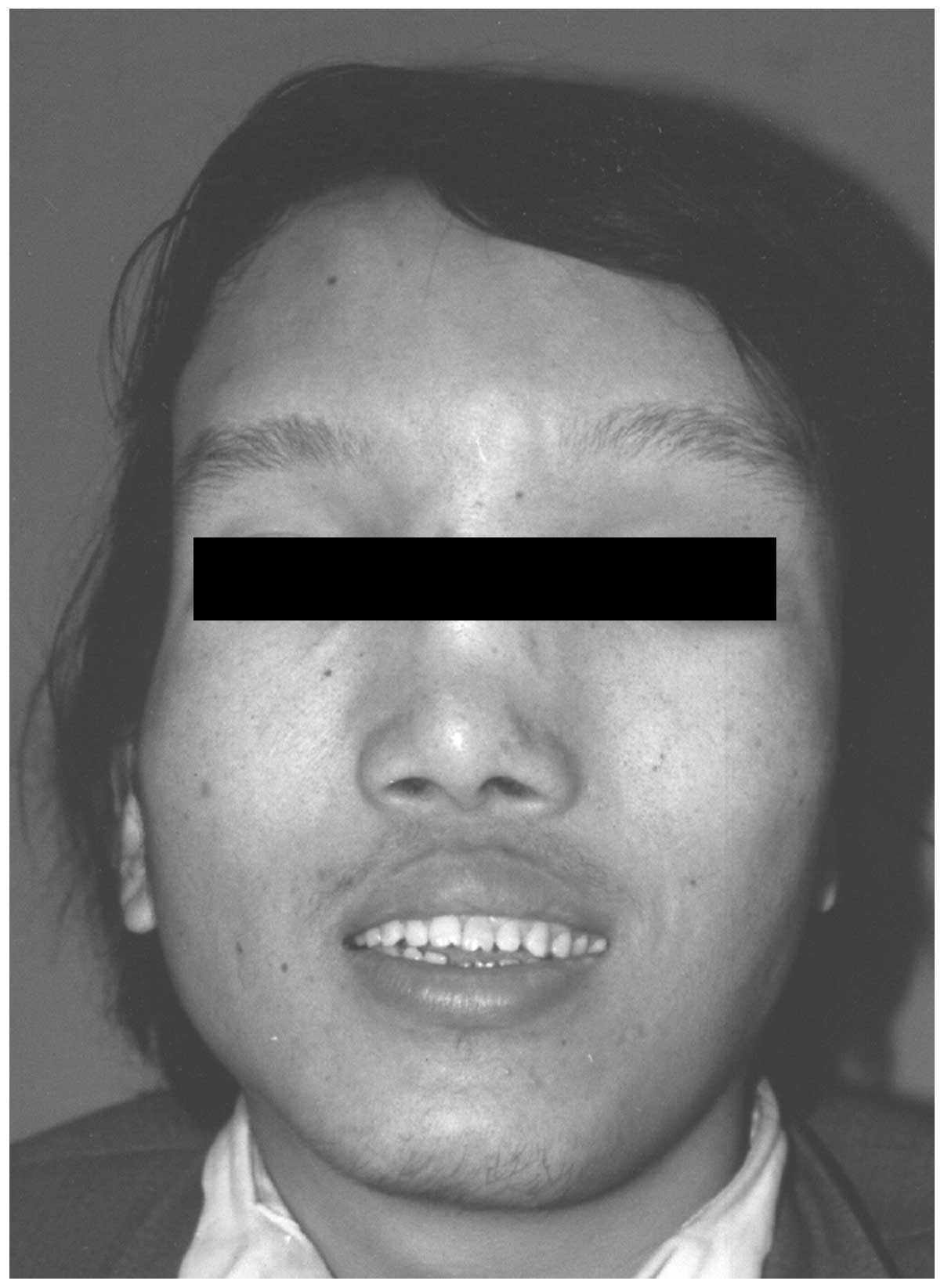
of 199.1 cm. Examination revealed an abnormally broad and prominent
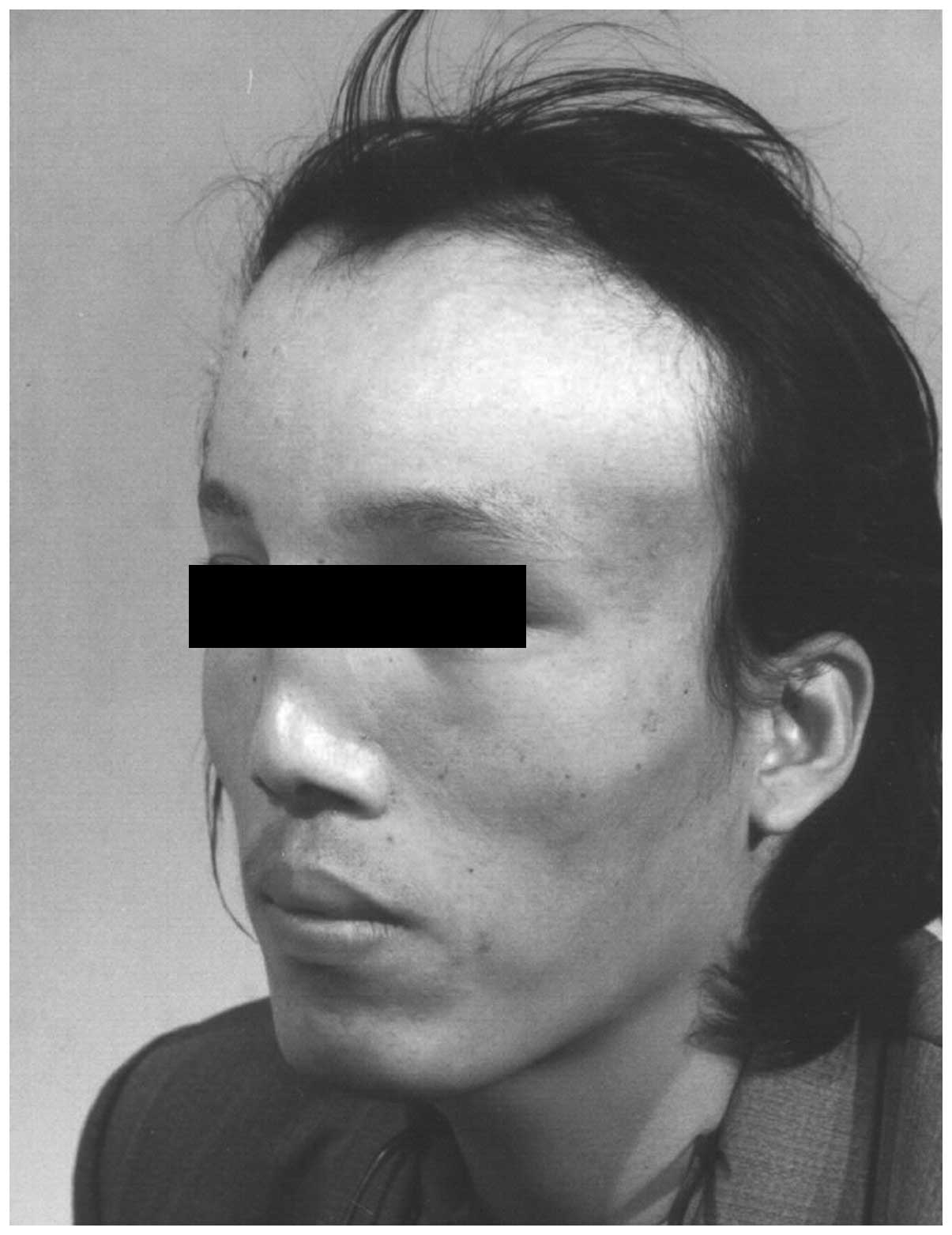
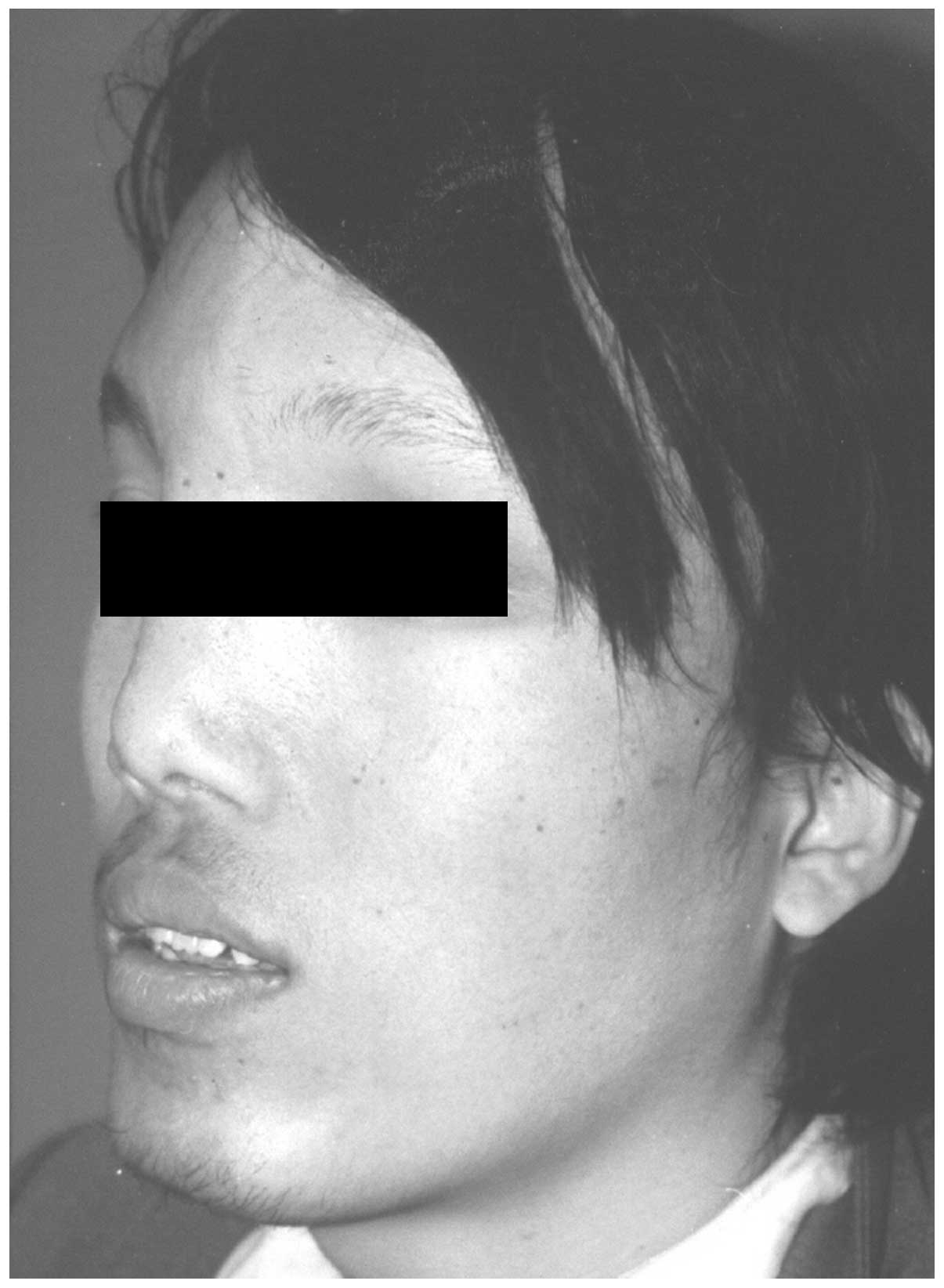
forehead (Fig. 1), which was more
marked on the left side (Fig. 2).
The bone near the sagittal suture was abnormally prominent;
however, no café-au-lait macules were found on the surface of the
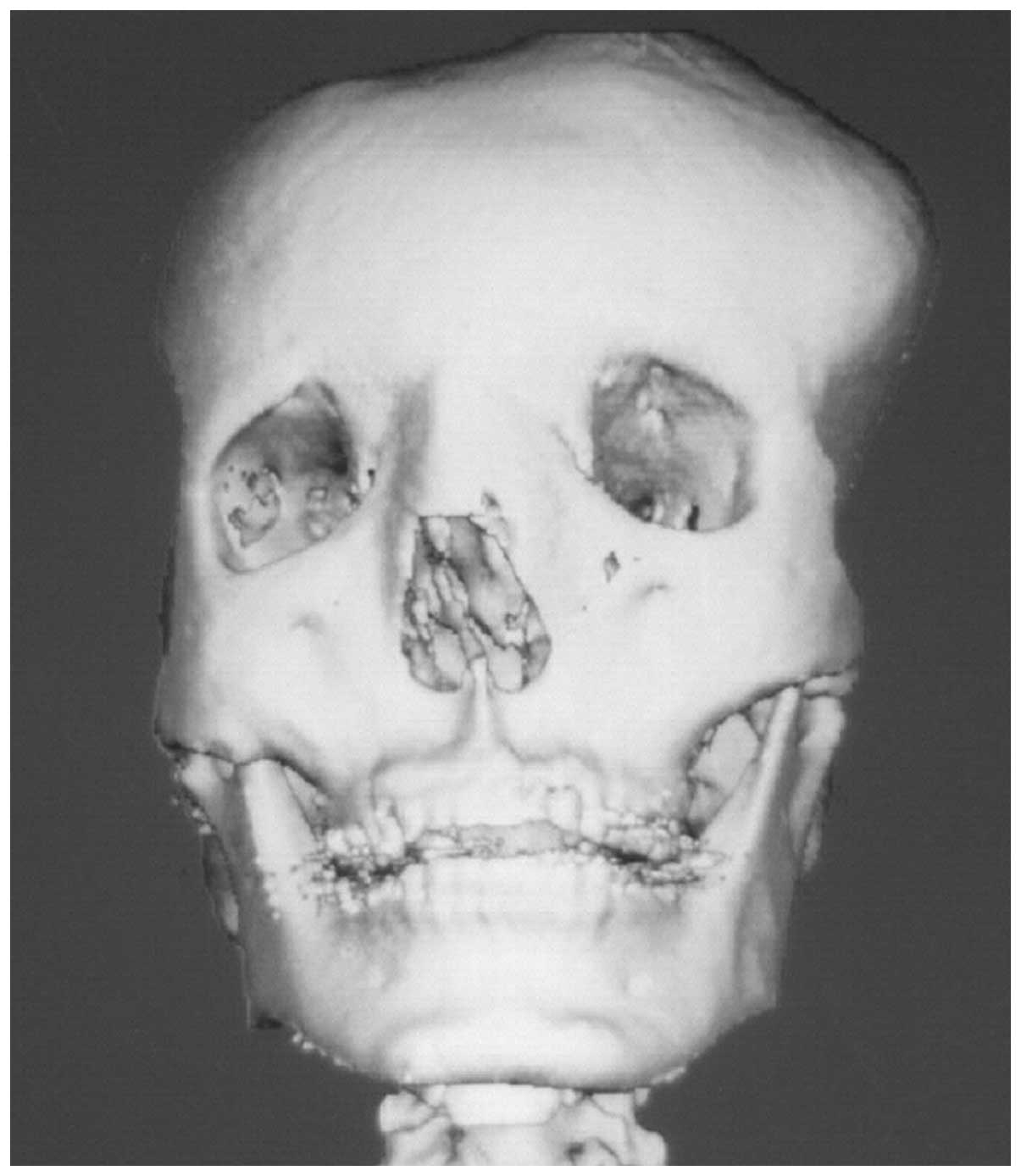
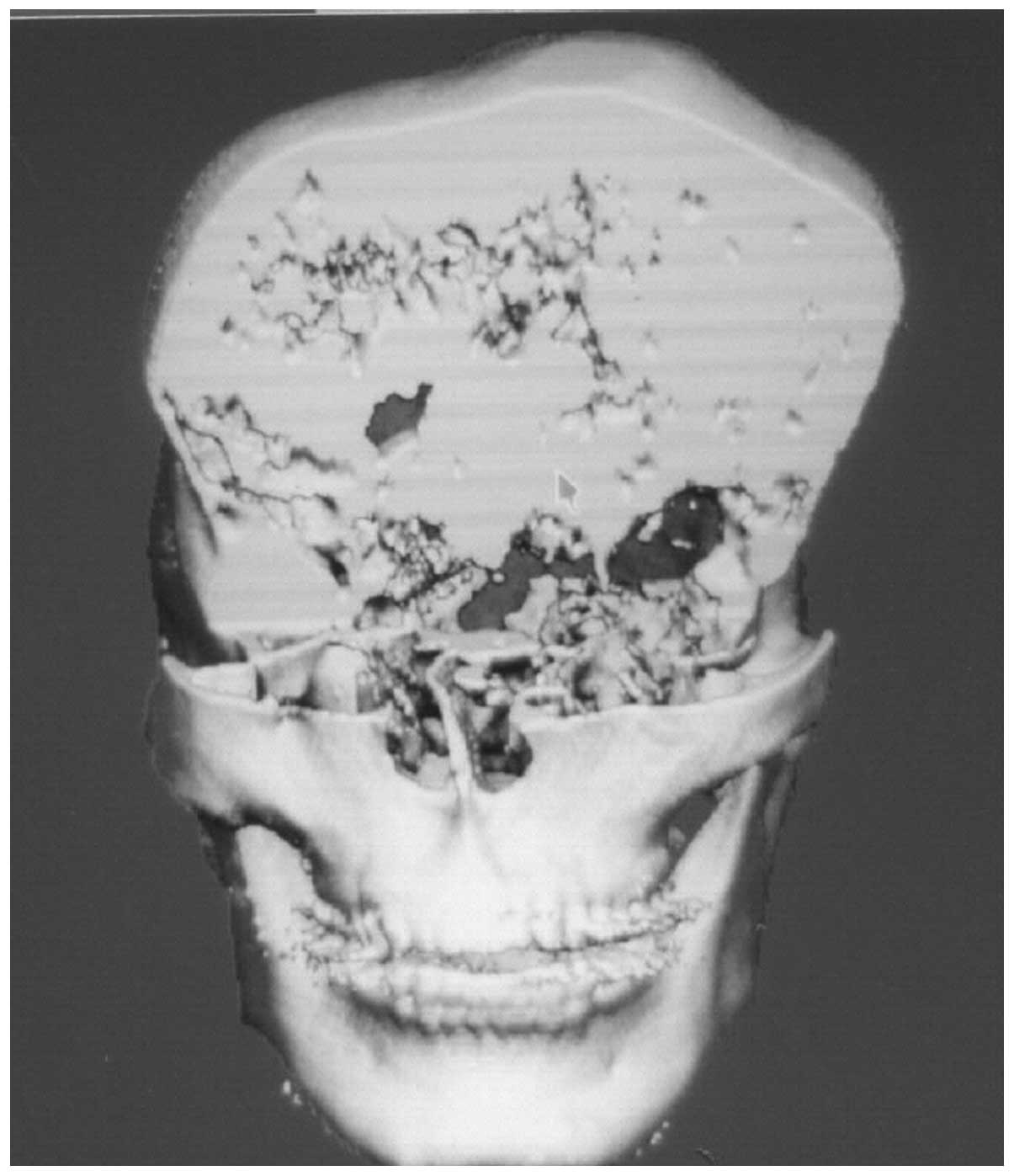
whole body. Computed tomography three-dimensional reconstruction
revealed the involvement of the frontal, temporal and parietal
bones, as well as the orbit and extensive skull base (Fig. 3–6). Since the polyostotic fibrous
dysplasia was associated with the pituitary adenoma, a diagnosis of
atypical MAS was considered.
A conservative shaving approach was selected for the
treatment of the deformity. Sections of the lesions were resected
using an osteotome, and the bones were then contoured with a
high-speed burr for esthetic reasons. A biopsy of the resected bone
was consistent with fibrous dysplasia, with areas of fibrosis and
woven bone.
The postoperative course was uneventful, and the
patient was satisfied with the appearance (Fig. 7 and 8). There was no evidence of postoperative
dysplastic recurrence during the one-year follow-up.
Discussion
MAS is a rare disorder, thus, is often
underdiagnosed and may be overlooked in patients with fibrous
dysplasia (8), particularly in
patients with atypical MAS. In patients with classic MAS, excessive
secretion of GHs may accelerate fibrous dysplasia, particularly
craniofacial fibrous dysplasia (3), resulting in visual and auditory
dysfunction and macrocephaly (9).
Therefore, the treatment of GH-secreting pituitary adenoma is the
first step in MAS treatment, which remains to be a challenge for
clinicians.
Surgery, radiotherapy and medication may be used for
the treatment of MAS; however, effective treatment protocols of
GH-secreting pituitary adenoma may be difficult to select due to
the complexity of MAS. The optimal current treatment for pituitary
adenoma, with the exception of prolactinomas, is surgical
resection. However, in patients with MAS, even subtotal pituitary
adenoma resection is often prohibited by severe skull base fibrous
dysplasia (10). Radiation therapy
remains controversial in patients with MAS due to the risk of
sarcomatous transformation of the fibrous dysplasia lesions within
the radiation portals (11,12).
However, in a previous study investigating fibrous dysplasia at the
Mayo Clinic, the results supported the use of radiotherapy
(13). The study demonstrated that
while 13 of the 1,122 patients with fibrous dysplasia (mostly
non-MAS patients) developed sarcomatous bone transformation within
the radiation portals, an almost equal number of patients did so
with (n=13) and without (n=14) previous radiation to the region.
However, to the best of our knowledge, no previous studies have
specifically addressed the use of radiosurgery in the treatment of
pituitary adenomas in patients with MAS; thus, radiosurgery may be
a viable option. In the present case study, due to the severe skull
base fibrous dysplasia, the patient received effective radiotherapy
for the pituitary adenoma, with no malignant transformation
observed through radiation imaging examination recently.
Surgery is the primary treatment option for
craniofacial fibrous dysplasia. The surgery varies according to the
different situations, including the degree of cranial nerve
involvement, the severity of the symptoms and the cosmetic demands
of the patient. Generally, the aim of treatment is to correct or
prevent functional problems and to achieve a normal facial
appearance (14–16). With regard to the management of
polyostotic fibrous dysplasia, a mixed approach may be considered,
using conservative shaving or radical resection. In the present
case, a conservative shaving approach was used for the following
reasons. Firstly, as the lesion was benign and the dysplasia
involved the skull base, the risks of complete resection were very
difficultly weighed; thus, conservative shaving or osseous
contouring was recommended. Secondly, the patient refused radical
excision for esthetic reasons and hoped for minimal surgical risks.
Finally, although the recurrence rate following contouring has been
reported to be as high as 25% (17), the patient was beyond adolescence
and the tumor had not grown for five years, reaching a static
phase; therefore this approach was selected for the patient.
In conclusion, the present study reported a case of
a 27-year-old male with MAS combined with GH-secreting pituitary
adenoma. The patient was successfully treated with radiosurgery and
a conservative shaving approach. The patient continues to undergo
follow-up observations by the methods of photography and radiation
imaging examination to assess any recurrent and malignant
transformation in the remaining lesion region.
Acknowledgements
The study was supported by grants from the National
Natural Science Fund (no. 81071583) of the National Natural Science
Foundation of China and from the Graduate Innovation Fund of the
Chinese Academy of Medical Sciences and Peking Union Medical
College (no. 2012-1002-011).
References
|
1
|
Dumitrescu CE and Collins MT:
McCune-Albright syndrome. Orphanet J Rare Dis. 3:122008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou J, Sun LH, Cui B, et al: Genetic
diagnosis of multiple affected tissues in a patient with
McCune-Albright syndrome. Endocrine. 31:212–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akintoye SO, Chebli C, Booher S, et al:
Characterization of gsp-mediated growth hormone excess in the
context of McCune-Albright syndrome. J Clin Endocrinol Metab.
87:5104–5112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weinstein LS, Shenker A, Gejman PV, et al:
Activating mutations of the stimulatory G protein in the
McCune-Albright syndrome. N Engl J Med. 325:1688–1695. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Obuobie K, Mullik V, Jones C, et al:
McCune-Albright syndrome: growth hormone dynamics in pregnancy. J
Clin Endocrinol Metab. 86:2456–2458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bercaw-Pratt JL, Moorjani TP, Santos XM,
Karaviti L and Dietrich JE: Diagnosis and management of precocious
puberty in atypical presentations of McCune-Albright syndrome: a
case series review. J Pediatr Adolesc Gynecol. 25:e9–e13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yavuzer R, Khilnani R, Jackson IT and
Audet B: A case of atypical McCune-Albright syndrome requiring
optic nerve decompression. Ann Plast Surg. 43:430–435. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hannon TS, Noonan K, Steinmetz R, et al:
Is McCune-Albright syndrome overlooked in subjects with fibrous
dysplasia of bone? J Pediatr. 142:532–538. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhansali A, Sharma BS, Sreenivasulu P, et
al: Acromegaly with fibrous dysplasia: McCune-Albright Syndrome -
clinical studies in 3 cases and brief review of literature. Endocr
J. 50:793–799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dou W, Lin N, Ma W, et al: Transsphenoidal
surgery in a patient with acromegaly and McCune-Albright syndrome:
application of neuronavigation. J Neurosurg. 108:164–169. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abs R, Beckers A, Van de Vyver FL, et al:
Acromegaly, multinodular goiter and silent polyostotic fibrous
dysplasia. A variant of the McCune-Albright syndrome. J Endocrinol
Invest. 13:671–675. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanner HC Jr, Dahlin DC and Childs DS Jr:
Sarcoma complicating fibrous dysplasia: probable role of radiation
therapy. Oral Surg Oral Med Oral Pathol. 14:837–846. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruggieri P, Sim FH, Bond JR and Unni KK:
Malignancies in fibrous dysplasia. Cancer. 73:1411–1424. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osada Y, Iwasawa M and Tanaka Y: Use of
image-guiding template for contouring surgery of midfacial fibrous
dysplasia. Ann Plast Surg. 59:459–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YR and Fairholm D:
Fronto-orbito-sphenoidal fibrous dysplasia. Ann Plast Surg.
15:190–203. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Munro IR and Chen YR: Radical treatment
for fronto-orbital fibrous dysplasia: the chain-link fence. Plast
Reconstr Surg. 67:719–730. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozek C, Gundogan H, Bilkay U, et al:
Craniomaxillofacial fibrous dysplasia. J Craniofac Surg.
13:382–389. 2002. View Article : Google Scholar
|