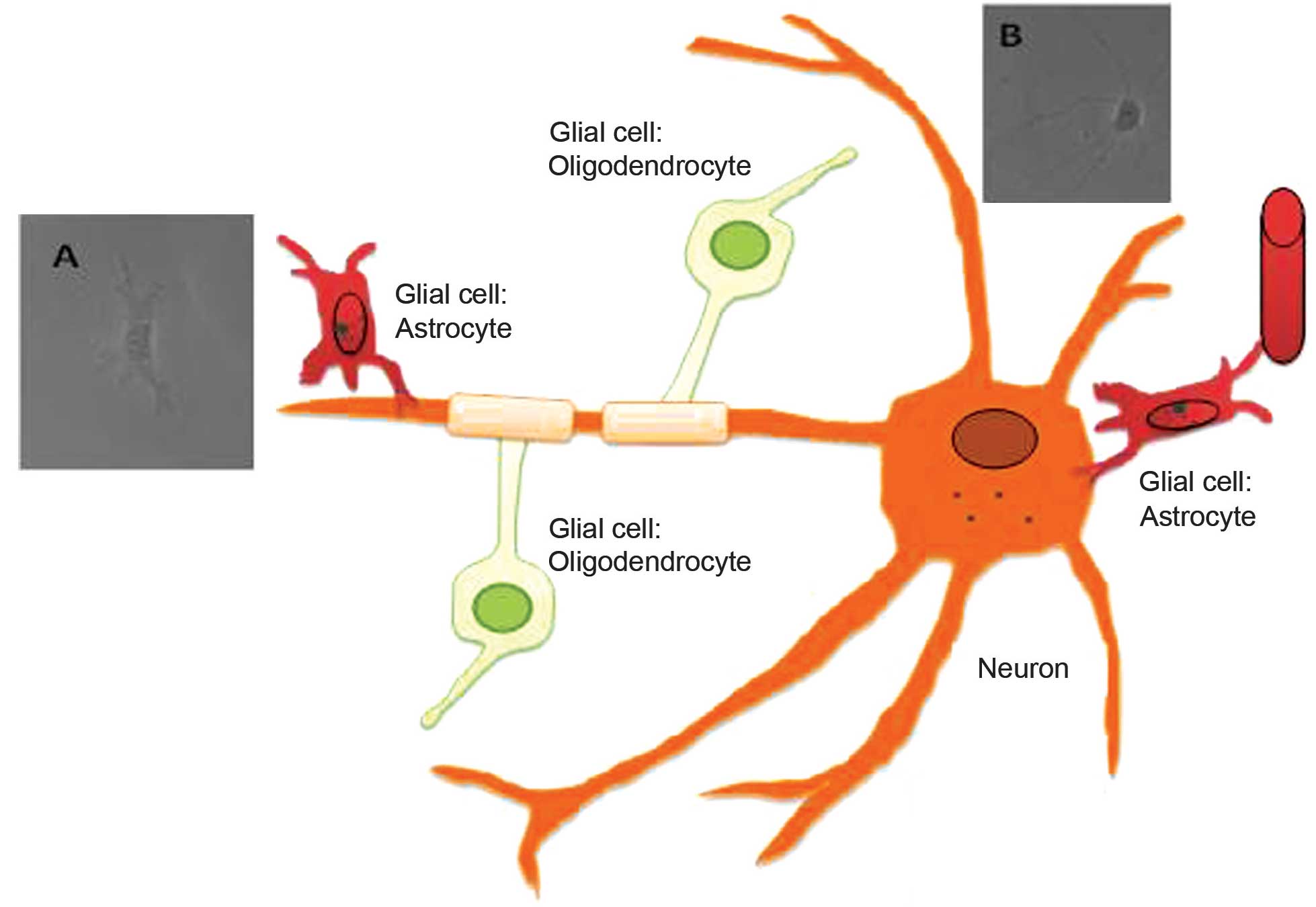
Introduction
Radiation therapy is among the essential treatment
modalities for primary and metastatic brain tumors. However,
subsequent cognitive function decline and developmental disorders
following radiation therapy to the brain must be overcome,
particularly in pediatric patients (1–5).
The central nervous system (CNS) is composed mainly
of neurons, glial cells and vascular endothelial cells (Fig. 1). Neurons, the majority of which
cease cell proliferation during fetal development, have been
considered to be more radioresistant than glial and vascular
endothelial cells, which continue to proliferate subsequent to
birth. Molecular studies have provided evidence that glial cells
are essential for the survival of neurons by supplying trophic
factors to the neurons (6–9). Thus, the mechanism underlying the
late adverse brain effects of radiation therapy has been believed
to mainly be the insufficient supply of nutrients and blood to
neurons due to the impaired functions of irradiated glial and
vascular endothelial cells, rather than a direct effect of the
radiation itself on neurons.
It was shown in the late 1990s that adult
neurogenesis occurs in certain areas of the brain, including the
subventricular zone (SVZ) and subgranular layer (SGL) (10). In addition, radiation-induced
apoptosis of neural progenitor cells was observed in the SVZ and
SGL, and relatively high radiosensitivity was demonstrated in
neurons residing in the areas where neurogenesis occurs (11,12).
These findings have raised the possibility that radiation-induced
neuronal death may be one of the causes of the late adverse effects
of radiation therapy, such as functional and developmental
disorders. Therefore, attempts have been made to prevent adverse
effects from developing following cranial irradiation using
intensity-modulated radiation therapy to reduce the dose to areas
that may be highly radiosensitive, such as the SVZ and SGL
(13–16).
Radiation affects neurons and glial and vascular
endothelial cells. It is therefore difficult to evaluate the
radiation sensitivities of these cell types separately in in
vivo studies. Furthermore, the majority of the previously
reported in vitro studies were conducted on a mixture of
neurons and glial cells (17).
However, due to technical difficulties, only a few investigations,
including our previous studies (18,19),
have examined the radiosensitivity of neurons by employing
monocultures of this cell type alone.
To estimate the extent of the involvement of neurons
and glial cells in the adverse brain effects of radiation therapy,
it is essential to compare radiosensitivities between glial cells
and neurons. A previous study using glial cells and neurons
cultured separately for such a comparison demonstrated the
radiosensitivity of glial cells to be comparable to that of neurons
(17). However, the cells used in
that study were isolated from different individuals (different
genetic backgrounds) and cultured for different lengths of time
(different developmental stages), such that the results are not
entirely convincing. In the present study, neurons and glial cells
were therefore obtained from the same rat to ensure uniform
conditions (identical genetic backgrounds and developmental stages)
and their radiosensitivities were investigated separately.
Materials and methods
Cell culture
The modified Banker’s method was used for primary
neuronal cultures (20). Briefly,
cells were obtained from the hippocampi of Wistar rat fetuses (Imai
Jikkendobutu Shiikujo, Saitama, Japan) at embryonic day 18, treated
with trypsin and mechanically dispersed by trituration with Pasteur
pipettes. The cells were then seeded at a density of 5,000
cells/cm2 on glass coverslips coated with poly-L-lysine
and cultured in minimum essential medium (MEM; Invitrogen Life
Technologies, San Diego, CA, USA) for 3 h. The coverslips were then
transferred to culture dishes containing a monolayer of supporting
glial cells maintained in serum-free MEM supplemented with B27
(Invitrogen Life Technologies). Cytosine β-D-arabinofuranoside
(Sigma, St. Louis, MO, USA) (10 μM) was added to the culture medium
at 3 days in vitro (DIV) to inhibit glial cell
proliferation. Neurons were irradiated at 7 or 21 DIV. For
X-irradiation of the cells, the cover slips were transferred to
another culture dish containing only medium and no glial cells.
Immediately subsequent to the irradiation of the neurons, the cover
slips were returned to the original culture dishes. Although the
neurons were in direct contact with the glial cells, they were
easily separable; thus, it was possible to irradiate and observe
only neurons.
Glial cells were obtained from the cerebral cortex
of the same Wistar rat fetus as that used for obtaining neurons at
embryonic day 18. Briefly, the cells were treated with trypsin,
dispersed by trituration with Pasteur pipettes and then seeded at a
density of 5,000 cell/cm2 on glass coverslips coated
with poly-L-lysine and cultured in MEM. Four days later, the cells
were again treated with trypsin, dispersed with Pasteur pipettes
and then cultured in new MEM. Glial cells were also irradiated at 7
DIV or 21 DIV. For X-irradiation of the cells, the cover slips were
transferred to another culture dish containing only medium and no
glial cells. Following irradiation of the glial cells, the cover
slips were returned to the original culture dishes.
All animal experiments were performed in accordance
with the guidelines set by the Animal Care and Experimentation
Committee (Gunma University, Maebashi, Japan).
Irradiation and cell fixation
At 7 and 21 DIV, neural and glial cells were
irradiated with 200 kV X-rays (Siemens-Asahi Medical Technologies
Ltd., Tokyo, Japan) at a dose of 50 Gy. At 24 h after irradiation,
the cells were fixed in 4% paraformaldehyde for 24 h at 4°C.
Non-irradiated culture cells were handled in parallel with the
irradiated samples as a control.
Assessment of apoptosis
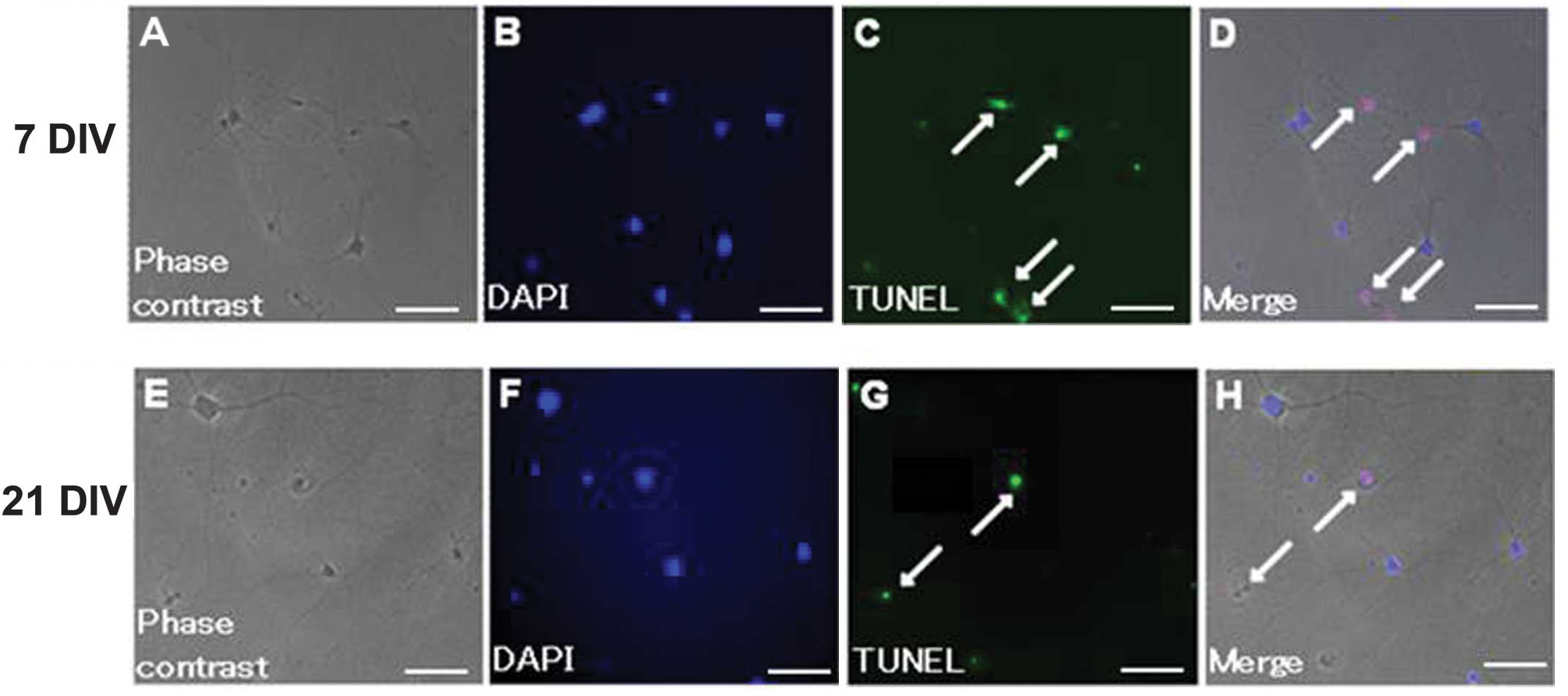
Apoptosis was determined by terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) assay using the ApopTag® Plus In Situ
Apoptosis Fluorescein Detection kit (Chemicon International,
Temecula, CA, USA). Fixed cells on coverslips were permeabilized in
ethanol:acetic acid (2:1) for 15 min at −20°C. The cells were then
washed twice with phosphate-buffered saline (pH 7.4) for 5 min and
incubated with ApopTag equilibration buffer for 5 min, followed by
terminal deoxynucleotidyl transferase linkage of digoxigenin-tagged
dUTP to the 3′-OH termini of DNA fragments at 37°C for 60 min. The
reaction was terminated at 37°C in stop/wash buffer for 30 min and
the cells were then washed. Subsequent to washing, the cells were
incubated with anti-digoxigenin fluorescein antibody for 30 min and
the coverslips were then mounted on slides with
Vectashield® Mounting Medium with DAPI (Vector
Laboratories, Burlingame, CA, USA).
Evaluation method
Fluorescein-labeled cells were observed under a
Zeiss Axioplan microscope (Carl Zeiss AG, Jena, Germany) equipped
with a Photometrics CoolSnap FX cooled CCD camera (Photometrics,
Tucson, AZ, USA) using MetaMorph software (Universal Imaging Corp.,
West Chester, PA, USA). Apoptotic cells were counted on each slide.
The apoptotic index (AI) was calculated as the number of DAPI- and
TUNEL-positive cells divided by the number of DAPI-positive cells.
The cells positive for TUNEL and negative for DAPI were excluded
from the calculations.
Statistical analysis
Statistical analysis was performed using StatMate
software (GraphPad Software, Inc., San Diego, CA, USA). P<0.01,
as determined using a Student’s t-test, was considered to indicate
a statistically significant difference. All results are shown as
the mean ± standard deviation.
Results
The average numbers of neurons and glial cells
counted in each coverslip were 332 (range, 211–556) and 273 (range,
131–292), respectively. Representative images of irradiated neurons
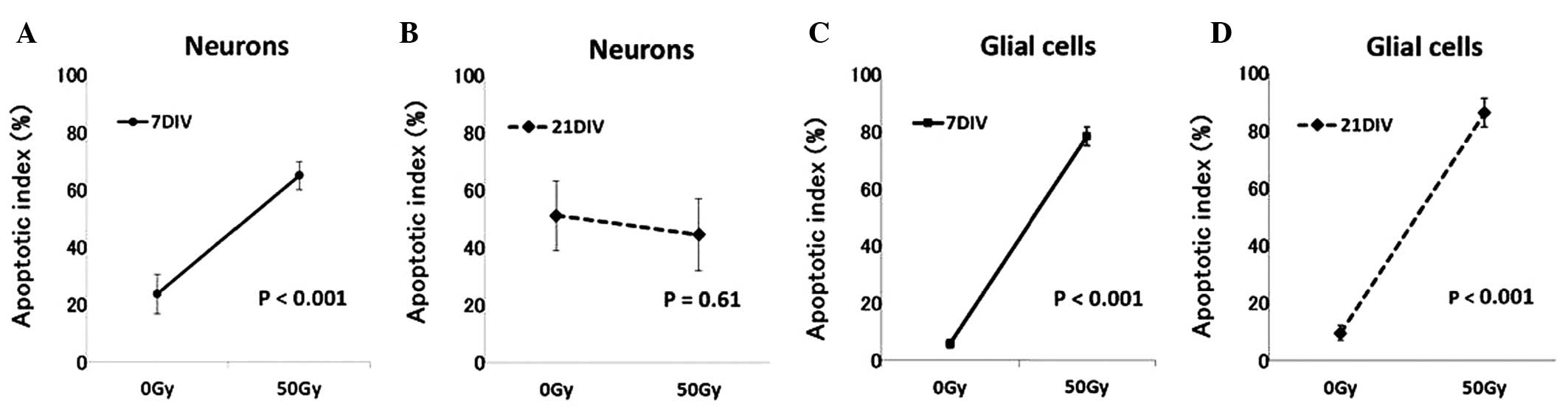
are shown in Fig. 2. The AI of 7
DIV neurons was 23.7±6.7% (n=3) in the control group and
significantly higher, 64.9±4.8% (n=3), in the 50 Gy irradiated
group (P<0.001) (Fig. 3A). At
21 DIV, the AI of neurons was 52.1±17.4% (n=9) in the control group
and 44.6±12.5% (n=8) in the irradiated group; no significant
difference was identified in the number of apoptotic cells between
the two groups (P=0.61) (Fig. 3B).
The average AI of 7 DIV glial cells was 5.8±1.5% (n=3) in the
control group and 78.4±3.3% (n=3) in the 50 Gy irradiated group
(Fig. 3C), and the average AI of
21 DIV glial cells was 9.6±2.6% (n=4) in the control group and
86.3±4.9% (n=4) in the 50 Gy irradiated group (Fig. 3D). The differences between the
control and 50 Gy irradiated groups were significant at 7 DIV
(P<0.001) as well as at 21 DIV (P<0.001).
Comparisons at the corresponding time-points
revealed both glial cells and neurons to be radiosensitive at 7
DIV, whereas glial cells but not neurons were radiosensitive at 21
DIV.
Discussion
Our previous study revealed 7 DIV neurons
(morphologically and functionally immature cells) to be relatively
radiosensitive, while 21 DIV neurons (morphologically and
functionally mature cells) were found to be extremely
radioresistant, showing no increase in apoptosis even following
high-dose irradiation (19).
Furthermore, when 7 DIV neurons were exposed to low doses of
X-irradiation (0, 5, 4 and 10 Gy) and further cultured for 14 and
21 days in total, the number of apoptotic cells increased, and the
clustering of synaptic proteins, indicative of the maturation of
synapses, decreased dose-dependently following irradiation
(18). Consistent with our
previous findings, the present results showed that the number of 7
DIV neurons undergoing apoptosis increased following irradiation,
whereas radiation did not significantly increase apoptosis in 21
DIV neurons. These results indicate that radiosensitive immature
neurons become radioresistant with maturation and that mature
neurons are radioresistant.
The AI of glial cells did not differ significantly
between 7 and 21 DIV in this study. Although no study has focused
on the association between the maturity and radiosensitivity of
glial cells, if the maturities of these cells reflect their
radiosensitivity, our present results may suggest their maturities
to be similar at 7 and 21 DIV. In other words, since glial cells
have the ability to proliferate (gliogenesis), unlike neurons, it
is assumed that a glial cell population would represent a mixture
of cells with differing maturities due to this proliferation. Thus,
their similar radiosensitivities suggest that the glial cells in
this study may have been at similar maturation stages.
Following irradiation, glial cells may undergo
mitotic cell death. Furthermore, we observed in a previous study
that a large percentage of neurons underwent delayed apoptosis
subsequent to irradiation (18).
Thus, comparing the radiosensitivities of neurons and glial cells
based on their AIs at 24 h after irradiation can be difficult. A
number of studies have shown that the ability to repair radiation
damage differs among sites (10,21).
Eriksson et al (10)
reported that adult neurogenesis occurs in both the SVZ and the
SGL, and Seaberg et al (21) showed that stem cells with
pluripotency and self-renewal ability were present in the SVZ,
while the SGL contained predominantly neural progenitor cells
without pluripotency and fewer stem cells. In other words, due to
the presence of radioresistant and pluripotent stem cells in the
SVZ, neurogenesis may occur following irradiation in this area,
whereas recovery subsequent to irradiation may be poor in the SGL
where the number of the stem cells is limited. In a study by
Hellström et al (22), the
volume and rate of DNA synthesis following whole brain irradiation
were reported to be significantly higher in the SVZ than in the SGL
(22). Glial cells have the
ability to proliferate, such that damaged glial cells can be
replaced by gliogenesis. Therefore, to understand the mechanisms
underlying the adverse effects of radiation therapy on the brain,
the neurogenesis, restoration of glial cells and secondary effects
due to brain blood vessels being impaired by irradiation must all
be taken into consideration. Furthermore, radiation effects on the
brain may vary according to the irradiation site, extent and dose,
due to the heterogeneous distribution of neural stem cells.
In conclusion, the radiosensitivities of neurons and
glial cells, obtained from the same rat brain, were evaluated by
examining the number of cells undergoing radiation-induced
apoptosis. The results showed both glial cells and neurons to be
radiosensitive at 7 DIV, whereas only glial cells were
radiosensitive at 21 DIV; neurons exhibited radioresistance at 21
DIV. Further studies are required to elucidate the mechanisms
underlying the late adverse effects of radiation therapy on the
CNS.
References
|
1
|
Hall EJ and Giaccia AJ: Radiobiology for
the Radiologist. Sixth edition. Lippincott Williams & Wilkins;
Philadelphia, PA: 2006
|
|
2
|
Chang EL, Wefel JS, Hess KR, et al:
Neurocognition in patients with brain metastases treated with
radiosurgery or radiosurgery plus whole-brain irradiation: a
randomised controlled trial. Lancet Oncol. 10:1037–1044. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakaya K, Hasegawa T, Flickinger JC,
Kondziolka DS, Fellows-Mayle W and Gobbel GT: Sensitivity to
radiation-induced apoptosis and neuron loss declines rapidly in the
postnatal mouse neocortex. Int J Radiat Biol. 81:545–554. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi L, Molina DP, Robbins ME, Wheeler KT
and Brunso-Bechtold JK: Hippocampal neuron number is unchanged 1
year after fractionated whole-brain irradiation at middle age. Int
J Radiat Oncol Biol Phys. 71:526–532. 2008.PubMed/NCBI
|
|
5
|
Takadera T, Sakamoto Y and Ohyashiki T:
NMDA receptor 2B-selective antagonist ifenprodil-induced apoptosis
was prevented by glycogen synthase kinase-3 inhibitors in cultured
rat cortical neurons. Brain Res. 1020:196–203. 2004. View Article : Google Scholar
|
|
6
|
Allen NJ and Barres BA: Signaling between
glia and neurons: focus on synaptic plasticity. Curr Opin
Neurobiol. 15:542–548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Volterra A and Meldolesi J: Astrocytes,
from brain glue to communication elements: the revolution
continues. Nat Rev Neurosci. 6:626–640. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamazaki Y, Hozumi Y, Kaneko K, et al:
Direct evidence for mutual interactions between perineuronal
astrocytes and interneurons in the CA1 region of the rat
hippocampus. Neuroscience. 134:791–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye ZC and Sontheimer H: Astrocytes protect
neurons from neurotoxic injury by serum glutamate. Glia.
22:237–248. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eriksson PS, Perfilieva E, Björk-Eriksson
T, et al: Neurogenesis in the adult human hippocampus. Nat Med.
4:1313–1317. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peissner W, Kocher M, Treuer H and
Gillardon F: Ionizing radiation-induced apoptosis of proliferating
stem cells in the dentate gyrus of the adult rat hippocampus. Brain
Res Mol Brain Res. 71:61–68. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tada E, Parent JM, Lowenstein DH and Fike
JR: X-irradiation causes a prolonged reduction in cell
proliferation in the dentate gyrus of adult rats. Neuroscience.
99:33–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barani IJ, Cuttino LW, Benedict SH, et al:
Neural stem cell-preserving external-beam radiotherapy of central
nervous system malignancies. Int J Radiat Oncol Biol Phys.
68:978–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghia A, Tomé WA, Thomas S, et al:
Distribution of brain metastases in relation to the hippocampus:
implications for neurocognitive functional preservation. Int J
Radiat Oncol Biol Phys. 68:971–977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gutiérrez AN, Westerly DC, Tomé WA, et al:
Whole brain radiotherapy with hippocampal avoidance and
simultaneously integrated brain metastases boost: a planning study.
Int J Radiat Oncol Biol Phys. 69:589–597. 2007.PubMed/NCBI
|
|
16
|
Jaganathan A, Tiwari M, Phansekar R, Panta
R and Huilgol N: Intensity-modulated radiation to spare neural stem
cells in brain tumors: a computational platform for evaluation of
physical and biological dose metrics. J Cancer Res Ther. 7:58–63.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gobbel GT, Bellinzona M, Vogt AR, Gupta N,
Fike JR and Chan PH: Response of postmitotic neurons to
X-irradiation: implications for the role of DNA damage in neuronal
apoptosis. J Neurosci. 18:147–155. 1998.PubMed/NCBI
|
|
18
|
Okamoto M, Suzuki Y, Shirai K, et al:
Effect of radiation on the development of immature hippocampal
neurons in vitro. Radiat Res. 172:718–724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shirai K, Mizui T, Suzuki Y, Kobayashi Y,
Nakano T and Shirao T: Differential effects of x-irradiation on
immature and mature hippocampal neurons in vitro. Neurosci Lett.
399:57–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goslin K, Asmussen H and Banker G: Rat
hippocampal neurons in low-density culture. Banker G and Goslin K:
Culturing Nerve Cells. Second edition. The MIT Press; Cambridge,
MA: pp. 339–370. 1998
|
|
21
|
Seaberg RM and van der Kooy D: Adult
rodent neurogenic regions: the ventricular subependyma contains
neural stem cells, but the dentate gyrus contains restricted
progenitors. J Neurosci. 22:1784–1793. 2002.
|
|
22
|
Hellström NA, Björk-Eriksson T, Blomgren K
and Kuhn HG: Differential recovery of neural stem cells in the
subventricular zone and dentate gyrus after ionizing radiation.
Stem Cells. 27:634–641. 2009.PubMed/NCBI
|