Introduction
Linear porokeratosis (LP), punctate porokeratosis
(PP), disseminated palmoplantar porokeratosis (DPP) and genital
porokeratosis are uncommon disorders resulting from epidermal
keratinization. Porokeratosis of Mibelli (PM), disseminated
superficial porokeratosis (DSP) and disseminated superficial
actinic porokeratosis (DSAP) are more common subtypes of
porokeratosis, from which an increasing number of people suffer.
Porokeratosis includes several clinical variants with a wide range
of clinical presentations, which all have histologically in common
the presence of a cornoid lamella. At present, several variants
have been reported to be inherited as an autosomal dominant trait
(1,2). According to the distribution of the
lesion, porokeratosis may be classified into two types, the
localized variants, including PM, LP and PP, and the extensive
variants, including DSP, DSAP and DPP (1).
Although, porokeratosis was first reported more than
a century ago, the etiology and pathogenesis remains unclear and
the results from different studies are contradictory. Certain
mutations that are associated with porokeratosis, including
frameshift mutations, have been identified (2,3).
However, these events are confined to certain pedigrees (4). Clinical and molecular evidence has
demonstrated that porokeratosis can be considered to be a
premalignant skin condition (5,6).
However, effective treatments to cure porokeratosis are currently
lacking. In the last twenty years, there were numerous cases of
porokeratosis reported in families (2,3,7).
Furthermore, there have also been a number of case reports,
nevertheless, no more than 31 cases have been investigated
individually.
Genital porokeratosis is extremely rare. To date,
only 26 cases have been reported in previous studies (8–22). A
case report of 10 cases of genital porokeratosis in Taiwan revealed
that 30% had diabetes mellitus (18). More cases are required to determine
whether this is associated with genital porokeratosis.
The aim of the present study was to define the
clinical features of porokeratosis, with particular emphasis on
genital porokeratosis, and to investigate the potential etiology. A
total of 55 cases of porokeratosis were analyzed in the present
study. To the best of our knowledge, this may be the largest scale
survey of porokeratosis, including for genital porokeratosis.
Subjects and methods
Subjects
In the present study, cases were identified from
Huashan Hospital (Shanghai, China). All subjects received careful
examination by three dermatologists, and the diagnosis of
porokeratosis was confirmed by histological examination of a skin
biopsy.
Statistical analysis
Statistical analysis was performed using SPSS
version 11.0.0 (SPSS, Inc., Chicago, IL, USA). The Chi-square test
was performed to analyze the effect of inheritance factors and
gender on the clinical variants. The Student-Newman-Keuls test was
used for the analysis of the age of onset of the clinical variant.
The age of onset of the clinical variant is presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographics
A total of 55 cases of porokeratosis diagnosed by
histopathology between 2000 and 2007 from Huashan Hospital
(Shanghai, China) were reviewed retrospectively. The present study
enrolled 39 males and 16 females, with an average age of 51.33
years (range, 5–84 years). Among them, 12 individuals had a family
history of porokeratosis, with ≥1 first-degree relatives suffering
from porokeratosis, however, the pattern of inheritance was not
determined. The mean age of onset was 39.42 years (range, 5–76
years), with a mean age of onset of 25.0 years in inherited cases
and 43.42 years in uninherited cases.
Clinical manifestations
In the present study, patients were classified into
four clinical variants: i) 22 cases of PM; ii) 17 cases of DSAP
(with the main part of the lesions developed in sun-exposed areas);
iii) 15 cases of DSP; and iv) one case of LP. PP and DPP were not
detected in the present study. The number of uninherited cases with
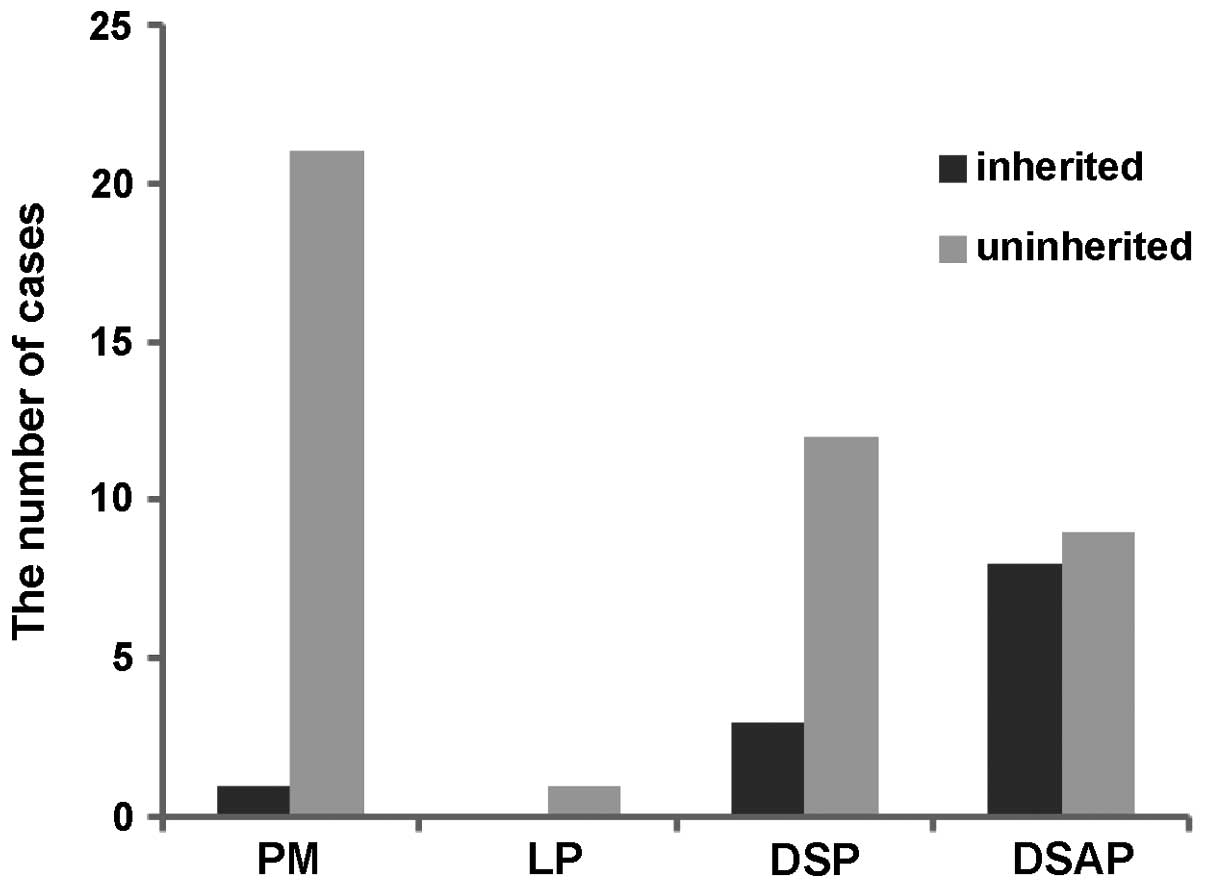
PM and DSP was significantly higher than inherited cases (Fig. 1), however, no difference was
observed in the DSAP cases (Fig.
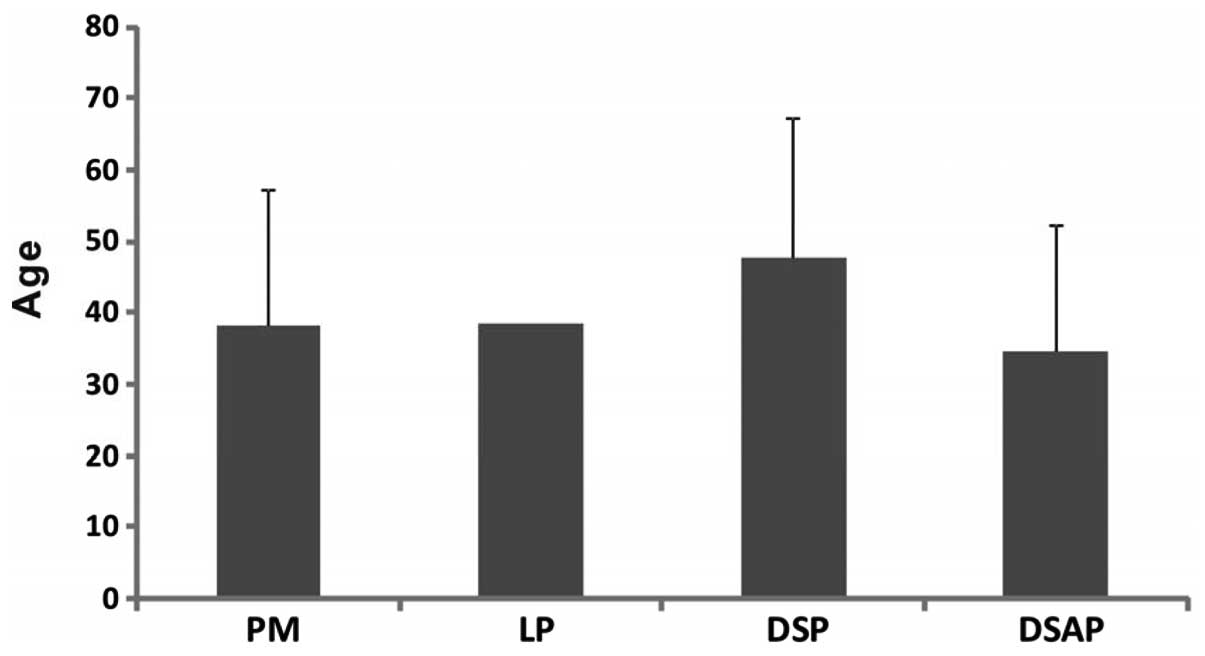
1). In the analysis of the age of onset and clinical variant,
no significant difference among these variants was identified
(Fig. 2). The number of cases of
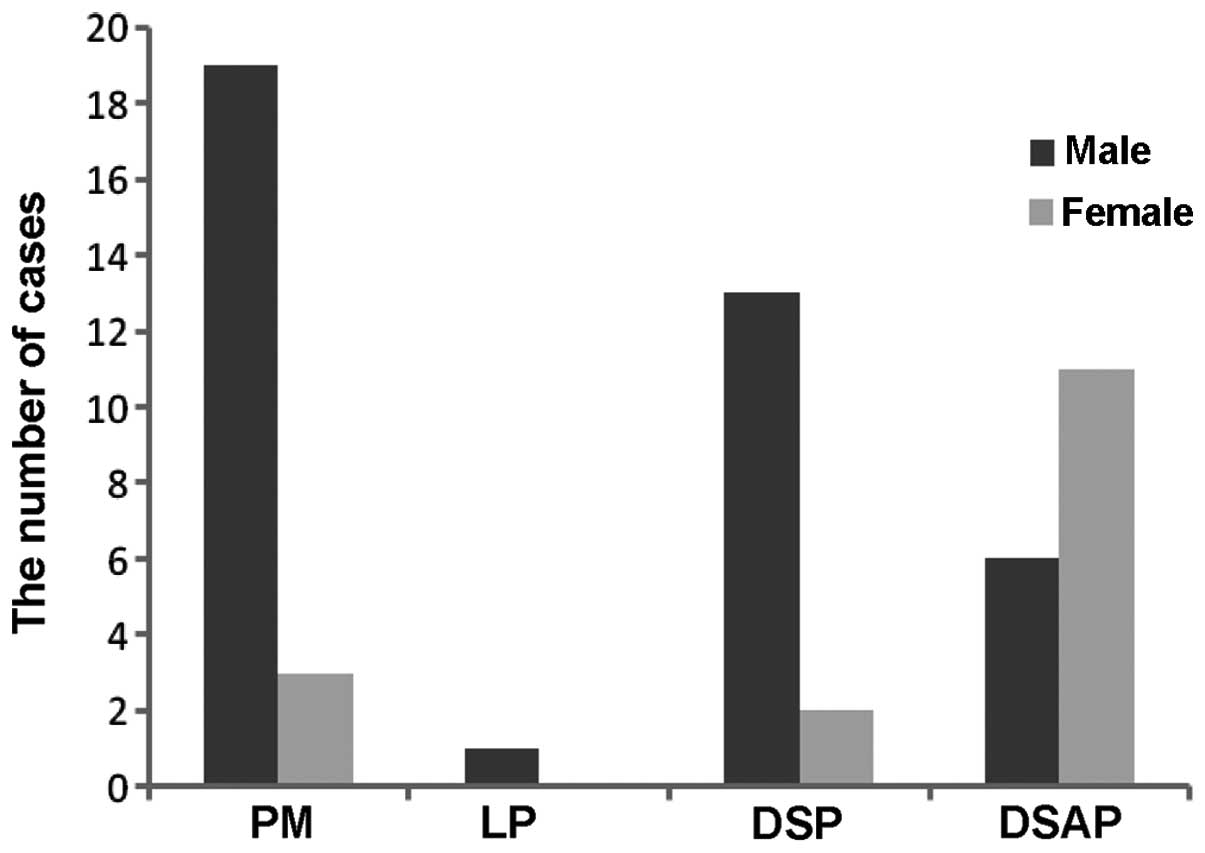
PM and DSP was higher in males, whilst the number of cases of DSAP
was higher in females (Fig. 3).
The lesions initiated on the face in 17 cases, on the body in 5
cases, on the limbs in 20 cases, on the buttock in 4 cases and in
the genital area in 9 cases (Table
I).
 | Table IAnalysis of the initial regions of the
lesions from different clinical variants of porokeratosis. |
Table I
Analysis of the initial regions of the
lesions from different clinical variants of porokeratosis.
| | | | Initial region |
|---|
| | | |
|
|---|
| Clinical type | Number of cases | Age of onset, range
(years) | Face, n (%) | Body, n (%) | Limb, n (%) | Buttock, n (%) | Genital area, n
(%) |
|---|
| PM | 22 | 22–76 | 2 (9.09) | 1 (4.55) | 7 (31.82) | 1 (4.55) | 11 (50.00) |
| LP | 1 | 39 | 0 (0.00) | 1 (100) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| DSP | 15 | 16–68 | 1 (6.67) | 3 (20) | 10 (66.67) | 1 (6.67) | 0 (0.00) |
| DSAP | 17 | 5–52 | 14 (82.35) | 1 (5.88) | 2 (11.76) | 0 (0.00) | 0 (0.00) |
In total, 40 patients investigated in the present
study suffered from pruritus, with no significant difference
observed between the clinical variants. Eight patients, including
six patients with PM and two patients with DSP, also had verrucous
hyperplasia centralized in the buttock and pubic region, and all
eight patients suffered from conspicuous pruritus. The clinical
features of the clinical variants are shown in Table II.
 | Table IIClinical manifestations of the
clinical variants. |
Table II
Clinical manifestations of the
clinical variants.
| Clinical variant | Number of cases | Obvious keratotic
border, n (%) | Verrucous hyperplasia
n (%) | Pruritus n (%) |
|---|
| PM | 22 | 15 (68.18) | 6 (27.27) | 19 (86.36) |
| LP | 1 | 0 (0.00) | 0 (0.00) | 1 (100) |
| DSP | 15 | 12 (80) | 2 (13.33) | 11 (73.33) |
| DSAP | 17 | 11 (64.71) | 0 (0.00) | 9 (52.94) |
Associated systemic conditions
Two patients with DSP had a long-term history of
corticosteroid use. The lesions increased following acute renal
failure in one patient with inherited DSP, with a darkening in
color of the lesion observed and a raising of the hyperkeratotic
margin. The lesions of one patient with PM was in burn scar tissue,
whilst another patient had PM lesions in the compressed region of
infected folliculitis. One patient had lesions which spread all
over the body and four limbs diagnosed as DP three months after
suffering from infecting viral pneumonia. One patient with genital
porokeratosis also suffered from condyloma acuminatum. A total of
14 patients, including 11 patients with DSAP lesions, found that
the lesions became darker and pruritus was aggravated when exposed
to the sun, however, in winter, the symptoms were alleviated.
Treatment and follow-up
Surgical excision was performed on seven patients
with PM and no recurrence of porokeratosis was observed. Topical
treatments were used in the remaining 48 patients, including
isotretinoin, vitamin D3 and its derivatives, corticosteroid,
imiquimod, hypericin and diclofenac gel.
The choice and use of the drug depended on the
individual patient. During the five-year follow-up period, no
noticeable aggravation of the lesions was detected.
Genital porokeratosis
In the present study, 11 patients, 10 males and one
female, suffered from genital porokeratosis (Table III). The lesions were observed in
the genital area only in nine patients, whilst the other two
patients suffered from lesions in the genital and adjacent areas.
All 11 patients had no family history of porokeratosis.
Hypertrophic plaques were found in five patients, whilst multiple
papules with verrucous hyperplasia were observed in three patients.
Nine patients suffered from pruritus and this symptom was
exacerbated at high temperatures. One patient had a medical history
of condyloma acuminatum and one patient had a medical history of
folliculitis. Several cases of genital porokeratosis coexisting
with genital diseases, for example condyloma acuminatum and
syphilis, were reported, however, whether these diseases are
associated with genital porokeratosis remains to be elucidated
(18). Surgical excision was
performed on two patients and no recurrence was identified during
the follow-up period. The remaining nine patients received retinoic
acid and corticosteroid treatment on the lesions, twice a day. The
treatment relieved pruritus, however, the lesions showed no obvious
disappearance. During the follow-up period, no obvious clinically
suspected malignant transformation was noted and no further growth
from previous lesions was observed.
 | Table IIIPatient demographics for genital
porokeratosis. |
Table III
Patient demographics for genital
porokeratosis.
| Patient | Gender | Age of onset
(years) | Course of disease
(years) | Affected regions | Symptoms | Medical
History | Clinical
manifestation |
|---|
| 1 | Male | 8 | 1 | Perianal
region | Pruritus | No | Reddish plaque
(1.5×10 cm) with raised border |
| 2 | Male | 22 | 8 | Scrotum and
penis | Pruritus | No | Verrucous
papule |
| 3 | Female | 25 | 6 | Labia majora | Pruritus | No | Verrucous papule
(3×4 mm) |
| 4 | Male | 28 | 5 | Scrotum and
penis | Pruritus | No | Multiple verrucous
papules 3×3 mm |
| 5 | Male | 26 | 8 | Perianal
region | Pruritus | No | Reddish plaques
with raised borders |
| 6 | Male | 36 | 10 | Scrotum | Pruritus | Condyloma
acuminatum | Reddish plaque (1.5
cm) with raised border |
| 7 | Male | 52 | 3 | Perianal
region | No | No | Reddish papule (3×4
mm) |
| 8 | Male | 48 | 8 | Perianal
region | Pruritus | No | Reddish and
keratotic plaque (1×3 cm) |
| 9 | Male | 61 | 2 | Perianal region and
buttock | Pruritus | Folliculitis | Multiple reddish
papules with raised borders |
| 10 | Male | 50 | 18 | Perianal region and
buttock | Pruritus | No | Multiple reddish
plaques with raised borders |
| 11 | Male | 50 | 34 | Scrotum | No | No | Keratotic plaque (4
mm) |
Discussion
Several family reports for porokeratosis have been
previously published (2,3,23)
and a considerable portion of the reports described DSAP families.
Our previous studies demonstrated that mutations in SSH1 or ARPC3
are responsible for DSAP in certain pedigrees (2,3).
Furthermore, in the present study, it was observed that among the
inherited cases (12 cases), eight cases were DSAP, which accounted
for 66.7% of the inherited cases. Therefore, this suggests that
inheritance may potentially be important in DSAP, however, the
potential genetic mechanisms of DSAP remain to be elucidated. As
with other inherited diseases, inherited cases of porokeratosis had
a significantly earlier average age of onset (25.08 years),
compared with sporadic cases (43.42 years).
Previous studies have demonstrated that males more
frequently suffer from porokeratosis, compared with females
(1,24). A total of 39 males and 16 females
were enrolled in the present study. It is generally accepted that
PM is more common in males than in females (1), and the results from the present study
suggest that this may also be true for DSP.
Porokeratosis may involve every part of the body,
including the oral mucosa. According to previous studies, PM is
usually confined to the four limbs (25). However, in the cases included the
present study, the initial region was primarily the buttock and
genital area. Previous studies have also found that the initial
region of DSAP was usually the skin often exposed to sunlight, but
rarely the face. By contrast, in the present study, it was observed
that 82.4% of cases with DSAP lesions originated from the face. A
family report of DSAP in China with 100 cases described that the
lesions all originated from the face (23). This suggests that the initial
region of origin of DSAP in the Chinese population is the face,
which differs from the results observed in Caucasians (26).
In eight cases, including six PM and two DSP,
verrucous hyperplasia of plaques and multiple papules were observed
associated with obvious pruritus (Table II). This suggested that verucous
hyperplasia may be caused by friction, scratching, long-term
compression, partial moisture and chronic diseases.
The etiology and pathogenesis of porokeratosis are
complex and multifactorial (27).
At present, genetic factors, ultraviolet light, trauma,
immunosuppression and infectious agents have been implicated in
porokeratosis (27). A prospective
study revealed that 10.68% of renal transplant recipients (103
patients in total) suffered from porokeratosis (28). In the present retrospective
analysis, two patients developed lesions following long-term
systemic corticosteroids treatment for allergic asthma and chronic
nephritis. In addition, in one patient with inherited
porokeratosis, the lesions aggravated following acute renal
failure. These cases imply that immune disorders may be associated
with porokeratosis.
Although no direct association between porokeratosis
and ultraviolet light has been verified, it is generally accepted
that exposure of lesions to ultraviolet light should be avoided. In
the present study, in 14 cases, including 11 cases of DSAP, it was
reported that pruritus was aggravated, along with a darkening of
the lesions, when lesions were exposed to sunlight. In the winter,
the symptoms were alleviated. A previous study has suggested that
ultraviolet light may affect the immune function in localized skin
(29). In any case, it is clear
that exposure of lesions to ultraviolet light should be avoided
(29–31).
Certain studies have revealed that porokeratosis may
also be caused by burns and infection (32,33).
In the present study, it was observed that porokeratosis occurred
in one patient with burn scar tissue, one patient suffering from
folliculitis and another patient suffering from viral pneumonia.
Surgical removal of the lesions was performed in these cases and no
recurrence was observed.
In the present study, all the patients suffering
from genital porokeratosis had no family history of porokeratosis
and no obvious contributing factor was identified. An analysis of
all the reported cases of genital porokeratosis (29 previously
reported cases and 11 cases reported in the present study) found
that 65% of patients were Chinese and 92.5% of patients were males
(18–21,34,35).
However, whether the Chinese population and males are more
susceptible to genital porokeratosis requires further
investigation. A retrospective analysis of 10 cases performed in
Taiwan suggested that the humid climate may be associated with
genital porokeratosis (18). It
was found that 78.4% of cases had pruritus and 62.2% of cases had
hyperplastic lesions. Compared with cases from previous studies,
the present study found that the majority of cases in China
suffered from pruritus, however, the majority of cases in western
countries did not (8,20).
Due to its distinct clinical characteristics, it has
been suggested that genital porokeratosis should be classified as a
new clinical variant (18).
According to the characteristics and the region of the lesions,
five subtypes of variations in morphology in the 11 cases of
genital porokeratosis were observed: : i) classical porokeratosis
of Mibelli, with classical round-shaped lesions, characterized by
atrophic patches surrounded by an elevated border, which was often
distributed in the penis, scrotum and pubic region; ii)
hyperkeratotic variant, characterized by a thickening of the
central region and a slightly raised hyperkeratotic border, often
distributed in the perianal region and buttock; iii) porokeratosis
ptychotropica (36), characterized
by butterfly-like plaques, with verrucous hyperplasia, primarily
distributed on the buttock, however, occasionally located in other
wrinkling regions; iv) ulceroproliferative porokeratosis, with
ulcerative round-shaped plaques, often distributed on the penis and
scrotum; v) verrucous hyperplasia, characterized by keratotic and
hypertrophic plaques and nodules in the genital region.
Due to similar clinical manifestations, genital
porokeratosis may easily be misdiagnosed as psoriasis, verrucous
tuberculosis of the skin, verrucous lichen planus, syphilis and
condyloma acuminatum.
The rate of malignant transformation is high in PM,
(~6.8–11% of cases) (1). If the
lesions spread excessively, surgery, laser treatment and
cryotherapy are suggested. Surgery is the most effective treatment;
however, due to the size, number and location of the lesions
surgery may not always be possible. In the present study, surgical
excisions were performed in seven cases and, during the five-year
follow-up period, no recurrence was observed. For cases where
surgery is unavailable, the use of tropical drugs and other
treatment modalities, including laser treatment and cryotherapy,
are required as well as regular follow-up care in order to manage
and control the disease. In cases where malignant transformation
was detected, localized surgery was able to be performed.
In conclusion, it was observed that DSAP may
originate from the face in the Chinese population, which differs
from what is observed in Caucasians. The present study also found
that genital porokeratosis was often associated with pruritus.
Since no recurrence of porokeratosis was observed following
surgical excision, this suggests that surgical excisions may
completely cure porokeratosis. Therefore, it is suggested that
surgical excisions should be performed for the treatment of
porokeratotic lesions if possible and regular follow-up is
required, given that malignancy is possible in porokeratosis.
References
|
1
|
Schamroth JM, Zlotogorski A and Gilead L:
Porokeratosis of Mibelli. Overview and review of the literature.
Acta Derm Venereol. 77:207–213. 1997.PubMed/NCBI
|
|
2
|
Zhang Z, Niu Z, Yuan W, et al: Fine
mapping and identification of a candidate gene SSH1 in disseminated
superficial actinic porokeratosis. Hum Mutat. 24:4382004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang ZH, Huang W, Niu ZM, et al: Two
closely linked variations in actin cytoskeleton pathway in a
Chinese pedigree with disseminated superficial actinic
porokeratosis. J Am Acad Dermatol. 52:972–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frank J, van Steensel MA and van Geel M:
Lack of SSH1 mutations in Dutch patients with disseminated
superficial actinic porokeratosis: is there really an association?
Hum Mutat. 28:1241–1242; author reply 1243–1244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Otsuka F, Shima A and Ishibashi Y:
Porokeratosis as a premalignant condition of the skin. Cytologic
demonstration of abnormal DNA ploidy in cells of the epidermis.
Cancer. 63:891–896. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Magee JW, McCalmont TH and LeBoit PE:
Overexpression of p53 tumor suppressor protein in porokeratosis.
Arch Dermatol. 130:187–190. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia JH, Yang YF, Deng H, et al:
Identification of a locus for disseminated superficial actinic
porokeratosis at chromosome 12q23.2-24.1. J Invest Dermatol.
114:1071–1074. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levell NJ, Bewley AP and Levene GM:
Porokeratosis of Mibelli on the penis, scrotum and natal cleft.
Clin Exp Dermatol. 19:77–78. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lucker GP, Happle R and Steijlen PM: An
unusual case of porokeratosis involving the natal cleft:
porokeratosis ptychotropica? Br J Dermatol. 132:150–151. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neri I, Marzaduri S, Passarini B and
Patrizi A: Genital porokeratosis of Mibelli. Genitourin Med.
71:410–411. 1995.
|
|
11
|
Tangoren IA, Weinberg JM, Ioffreda M,
Werth VP and James WD: Penile porokeratosis of Mibelli. J Am Acad
Dermatol. 36:479–481. 1997. View Article : Google Scholar
|
|
12
|
Trcka J, Pettke-Rank CV, Bröcker EB and
Hamm H: Genitoanocrural porokeratosis following chronic exposure to
benzene. Clin Exp Dermatol. 23:28–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe T, Murakami T, Okochi H, Kikuchi
K and Furue M: Ulcerative porokeratosis. Dermatology. 196:256–259.
1998. View Article : Google Scholar
|
|
14
|
Stone N, Ratnavel R and Wilkinson JD:
Bilateral perianal inflammatory verrucous porokeratosis
(Porokeratosis ptychotropica). Br J Dermatol. 140:553–555. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porter WM, Du P, Menagé H, Philip G and
Bunker CB: Porokeratosis of the penis. Br J Dermatol. 144:643–644.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wallner JS, Fitzpatrick JE and Brice SL:
Verrucous porokeratosis of Mibelli on the buttocks mimicking
psoriasis. Cutis. 72:391–393. 2003.PubMed/NCBI
|
|
17
|
Laino L, Pala S, Innocenzi D,
Accappaticcio G and Van Steensel MA: Genital porokeratosis. Eur J
Dermatol. 14:190–192. 2004.PubMed/NCBI
|
|
18
|
Chen TJ, Chou YC, Chen CH, Kuo TT and Hong
HS: Genital porokeratosis: a series of 10 patients and review of
the literature. Br J Dermatol. 155:325–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sengupta S, Das JK and Gangopadhyay A:
Porokeratosis confined to the genital area: a report of three
cases. Indian J Dermatol Venereol Leprol. 74:802008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valdivielso-Ramos M: (Genital
porokeratosis). Actas Dermosifiliogr. 99:217–220. 2008.(In
Spanish).
|
|
21
|
Benmously Mlika R, Kenani N, Badri T, et
al: Localized genital porokeratosis in a female patient with
multiple myeloma. J Eur Acad Dermatol Venereol. 23:584–585.
2009.PubMed/NCBI
|
|
22
|
Schiffman LA, Berry R, Watsky M and
Heilman E: Annular patches and plaques on the scrotum and buttocks.
Genital porokeratosis. Arch Dermatol. 145:715–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XQ, Yang S, Guo Y, et al: Clinical
manifestation and heredity feature in five pedigrees with
porokeratosis. Zhonghua Pifuke Zazhi. 36:549–552. 2003.(In
Chinese).
|
|
24
|
Chernosky ME and Freeman RG: Disseminated
superficial actinic porokeratosis (DSAP). Arch Dermatol.
96:611–624. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan LS and Chong WS: Porokeratosis in
Singapore: an Asian perspective. Australas J Dermatol. 53:e40–44.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chernosky ME: Disseminated superficial
actinic porokeratosis (DSAP). Int J Dermatol. 12:152–157. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanitakis J, Euvrard S, Faure M and Claudy
A: Porokeratosis and immunosuppression. Eur J Dermatol. 8:459–465.
1998.
|
|
28
|
Herranz P, Pizarro A, De Lucas R, et al:
High incidence of porokeratosis in renal transplant recipients. Br
J Dermatol. 136:176–179. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bencini PL, Tarantino A, Grimalt R,
Ponticelli C and Caputo R: Porokeratosis and immunosuppression. Br
J Dermatol. 132:74–78. 1995. View Article : Google Scholar
|
|
30
|
Chernosky ME and Anderson DE: Disseminated
superficial actinic porokeratosis. Clinical studies and
experimental production of lesions. Arch Dermatol. 99:401–407.
1969. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neumann RA, Knobler RM, Jurecka W and
Gebhart W: Disseminated superficial actinic porokeratosis:
experimental induction and exacerbation of skin lesions. J Am Acad
Dermatol. 21:1182–1188. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nova MP, Goldberg LJ, Mattison T and
Halperin A: Porokeratosis arising in a burn scar. J Am Acad
Dermatol. 25:354–356. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung JY, Yeon JH, Ryu HS, et al:
Disseminated superficial porokeratosis developed by
immunosuppression due to rheumatoid arthritis treatment. J
Dermatol. 36:466–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kienast AK and Hoeger PH: Penile linear
porokeratosis in a child: a case report. Pediatric Dermatol.
26:216–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wanat KA, Gormley RH, Bennett DD and
Kovarik CL: Genitogluteal porokeratosis involving the scrotum: an
unusual presentation of an uncommon disease. J Cutan Pathol.
39:72–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McGuigan K, Shurman D, Campanelli C and
Lee JB: Porokeratosis ptychotropica: a clinically distinct variant
of porokeratosis. J Am Acad Dermatol. 60:501–503. 2009. View Article : Google Scholar : PubMed/NCBI
|