Introduction
Pericarditis is an uncommon complication following
renal transplantation, with a reported incidence of 2.4% in the
first two months following renal transplantation (1). Multiple factors may contribute to the
development of this disorder, including catabolism abnormalities
secondary to surgery and drugs and an increased risk of infections
due to immunosuppressive therapy (1,2).
Pericardial tamponade, which is caused by effusion accumulation and
increased intrapericardial pressure, is a severe complication of
pericarditis (3). Therapeutic
strategies targeting pericarditis should focus on the etiology as
much as possible. However, when the etiology is unclear or
idiopathic, and inflammatory markers are elevated, nonsteroidal
anti-inflammatory drugs, colchicine and corticosteroids are the
most common therapeutic modalities for non-transplant patients
(4). The current study presents a
rare case of recurrent pericarditis that manifested as large
pericardial effusion and pericardial tamponade within the first
year following kidney transplantation. The condition was
successfully treated with colchicine. To the best of our knowledge,
the use of colchicine for the treatment of recurrent pericarditis
in kidney transplant patients has rarely been reported.
Case report
A 31-year-old male with end-stage renal disease,
secondary to chronic glomerulonephritis (IgA nephropathy), received
a kidney transplant in December 2010 from a 25-year-old male donor
who succumbed to cardiac death as a result of craniocerebral
trauma. Written informed consent for inclusion in the case report
was provided by the patient’s family. The patient had been
maintained on hemodialysis for one year prior to the
transplantation and had a history of tuberculosis contact and
digestive tract hemorrhage. Preoperative chest X-rays,
electrocardiograms and echocardiography examinations revealed no
abnormalities. Cytomegalovirus and hepatitis B and C viruses were
seronegative in the donor and recipient. Surgical follow-up was
uncomplicated. The immunosuppressive regimen included 0.5 g
intravenous methylprednisolone during surgery and 0.5 g/day for the
first three days following the transplantation. Tacrolimus was
administered according to the patient’s body weight. As the body
weight was 65 kg, tacrolimus was administered at 6.5 mg/day for the
initial three days. Subsequently, the dose was adjusted according
to the trough concentration of tacrolimus, and 6–8 ng/ml tacrolimus
was applied for the first six months following transplantation and
5–6 ng/ml was administered for months 6–12 following
transplantation. Mycophenolate mofetil (0.75 g) was administered
orally every 12 h, which was gradually reduced to 0.5 g per 12 h
for maintenance. Furthermore, prednisolone at a first dose of 80
mg/day was applied, which was gradually tapered during a
six-month-period to 10 mg/day for long-term maintenance. The
allograft function was excellent immediately following surgery.
However, at day 11 following the transplantation,
the patient developed symptoms of chest pain and dyspnea, and a
decrease in urinary volume (from >2,000 to 1,000 ml/day) was
observed. A physical examination revealed tachycardia, a decrease
in systolic pressure and jugular venous distention.
Echocardiography and chest computed tomography scans revealed an
abundant compressive circumferential pericardial effusion
(echo-free space, 62.3 mm) and pleural effusion (Fig. 1) with 49% of the left ventricular
ejection fraction. Pericardiocentesis was performed and a
lemon-colored exudative fluid (3,000 ml; protein level, 51.4 g/l;
fluid/serum ratio, >0.5) with abundant polymorphonuclear cells
was removed, which relieved the patients symptoms. Further
investigations revealed that the erythrocyte sedimentation rate was
6 mm/h, the serum creatinine level was 200 μmol/l, the serum
lactate dehydrogenase level was 339.3 U/l (serum/fluid ratio,
>0.6) and the adenosine deaminase level in the pericardial fluid
was 3.5 U/l. Negative results were observed for serum anti-nuclear
antibodies and tumor markers, including carcinoembryonic antigen,
carbohydrate antigen (CA) 125, CA 15-3 and CA 19-9. Serological
tests for cytomegalovirus, Epstein-Barr virus, mycoplasma,
chlamydia, coxsackie virus, herpes simplex virus, Mycobacterium
tuberculosis and toxoplasma were negative. In addition, direct
examination of the pericardial fluid for mycobacteria,
Aspergillus and yeast, as well as a pericardial fluid
culture for bacteria, were negative. The drainage was maintained
for 12 days. A repeated echocardiogram scan did not detect any
pericardial effusion. Thus, the patient was discharged following
removal of the drainage tube, and once the serum creatinine level
had reached 150 μmol/l.
However, the patient was readmitted after 20 days
with pericardial tamponade, which required pericardiocentesis to be
performed. A lemon-colored exudative fluid (600 ml) was removed and
a catheter was maintained. The aforementioned etiological
examinations were performed again, with all tests negative, with
the exception of the enzyme-linked immunospot test (ELISPOT) for
Mycobacterium tuberculosis. Therefore, at day 48 following
transplantation, a two-month period of empirical antituberculous
therapy (rifampicin, isoniazid, ethambutol, pyrazinamide and
moxifloxacin) was proposed, which was prolonged to three months.
However, during this time period, the volume of pericardial
drainage fluid remained at 200–600 ml per day. At day 77 following
transplantation, a pericardial window with thoracic close drainage
was performed due to the frequent obstruction of the catheter.
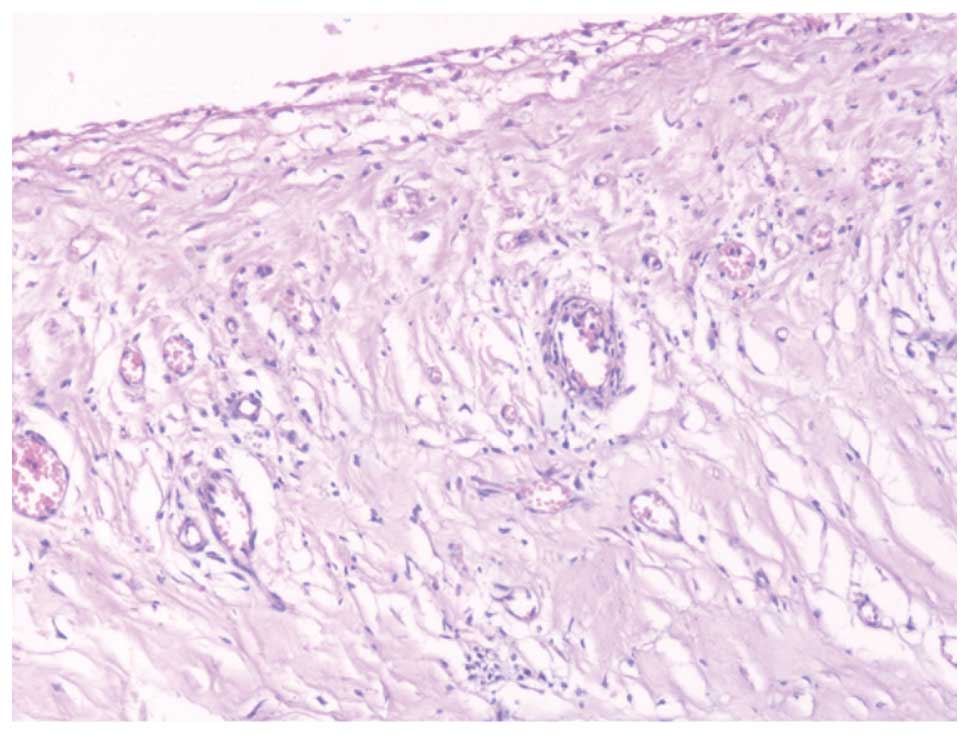
Postoperative pericardial histopathological assessment revealed
chronic inflammatory changes without any evidence of malignancy or
tuberculosis (Fig. 2).
Furthermore, the culture of pericardial fluid tested negative for
Mycobacterium tuberculosis after 60 days. At day 127
following transplantation, a pericardiocentesis was performed again
to replace the blocked thoracic close drainage with a catheter. The
ELISPOT was repeated and negative results were achieved. Since no
specific causes were identified, including infections or
malignancy, and the prolonged (three months) empirical
antituberculous therapy failed to ameliorate the symptoms, the
patient was diagnosed with idiopathic recurrent pericarditis.
Antituberculous therapy was stopped and colchicine (0.5 mg twice
daily) plus aspirin (500 mg twice daily, which was stopped after
five days due to suspicion of digestive tract hemorrhage) were
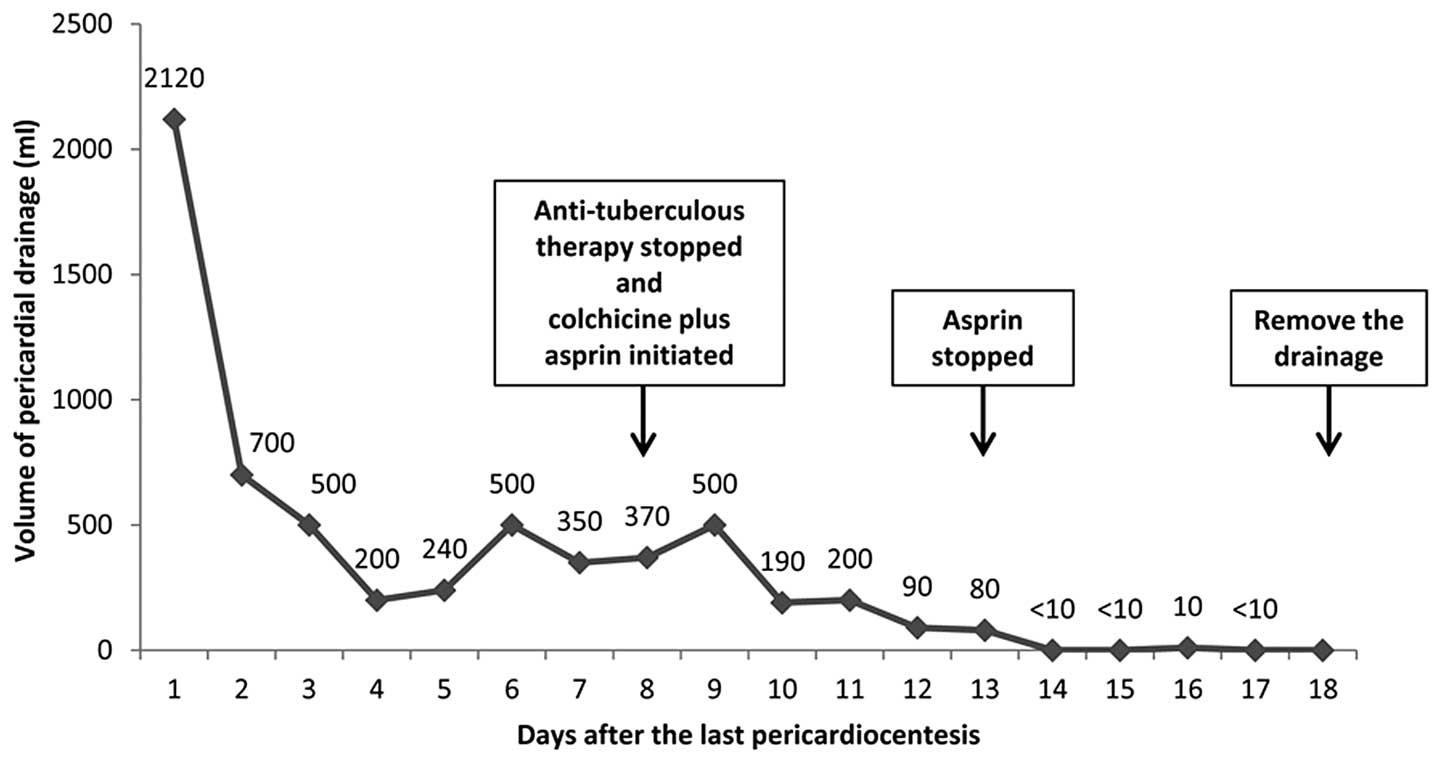
prescribed at day 135 following transplantation. The effusion
regressed rapidly thereafter. At day six following the initiation
of colchicine treatment, the volume of the drainage fluid decreased
to <10 ml per day. The drainage tubes were removed at day 10
following the initiation of treatment, since minimal residual
effusion was observed by echocardiography (Fig. 3). Treatment was maintained for 12
months with transient reversible elevation of alanine
aminotransferase (peak value, 80 U/l). Within the 30 months of
follow-up since the treatment was initiated, no relapse has been
observed. In addition, the serum creatinine levels have remained
between 130 and 145 μmol/l.
Discussion
Pericarditis is a common complication in patients
with end-stage renal disease, although the incidence rate decreases
significantly following renal transplantation (1,3). In
a study of 1,497 patients by Sever et al (1), it was demonstrated that during the
first two months following renal transplantation, the etiologies of
acute pericarditis included uremia, bacterial or viral infection,
idiopathy and drugs (1). Among the
etiologies, tuberculosis was the most common in developing
countries (4). In the present
case, extensive investigation into the etiology was performed to
exclude the majority of the potential causes, including infection
and malignancy. In particular, a prolonged three-month period of
empirical antituberculous therapy plus culture of pericardial fluid
for Mycobacterium tuberculosis were performed on the basis
of the positive result from the ELISPOT and the epidemiological
background of the patient to exclude a potential tuberculous
infection. Uremic pericarditis is not uncommon in renal transplant
patients, particularly among those with insufficient allograft
function (1); however, the
condition should regress along with the recovery of allograft
function. A number of drugs, including sirolimus and minoxidil,
have been reported to induce pericardial effusion and even
pericardial tamponade in renal transplant patients (1,5).
However, these drugs were not administered to the patient in the
present study. The pathogenesis of the idiopathic pericarditis
remains complicated and obscure. In certain cases, undiagnosed
viral infections may contribute to the development of the disorder
(6). Furthermore, due to the
limitation of the etiological tests, viral causes are unable to be
excluded in the present case study.
Acute pericarditis may recur in up to 50% of
patients following the first incidence (7), and is severe complication. Currently,
available therapeutic treatments include nonsteroidal
anti-inflammatory drugs (NSAID), colchicine and corticosteroid.
Colchicine, an inhibitor of microtubule polymerization, exerts
anti-inflammatory effects by inhibiting the migration and
activities of polymorphonuclear cells. The drug has been approved
for the treatment of several inflammatory diseases, including
familial Mediterranean fever, gout and Behcet’s disease (8). Previous studies have also
demonstrated that colchicine, applied alone or in combination with
conventional therapies, such as NSAIDs, may significantly reduce
the recurrence rate, the persistence of the symptoms and the mean
number of recurrences, as well as prolong the time to subsequent
recurrence (9,10). Despite results from additional
studies being contradictory, corticosteroids are commonly used for
the treatment of recurrent pericarditis (11). However, an international
multicenter study proposed that pretreatment with corticosteroids
impeded the efficacy of colchicine and was associated with a higher
percentage of relapse (12). In
the present case, as immunosuppression was required,
immunosuppressive regimens, including corticosteroids (10 mg/day),
were maintained during and following colchicine therapy. No relapse
occurred following the initiation of colchicine treatment. The
curative effects of colchicine are controversial due to the
previous administration of antituberculous therapy. However, during
the 30 months follow-up, no evidence of tuberculosis was observed
in the patient, although it is known that a three-month-period of
antituberculous therapy is not sufficient for curing
tuberculosis.
The use of colchicine in transplant patients is
limited and cautious due to the toxicity. Previous studies have
demonstrated that colchicine induces severe rhabdomyolysis in solid
organ transplant patients when used for the treatment of acute gout
arthritis (13,14). The causes may be attributed to the
concurrent use of P-glycoprotein inhibitors, such as cyclosporin,
pravastatin and azithromycin, high doses or impaired renal or
hepatic functions (13,14). However, in the present case, during
the 12-month period of colchicine treatment, only modest transient
reversible side effects were observed and the allograft function
remained stable. A recent study demonstrated that tacrolimus was
also an inhibitor of P-glycoprotein; however, less active than
cyclosporine (15). Thus, when
administered in combination, tacrolimus may reduce the likelihood
of severe colchicine intoxication in renal transplant patients.
However, further investigation is required.
In conclusion, the current study presented a rare
case of recurrent pericarditis and pericardial tamponade within one
year of renal transplantation, which was successfully treated with
colchicine. Therefore, low-dose colchicine may be a safe and
effective strategy for the treatment of recurrent pericarditis in
renal transplant patients.
References
|
1
|
Sever MS, Steinmuller DR, Hayes JM, Streem
SB and Novick AC: Pericarditis following renal transplantation.
Transplantation. 51:1229–1232. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alpert MA and Ravenscraft MD: Pericardial
involvement in end-stage renal disease. Am J Med Sci. 325:228–236.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maisch B, Seferović PM, Ristić AD, et al;
Task Force on the Diagnosis and Management of Pericardial Diseases
of the European Society of Cardiology. Guidelines on the diagnosis
and management of pericardial diseases executive summary; The Task
Force on the Diagnosis and Management of Pericardial Diseases of
the European Society of Cardiology. Eur Heart J. 25:587–610. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mayosi BM, Burgess LJ and Doubell AF:
Tuberculous pericarditis. Circulation. 112:3608–3616. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bertrand D, Desbuissons G, Pallet N, et
al: Sirolimus therapy may cause cardiac tamponade. Transpl Int.
26:e4–e7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maisch B: Recurrent pericarditis:
mysterious or not so mysterious? Eur Heart J. 26:631–633. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gianni F and Solbiati M; Gruppo di
Autoformazione Metodologica (GrAM). Colchicine is safe and
effective for secondary prevention of recurrent pericarditis.
Intern Emerg Med. 7:181–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Markel G, Imazio M, Brucato A and Adler Y:
Prevention of recurrent pericarditis with colchicine in 2012. Clin
Cardiol. 36:125–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imazio M, Brucato A, Cemin R, et al; CORP
(COlchicine for Recurrent Pericarditis) Investigators. Colchicine
for recurrent pericarditis (CORP): a randomized trial. Ann Intern
Med. 155:409–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Imazio M, Bobbio M, Cecchi E, et al:
Colchicine as first-choice therapy for recurrent pericarditis:
Results of the CORE (COlchicine for REcurrent pericarditis) trial.
Arch Intern Med. 165:1987–1991. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imazio M, Spodick DH, Brucato A, Trinchero
R and Adler Y: Controversial issues in the management of
pericardial diseases. Circulation. 121:916–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Artom G, Koren-Morag N, Spodick DH, et al:
Pretreatment with corticosteroids attenuates the efficacy of
colchicine in preventing recurrent pericarditis: a multi-centre
all-case analysis. Eur Heart J. 26:723–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bouquié R, Deslandes G, Renaud C, et al:
Colchicine-induced rhabdomyolysis in a heart/lung transplant
patient with concurrent use of cyclosporin, pravastatin, and
azithromycin. J Clin Rheumatol. 17:28–30. 2011.PubMed/NCBI
|
|
14
|
Garrouste C, Philipponnet C, Kaysi S, et
al: Severe colchicine intoxication in a renal transplant recipient
on cyclosporine. Transplant Proc. 44:2851–2852. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Llaudó I, Colom H, Giménez-Bonafé P, et
al: Do drug transporter (ABCB1) SNPs and P-glycoprotein function
influence cyclosporine and macrolides exposure in renal transplant
patients? Results of the pharmacogenomic substudy within the
symphony study. Transpl Int. 26:177–186. 2013.
|