Introduction
Cardiovascular disease (CVD) constitutes the leading
cause of mortality worldwide. The major modifiable risk factors for
ischemic heart disease principally include high blood pressure,
high total cholesterol and low-density lipoprotein cholesterol, low
high-density lipoprotein cholesterol, tobacco use, physical
inactivity, poor nutrition and obesity. All of these established
risk factors are known to cause endothelial activation and
dysfunction (1,2). The prevention and control of these
risk factors are associated with preserved endothelial function and
reduced risk of ischemic heart disease.
The normal endothelium exhibits anticoagulant and
anti-inflammatory properties, and promotes vasodilatation by the
production of nitric oxide (NO), prostacyclin and other
vasodilators (3). In various
diseases the endothelium can become dysfunctional and promote
thrombosis and inflammation, and lose its vasodilatory effects.
Endothelial cells (ECs) and the factors released by them play a
crucial role in maintaining the physiological functions of the
cardiovascular system and are also involved in the development of a
variety of human diseases. Oxidative stress is a molecular
dysregulation associated with reactive oxygen species (ROS)
metabolism, and is a crucial factor in the pathogenesis of
endothelial dysfunction, vascular inflammation and atherosclerosis
(3). Variations of the phagocytic
nicotinamide adenine dinucleotide phosphate (NADPH) oxidases have
been identified in all vascular cells, including ECs, vascular
smooth muscle cells and fibroblasts (4). The activity and expression of NADPH
oxidase can be regulated by cytokines, such as tumor necrosis
factor-α (TNF-α), transforming growth factor-β and platelet-derived
growth factor. NADPH oxidase activity has been confirmed to be
inversely correlated with endothelial function in humans. TNF-α has
been shown to stimulate the upregulation of NO synthase (NOS)
activity and NO production in ECV304 cells, which may be
accompanied by a burst in production of intracellular ROS,
including superoxide anion (O2−) and hydrogen
peroxide (5).
ECV304 cells are an abundant and easily accessible
EC type. ECV304 cells and human coronary vascular ECs have been
shown to exhibit similar sensitivities to the harmful effects of
inflammatory cytokines (6),
including TNF-α, and oxidative damage. Guanxin Shutong capsule
(GXSTC) is a Chinese medicinal formula that is widely used
clinically for the treatment of numerous symptoms, including
palpitation, restlessness, dyspnea, chest pain, dizziness and
fatigue. In addition, GXSTC is used to promote blood circulation,
remove blood stasis and prevent CVDs (7,8). Our
previous study suggested that oral GXSTC could protect the heart
against oxidative stress and apoptosis in rats with myocardial
infarction (9). However, the
effects of GXSTC on ECs are not well known. The present study was
therefore designed to evaluate whether or not GXSTC could protect
against TNF-α-induced endothelial dysfunction.
Materials and methods
Cell culture
ECV304 cells (Type Culture Collection of the Chinese
Academy of Sciences, Shanghai, China) were grown in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% bovine serum,
antibiotics (100 IU/ml penicillin and 100 mg/ml streptomycin), at
37°C in a 5% CO2 atmosphere.
Drug and reagents
GXSTC consists of five traditional Chinese drugs:
57.1% Fructus Choerospondiatis (Polyphagidae), 28.6% Salvia
miltiorrhiza (Labiatae), 7.1% Syzygium aromaticum
(Labiatae), 3.6% borneol (Leguminosae) and 3.6% tabasheer. GXSTC
(batch no. 20120125) was provided by Buchang Pharmaceuticals
(Xi’an, China). DMEM and fetal bovine serum were obtained from
Gibco-BRL (Grand Island, NY, USA). The recombinant human TNF-α was
obtained from Promega Corp. (Madison, WI, USA). The
anti-endothelial NOS (eNOS) and anti-AKT monoclonal antibodies were
purchased from Cell Signaling Technology, Inc. (Boston, MA, USA).
Antibodies for p47phox and GAPDH were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) and those for gp91phox
and NAPDH oxidase 4 (Nox4) were purchased from Epitomics
(Burlingame, CA, USA). Enhanced chemiluminescence reagent was
obtained from Pierce Biotechnology, Inc. (Rockford, IL, USA). All
primers used were provided by Takara Bio, Inc. (Shiga, Japan), and
the NO, NOS, superoxide dismutase (SOD), malondialdehyde (MDA) and
lactate dehydrogenase (LDH) assay kits were purchased from the
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Preparation of serum containing the
tested drugs
Subsequent to obtaining approval from the Ethics
Committee of Xi’an Jiaotong University (Xi’an, China), male Sprague
Dawley rats weighing 200–250 g were provided by the Experimental
Animal Center of Xi’an Jiaotong University School of Medicine and
housed in a room with a temperature of 21–25°C, a relative humidity
of 50–60% and a 12-h light/dark cycle. Following the removal of the
capsules, the GXSTC powder was dissolved in aseptic 0.5% sodium
carboxymethylcellulose. The study was conducted in accordance with
the Guidelines for the Care and Use of Laboratory Animals of Xi’an
Jiaotong University. Each group contained 10 rats and they were
used for the preparation of the serum containing the tested drugs.
Rats in the normal and low-, medium- and high-dose GXSTC serum
groups were intragastrically administered saline or 5, 10 or 20
g/kg GXSTC, respectively, for 14 days. Blood was aseptically
obtained from the abdominal aorta of the rats 2 h after the final
administration and the serum was then acquired by centrifugation of
the blood at 720 × g for 20 min. Following filtration twice with a
0.22-μm cellulose acetate membrane, the serum was bottled, calefied
in 56°C water for 30 min and stored at −20°C for use (10). ECV304 cells were subsequently
divided into five groups, as follows: GXSTC-L (treatment with 5
ng/ml TNF-α and GXSTC at 5 g/kg), GXSTC-M (treatment with 5 ng/ml
TNF-α and GXSTC at 10 g/kg), GXSTC-H (treatment with 5 ng/ml TNF-α
and GXSTC at 20 g/kg), TNF-α (treatment with 5 ng/ml TNF-α and
vehicle) and control (treatment with vehicle but without
TNF-α).
Measurement of NO levels and NOS
activity
Subsequent to the ECV304 cells reaching confluence
in a 96-well plate, DMEM containing various concentrations of TNF-α
(2.5, 5 and 10 ng/ml) was added. After 6 h of culture, the medium
from each sample was collected, and the levels of NO released by
the ECV304 cells and the NOS activity were calculated using the NO
and NOS assay kits according to the manufacturer’s instructions
(Nanjing Jiancheng Bioengineering Institute).
In order to determine the effects of GXSTC on NO
levels and NOS activity in TNF-α-stimulated ECV304 cells, the cells
were again cultured in a 96-well plate and were then treated with
the different concentrations of GXSTC drug-containing serum (low-,
medium- and high-dose GXSTC) for 24 h followed by treatment with
TNF-α (5 ng/ml) for 6 h. In all the groups, including the control
group, the same volume of vehicle serum in the DMEM was added. The
medium from each sample was collected after 6 h and the levels of
NO released by the ECV304 cells and the NOS activity were
calculated using the assay kits.
MTT assay for cell viability
Cell survival was quantified by the colorimetric MTT
assay (Sigma, St. Louis, MO, USA), which measures mitochondrial
activity in viable cells. Briefly, cells in the exponential growth
period were harvested and plated in 96-well plates at a
concentration of 1×104 cells/well, and incubated at 37°C
for 24 h. The cells were treated with the different concentrations
of GXSTC drug-containing serum (low-, medium- and high-dose GXSTC)
for 24 h, and subsequently treated with TNF-α (5 ng/ml) for 6 h.
MTT (5 mg/ml, 20 μl) was then added to each well and the cells were
incubated at 37°C for 4 h. Subsequent to the supernatant being
discarded, 150 μl dimethylsulfoxide was added to each well, and the
absorbance at 490 nm was determined by use of a microplate reader
(Bio-Rad, Hercules, CA, USA).
Assay for LDH release
Cytotoxicity was quantified by measuring LDH release
in the medium during the exposure to different reagents (11). Subsequent to ECV304 cells reaching
confluence in a 96-well plate, the cells were treated with the
different concentrations of GXSTC drug-containing serum (low-,
medium- and high-dose GXSTC) for 24 h and then treated with TNF-α
(5 ng/ml) for 6 h.
Determination of MDA levels and SOD
activity
Subsequent to ECV304 cells reaching confluence in a
96-well plate, the cells were treated with the different
concentrations of GXSTC drug-containing serum (low-, medium- and
high-dose GXSTC) for 24 h and then treated with TNF-α for 6 h. The
total SOD activity and MDA levels in the cell lysates were assayed
using reagent kits in accordance with the manufacturer’s
instructions (Nanjing Jiancheng Bioengineering Institute) (12).
RNA preparation and semi-quantitative
reverse transcription polymerase chain reaction (RT-PCR)
To determine mRNA levels, confluent ECV304 cells
grown in six-well plates were treated as previously described
(12). Total RNA was extracted
using TRIzol® (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. cDNA samples
were generated and amplified using a Takara RNA PCR kit (avian
myeloblastosis virus) Ver. 3.0 (Takara Bio, Inc.) following the
manufacturer’s instructions. Thirty-five cycles of PCR
amplification were performed for eNOS, inducible NOS (iNOS),
neuronal NOS (nNOS) and GAPDH (95°C for 35 sec, 62°C for 90 sec and
72°C for 90 sec) using specific primers (Table I). The PCR products were visualized
on 1.5% agarose gels stained with GoldView™ (Science and Technology
Ltd, Beijing, China) staining. GAPDH served as an internal
control.
 | Table IPrimers used for eNOS, iNOS, nNOS and
GAPDH. |
Table I
Primers used for eNOS, iNOS, nNOS and
GAPDH.
| mRNA | Primer | Sequence (5′-3′) |
|---|
| eNOS | Sense |
CACCGCTACAACATCCTG |
| Antisense |
GCCTTCTGCTCATTCTCC |
| iNOS | Sense |
GCTACCAGATGCCAGATG |
| Antisense |
CTCAAGCACAAGGTCAGG |
| nNOS | Sense |
GTGGAGGTGCTGGAGGAG |
| Antisense |
GTGCGGTAGGAAACGATGG |
| GAPDH | Sense |
CACCCACTCCTCCACCTTTG |
| Antisense |
CCACCACCCTGTTGCTGTAG |
Western blotting
Confluent ECV304 cells in six-well plates were
treated with GXSTC and then with TNF-α in accordance with the
aforementioned methods. The protein expression of AKT, eNOS,
p47phox, gp91phox, Nox4 and GAPDH was measured by western blot
analysis as previously described (13).
Statistical analysis
Values are presented as the mean ± standard error of
the mean. One-way analysis of variance followed by a Tukey’s
multiple comparison test were used to test the significance between
three or more groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of GXSTC on NO production by
ECV304 cells stimulated with TNF-α
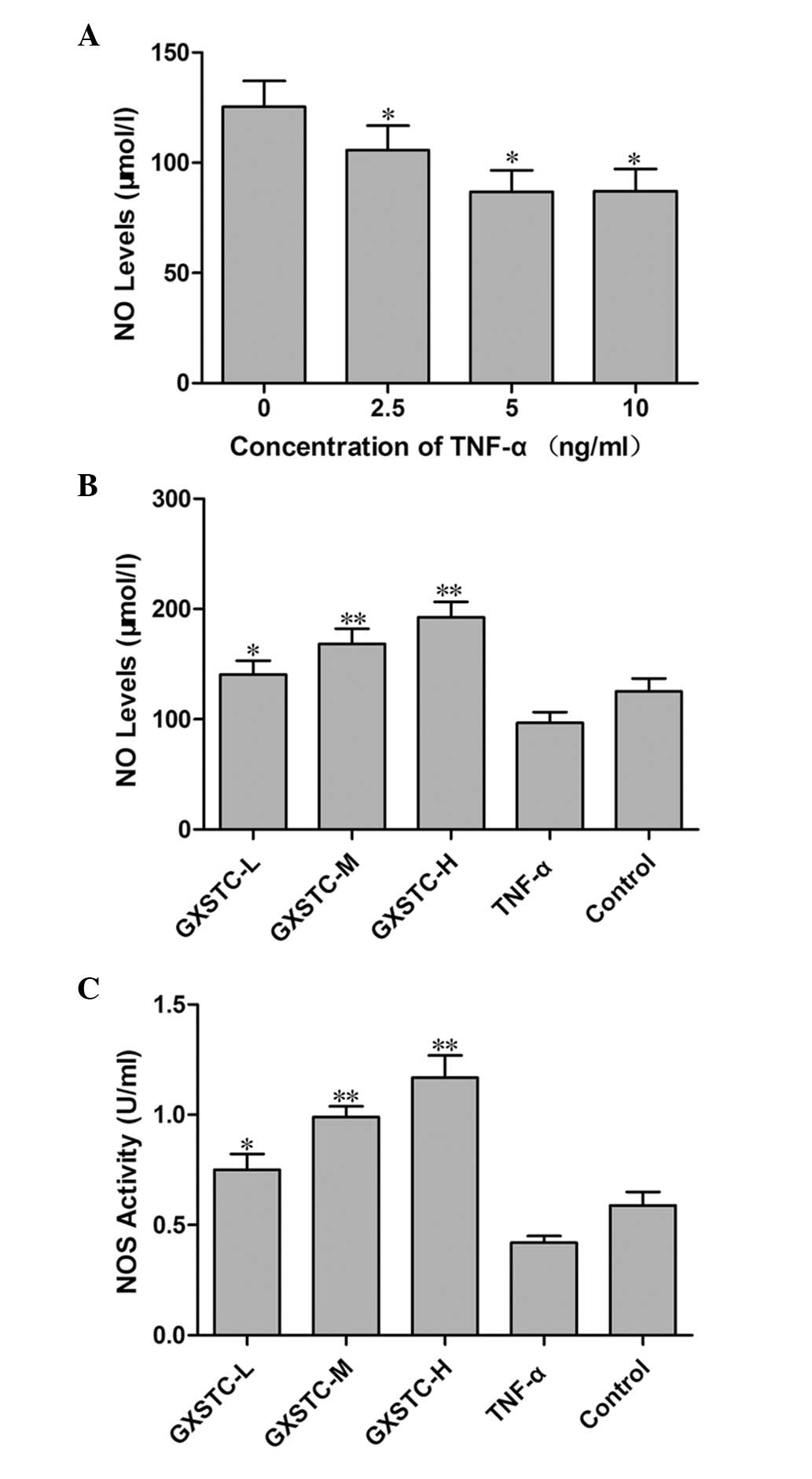
TNF-α treatment significantly reduced the NO
production by ECV304 cells, indicating that endothelial dysfunction
had occurred. The concentration of 5 ng/ml TNF-α was selected as an
in vitro endothelial dysfunction model (Fig. 1A), and GXSTC increased the level of
NO in a dose-dependent manner in TNF-α-stimulated ECV304 cells
(Fig. 1B). Compared with cells in
the TNF-α group, the activity of NOS was significantly increased in
the cells following treatment with GXSTC (P<0.05, Fig. 1C). NO is generated by three NOSs,
and the mRNA expression of these three NOSs was measured in each
experiment using RT-PCR. The expression of eNOS was decreased in
the TNF-α-treated group but increased following incubation with
GXSTC. The expression of iNOS was increased in the TNF-α-treated
group but decreased after incubation with GXSTC, whereas nNOS were
not detected by amplification
Effects of GXSTC on cell viability of
ECV304 cells stimulated with TNF-α
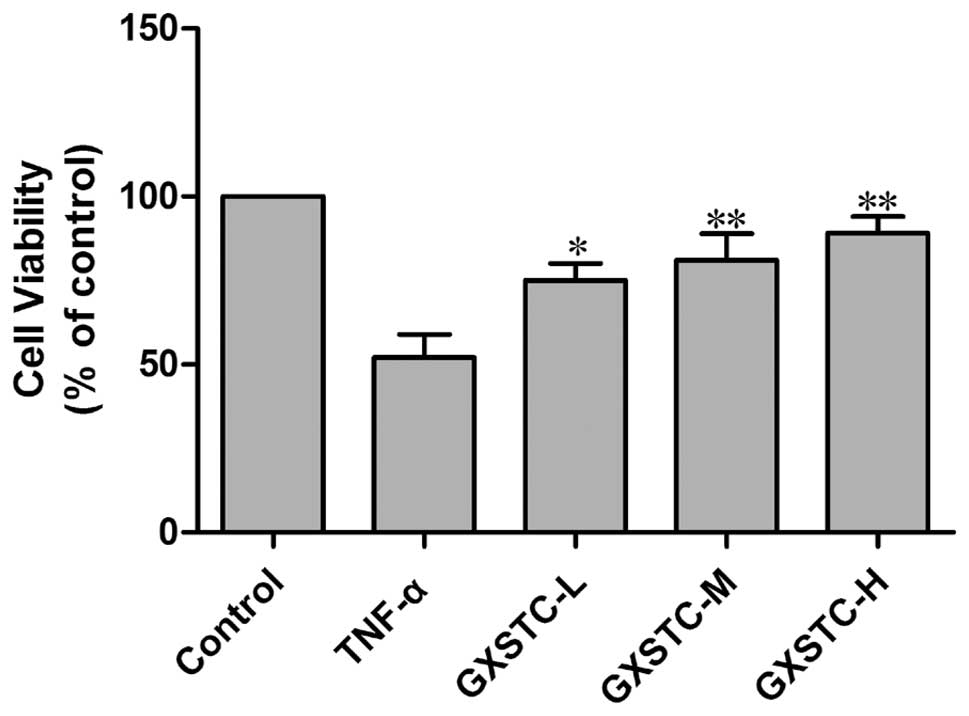
The exposure of cells to TNF-α for 6 h induced cell
death in more than one-third of the cells as compared with the
control group, as measured by the MTT assay (Fig. 3). GXSTC significantly attenuated
TNF-α-induced cell death.
Effects of GXSTC on LDH release by ECV304
cells stimulated with TNF-α
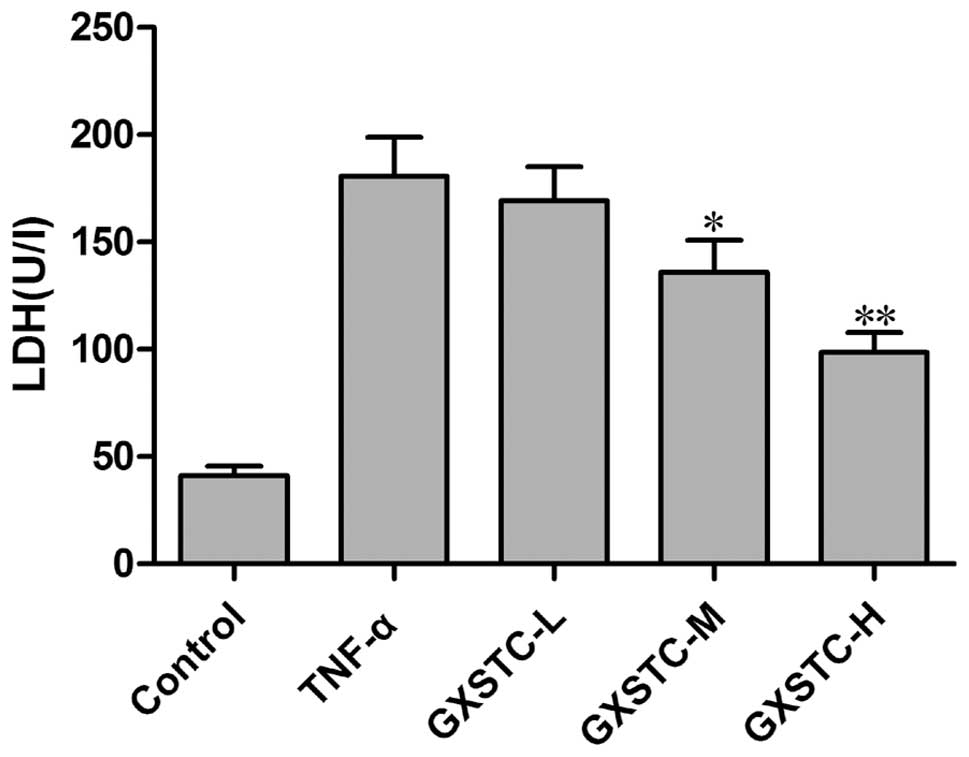
As shown in Fig. 4,
LDH release was minimal in the control group. The stimulation of
cells with TNF-α resulted in a marked increase in LDH release.
GXSTC significantly attenuated the TNF-α-induced increase in LDH
release.
Effects of GXSTC on MDA levels and SOD
activity in ECV304 cells stimulated with TNF-α
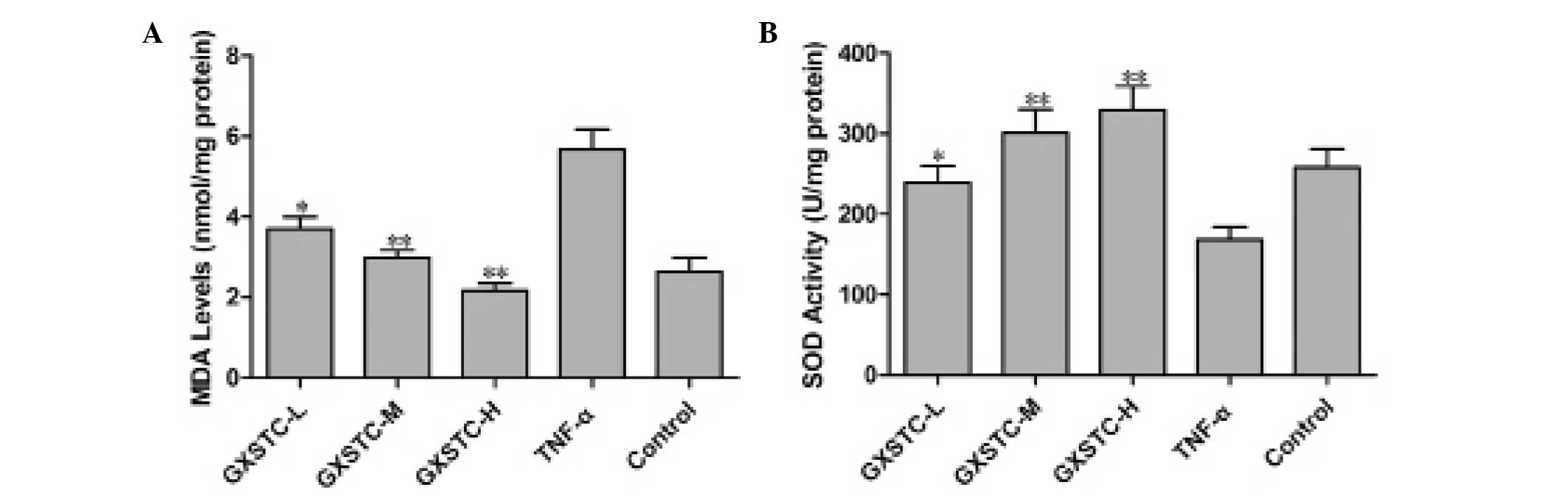
GXSTC treatment resulted in significant decreases in
MDA content (P<0.05, Fig. 5A)
and increases in SOD activity (P<0.05, Fig. 5B) in ECV304 cells exposed to TNF-α
as compared with cells exposed to TNF-α alone.
Effects of GXSTC on protein expression of
eNOS in ECV304 cells stimulated with TNF-α
Exposure of cells to TNF-α for 6 h resulted in a
significant decrease in eNOS protein expression (P<0.05,
Fig. 6); GXSTC restored eNOS
protein expression (Fig. 6).
Treatment of ECV304 cells with TNF-α for 6 h induced the
phosphorylation of AKT. However, when cells were preincubated with
GXSTC for 6 h, the phosphorylation of AKT decreased in a
dose-dependent manner (Fig.
6).
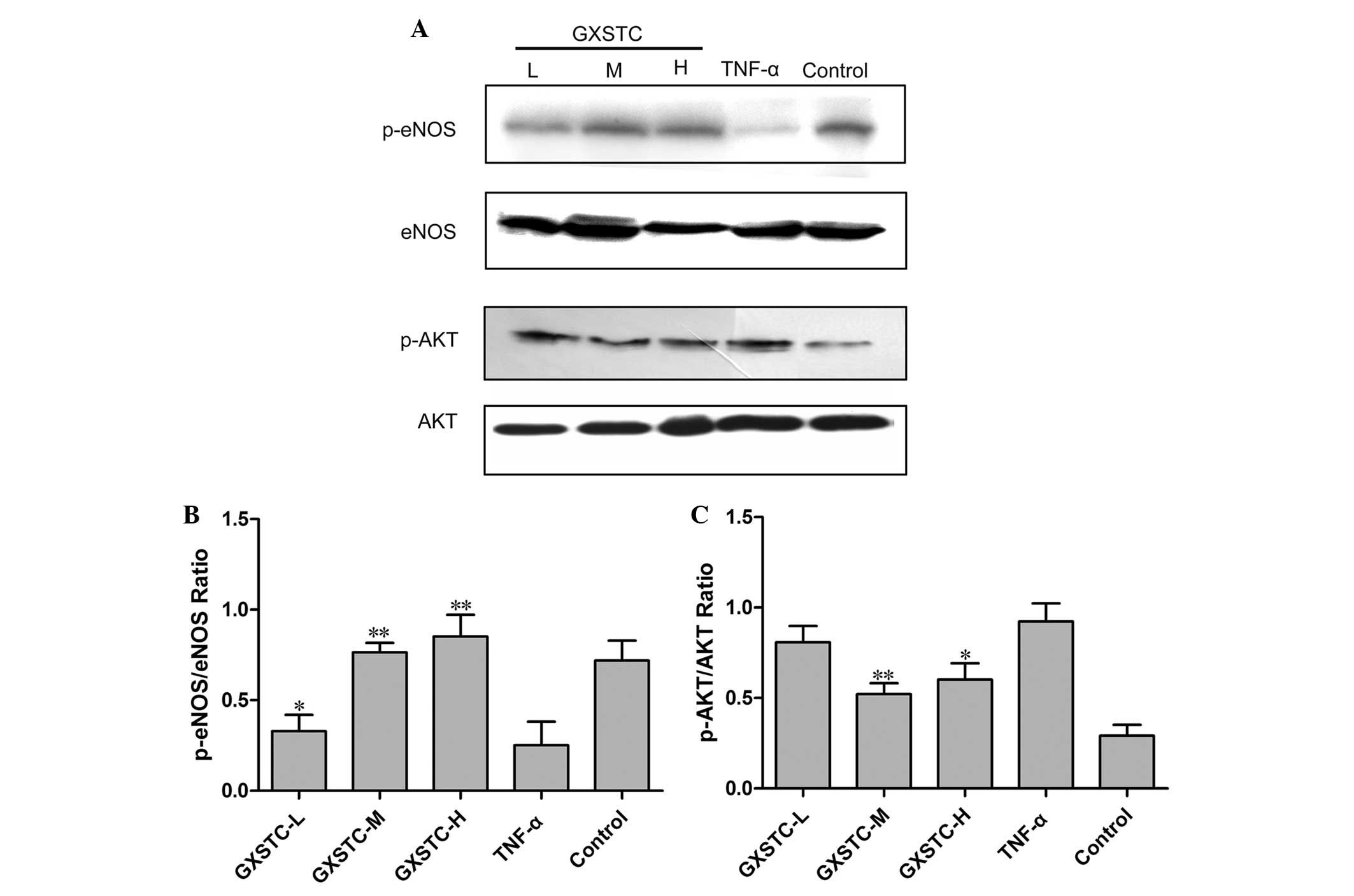 | Figure 6Effects of GXSTC on eNOS and AKT
protein expression in TNF-α-stimulated endothelial cells. ECV304
cells were grown to confluence in six-well plates and pre-treated
with GXSTC for 24 h. A total of 5 ng/ml TNF-α was then added for 6
h. (A) Bands corresponding to eNOS, p-eNOS, AKT and p-AKT. (B and
C) The results for (B) p-eNOS and (C) p-AKT were quantified by
densitometric analysis of the bands and then normalized to eNOS and
AKT, respectively, in ECV304 cells. Data are presented as the mean
± standard error of the mean of three independent experiments
duplicated in each run. *P<0.05 and
**P<0.01 vs. the TNF-α group. GXSTC, Guanxin Shutong
capsule; eNOS, endothelial nitric oxide synthase; TNF-α, tumor
necrosis factor-α; p-, phosphorylated-; GXSTC-L, low-dose GXSTC;
GXSTC-M, medium-dose GXSTC; GXSTC-H, high-dose GXSTC. |
Effects of GXSTC on NADPH oxidase
expression in ECV304 cells stimulated with TNF-α
Oxidative stress and increased
O2− production by NADPH oxidases are critical
in the development of CVDs. The expression of p47phox, gp91phox and
Nox4 protein was increased in TNF-α-stimulated ECV304 cells.
Administration of GXSTC significantly decreased p47phox, Nox-4, and
gp91phox protein expression compared with TNF-α treatment alone
(Fig. 7).
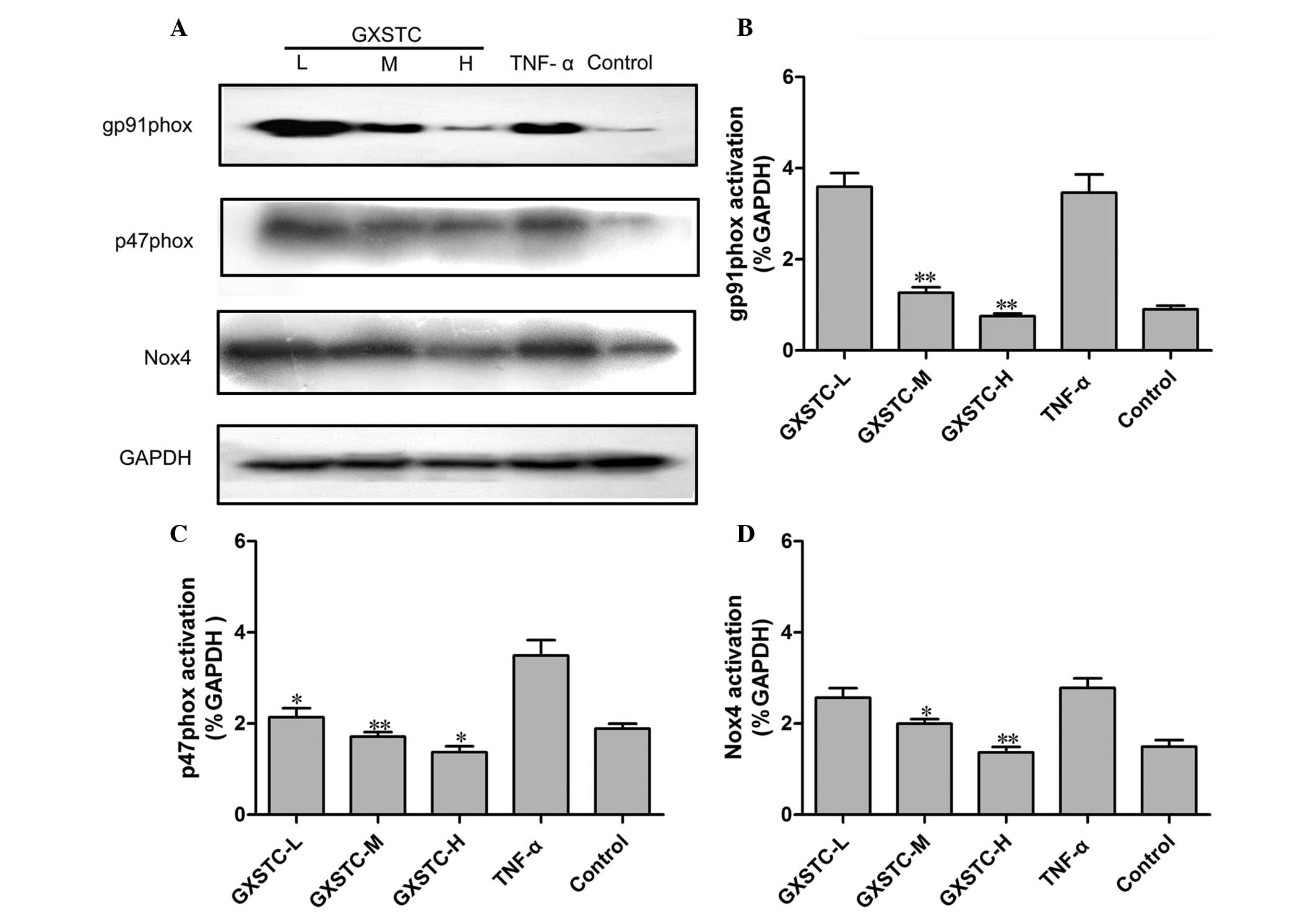 | Figure 7Effects of GXSTC on NADPH oxidase
protein expression in TNF-α-stimulated endothelial cells. ECV304
cells were grown to confluence in six-well plates and pre-treated
with GXSTC for 24 h. A total of 5 ng/ml TNF-α was then added for 6
h. (A) Bands corresponding to gp91phox, p47phox, Nox4 and GAPDH.
(B–D) The results for (B) gp91phox, (C) p47phox and (D) Nox4 were
quantified by densitometric analysis of the bands and then
normalized to GAPDH in ECV304 cells. Data are presented as the mean
± standard error of the mean (n=3). *P<0.05 and
**P<0.01 vs. the TNF-α group. GXSTC, Guanxin Shutong
capsule; NAPDH, nicotinamide adenine dinucleotide phosphate; TNF-α,
tumor necrosis factor-α; Nox4, NAPDH oxidase 4; GXSTC-L, low-dose
GXSTC; GXSTC-M, medium-dose GXSTC; GXSTC-H, high-dose GXSTC. |
Discussion
In the present study, TNF-α stimulation resulted in
reduced cell viability and increased the release of LDH and the
lipid peroxidation product MDA; this was associated with increased
production of NO and NADPH oxidase, suggesting that underproduction
of NO and downregulation of p-eNOS may have partially mediated the
cell death through enhancing oxidative stress and consequently
increasing the cellular lipid peroxidation. TNF-α stimulation
resulted in cellular injury and reduced NO production in ECV304
cells, and co-culture of ECV304 cells with GXSTC conferred
protection against cellular injury and significantly increased NO
production. The GXSTC-induced enhancement of NO production appeared
to be attributed to the restoration of eNOS production. GXSTC
enhanced cell viability and reduced the cellular LDH release
induced by TNF-α. This phenomenon may suggest that underproduction
of NO by ECs in response to TNF-α stimulation had the initial
function of cell protection rather than injury. However, under
circumstances where the ensuing burst in ROS production induced by
TNF-α is not counteracted by sufficient antioxidant defense, the
outcome may be endothelial injury. In the present study, GXSTC
significantly attenuated the TNF-α-induced reduction in the
activity of SOD, a major endogenous antioxidant, and attenuated the
TNF-α-induced increase in the lipid peroxidation product MDA. These
findings may represent major mechanisms by which GXSTC attenuates
TNF-α-induced endothelial injury.
The modulation of vascular tone and structure is
reliant on the multifunctional vascular endothelium (2). Cellular adhesion, thromboresistance,
smooth muscle cell proliferation and vessel wall inflammation are
regulated by a variety of factors produced by ECs (1). Thus, since the endothelium plays a
critical role in maintaining the homeostasis of the body,
endothelial dysfunction is associated with a number of
pathophysiological conditions, including atherosclerosis,
hypertension, diabetes and certain CVDs. There are three distinct
isoforms of NOS, which differ both in structure and in function.
eNOS and nNOS are constitutively expressed and are referred to as
Ca2+-dependent enzymes. iNOS is only expressed at high
levels following induction by cytokines or other inflammatory
agents, and its activity is independent of an increase in
intracellular Ca2+ (14). The main source of endothelial NO is
eNOS expressed by ECs. In particular, NO plays a key role in
protecting the endothelium against the initiation and progression
of CVD via its dilatory effect (2), and may also be involved in
cardioprotection (15). Since it
protects against CVDs, NO production by ECV304 cells was selected
as an indicator of endothelial dysfunction in the present study.
The nNOS isoform could not be detected by amplification. Therefore,
the effects of treatment with GXSTC drug-containing serum on NO
production in TNF-α-treated ECV304 cells were determined by
measuring NO generation, NOS activity and eNOS expression. TNF-α
decreased the secretion of NO into the medium, but GXSTC increased
eNOS expression and enhanced NO production and NOS activity. These
results suggest that TNF-α-stimulated ECV304 cells represent an
endothelial dysfunction model, and that GXSTC can improve the
endothelial dysfunction by inducing eNOS expression and increasing
NO production and NOS activity.
Endothelial NO is produced by eNOS in response to
physiological stimuli. In the absence of its substrate
(L-arginine), the eNOS generates O2− instead
of NO. NO exerts a number of beneficial antiatherogenic effects and
reacts with a variety of other targets, acting to modulate ion
channel function, intracellular signal transduction and gene
expression (7,16). Therefore, the loss of NO
bioavailability is a key feature of endothelial dysfunction, and
can occur due to multiple pathways altering NO synthesis or
biodegradation. The excessive production of ROS appears to be a
major mechanism underlying reduced vascular NO levels. There are
several mechanisms through which NO bioavailability can be modified
by ROS (2). Firstly,
O2− rapidly reacts with NO, leading to the
production of the strong oxidant peroxynitrite. Treatment with SOD
or SOD mimetics reduces vascular O2− levels
and restores endothelial function (1). NADPH oxidases, lipoxygenase, xanthine
oxidase, mitochondrial oxidases and NOSs are potential sources of
vascular O2− production (4,17,18).
The NADPH oxidase has been shown to be the principal source of
O2− production in certain animal models of
vascular disease. The enzyme was originally characterized in
neutrophils, and has been shown to be present in vascular smooth
muscle cells and ECs (19).
Furthermore, the nitration and nitrosylation of the NADPH oxidase
may lead to the inhibition of the enzyme. In the present study,
NADPH oxidase and AKT expression was inhibited subsequent to NOS
activity being enhanced by GXSTC. This association remained even
following correction for other major risk factors for
atherosclerosis, including diabetes, hypercholesterolemia and
coronary heart disease.
In conclusion, GXSTC inhibited the expression of
p47phox, gp91phox and Nox4, which are NADPH oxidase subunits and
major risk factors for CVDs. Furthermore, GXSTC was shown to reduce
SOD activity in TNF-α-stimulated ECV304 cells. eNOS mRNA and
protein levels in ECV304 cells were increased by GXSTC. Previous
studies have indicated that GXSTC exerts an anti-ischemic effect
during the early and late stages of myocardial infarction (20,21).
This study supports the hypothesis that GXSTC protects against
endothelial injury via the NO pathway and its antioxidant effects.
GXSTC may have an important role in preventing atherosclerosis or
myocardial infarction initiated by endothelial injury.
Acknowledgements
This study was supported by the Ministry of National
Science and Technology during the Significant New Drugs Creation
Special Project (2011ZX09401-308-6).
References
|
1
|
Boos CJ, Lip GY and Blann AD: Circulating
endothelial cells in cardiovascular disease. J Am Coll Cardiol.
48:1538–1547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: the role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schramm A, Matusik P, Osmenda G and Guzik
TJ: Targeting NADPH oxidases in vascular pharmacology. Vascul
Pharmacol. 56:216–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Griendling KK, Sorescu D and Ushio-Fukai
M: NAD(P)H oxidase: role in cardiovascular biology and disease.
Circ Res. 86:494–501. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia Z, Liu M, Wu Y, et al:
N-acetylcysteine attenuates TNF-alpha-induced human vascular
endothelial cell apoptosis and restores eNOS expression. Eur J
Pharmacol. 550:134–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Willam C, Koehne P, Jürgensen JS, et al:
Tie2 receptor expression is stimulated by hypoxia and
proinflammatory cytokines in human endothelial cells. Circ Res.
87:370–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Demosthenous M, Antoniades C, Tousoulis D,
Margaritis M, Marinou K and Stefanadis C: Endothelial nitric oxide
synthase in the vascular wall: Mechanisms regulating its expression
and enzymatic function. Artery Res. 5:37–49. 2011. View Article : Google Scholar
|
|
8
|
Huo Y, Yao TM, Liang Z, Li J, You Y and
Han YL: Effects of Guanxin Shutong capsule on lipid metabolism and
hemorrheology in rat model with experimental atherosclerosis.
Liaoning Zhongyiyao Daxue Xuebao. 13:248–250. 2011.(In
Chinese).
|
|
9
|
Cao YJ, He X, Lui F, Huang Z and Zhang Y:
Chinese medicinal formula Guanxin Shutong capsule protects the
heart against oxidative stress and apoptosis induced by ischemic
myocardial injury in rats. Exp Ther Med. 7:1033–1039.
2014.PubMed/NCBI
|
|
10
|
Zhang YH, Liu JT, Wen BY and Liu N:
Mechanisms of inhibiting proliferation of vascular smooth muscle
cells by serum of rats treated with Dahuang Zhechong pill. J
Ethnopharmacol. 124:125–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu L, Qiao H, Li Y and Li L: Protective
roles of puerarin and Danshensu on acute ischemic myocardial injury
in rats. Phytomedicine. 14:652–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Luo W, Zheng L, Li M and Zhang Y:
Construction of recombinant FGFR1 containing full-length gene and
its potential application. Plasmid. 64:60–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao YJ, He X, Wang N and He LC: Effects of
imperatorin, the active component from Radix Angelicae (Baizhi), on
the blood pressure and oxidative stress in 2K,1C hypertensive rats.
Phytomedicine. 20:1048–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pae HO, Son Y, Kim NH, Jeong HJ, Chang KC
and Chung HT: Role of heme oxygenase in preserving vascular
bioactive NO. Nitric Oxide. 23:251–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones SP and Bolli R: The ubiquitous role
of nitric oxide in cardioprotection. J Mol Cell Cardiol. 40:16–23.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pearson JD: The endothelium in vascular
pharmacology - an overview of 2011–2012. Vascul Pharmacol.
58:335–336. 2013.
|
|
17
|
Moulton PJ, Hiran TS, Goldring MB and
Hancock JT: Detection of protein and mRNA of various components of
the NADPH oxidase complex in an immortalized human chondrocyte
line. Br J Rheumatol. 36:522–529. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guzik TJ and Harrison DG: Vascular NADPH
oxidases as drug targets for novel antioxidant strategies. Drug
Discov Today. 11:524–533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aguado A, Galán M, Zhenyukh O, et al:
Mercury induces proliferation and reduces cell size in vascular
smooth muscle cells through MAPK, oxidative stress and
cyclooxygenase-2 pathways. Toxicol Appl Pharmacol. 268:188–200.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiaxin Q, Lin Z, Ge KL, et al: Influence
of Guanxin Shutong containing serum on vascular smooth muscle cells
proliferation induced by advanced glycation end products. Zhongyao
Yaoli Yu Linchuang. 28:155–158. 2012.(In Chinese).
|
|
21
|
Li GL and Li LX: Efficacy of Guanxin
Shutong capsules for treatment of chronic seable angina. Zhongxiyi
Jiehe Xinnaoxueguanbing Zazhi. 10:1401–1402. 2012.
|