Introduction
Liver disease, which refers to damage or disease of
the liver, includes hepatitis, alcoholic liver disease, fatty liver
disease, liver cirrhosis and liver cancer. The majority of these
diseases are able to cause liver tissue damage (1). Numerous drugs, the majority of which
are chemical, are used for the treatment of hepatic damage;
however, a number of these also exhibit side effects (2). Novel biological therapies have
demonstrated efficacy in treating liver damage and are also safe
for human administration.
Dendrobium is a large genus of orchids that
includes Dendrobium candidum Wall. ex Lindl. (D.
candidum), which is equivalent to Dendrobium moniliforme
(L.) Sw. (3). D. candidum
is a traditional Chinese medicinal herb that is used raw or
processed to produce health care products in China (4). The plant contains water-soluble
polysaccharides, phenanthrenes and numerous amino acids. In
addition, high contents of chrysotoxen and erianin may participate
in the inhibitory activities in liver cancer (5).
In the present study, the preventive effect of D.
candidum on hepatic damage was determined. The serum levels of
aspartate aminotransferase (AST), alanine aminotransferase (ALT),
lactate dehydrogenase (LDH), triglyceride (TG) and total
cholesterol (TC), as well as levels of the inflammation-associated
cytokines, interleukin (IL)-6, IL-12, tumor necrosis factor (TNF)-α
and interferon (IFN)-γ, were used to determine the preventive
effects of D. candidum on CCl4-induced hepatic
damage in imprinting control region (ICR) mice. Liver tissue
histology was also performed to determine the preventive effects of
D. candidum in vivo. The mRNA expression levels of nuclear
factor (NF)-κB, IκB-α, inducible nitric oxide synthase (iNOS) and
cyclooxygenase (COX)-2 in the liver tissue were also determined to
further investigate the preventive effects of D.
candidum.
Materials and methods
Preparation of D. candidum
D. candidum was purchased from Shanghai No. 1
Pharmacy Co., Ltd. (Shanghai, China), and stored at −80°C and
freeze-dried to produce a powder. A 20-fold volume of boiling water
was added to the powdered sample and D. candidum was
extracted twice by stirring overnight. The aqueous extract was
evaporated and concentrated using an N-1100 rotary evaporator
(Eyela; Tokyo Rikakikai Co., Ltd., Tokyo, Japan).
Induction of hepatic damage
In total, 50 male ICR mice (age, 7 weeks) were
purchased from the Experimental Animal Center of Chongqing Medical
University (Chongqing, China). The mice were divided into five
groups of 10 mice each. The experimental design was as follows. The
normal control group were administered distilled water for 14 days
and a single oral dose of vehicle [0.2 ml/kg body weight (bw) olive
oil]. The CCl4 control group received a 14-day repeated
oral administration of distilled water, followed by a single
administration of CCl4 (0.2 ml/kg bw dissolved in olive
oil, 1:1, v/v) on the final day to induce hepatic damage. The two
D. candidum groups received 200 or 400 mg/kg bw D.
candidum extract by gavage for 14 days, and hepatic damage was
induced in the same manner as the control group. Finally, the
positive control group received 100 mg/kg bw silymarin dissolved in
water for 14 days, with hepatic damage induced in the same manner
as for the control group mice. The mice were anesthetized 24 h
following the administration of CCl4 and sacrificed
using CO2 (6). Blood
and liver samples were collected and preserved at −70°C until
required for the biological assays. Experimental protocols were
approved by the Animal Ethics Committee of Chongqing Medical
University.
Serum levels of AST, ALT and LDH
Serum levels of AST and ALT were determined using
the aspartate aminotransferase, aminotransferase and lactate
dehydrogenase ELISA kits (Shanghai Institute of Biological Products
Co., Ltd., Shanghai, China). Serum levels of LDH were also
determined using a commercially available kit (Cayman Chemical Co.,
Ann Arbor, MI, USA).
Serum levels of TG and TC
Serum levels of TG and TC were determined using the
triglycerides reagent and the cholesterol total ELISA kits
(Shanghai Institute of Biological Products Co., Ltd.),
respectively.
Analysis of inflammation-associated
cytokines in the serum by enzyme-linked immunosorbent assay
(ELISA)
For the serum cytokine assay, blood from the
inferior vena cava was collected in a tube and centrifuged at 1,100
× g for 10 min at 4°C. The concentrations of the
proinflammatory-associated cytokines, IL-6, IL-12, TNF-α and IFN-γ,
were measured using ELISA, according to the manufacturer’s
instructions (BioLegend ELISA MAX™ Deluxe kit; BioLegend, San
Diego, CA, USA).
Histological examination of the liver
tissue
For histological investigations, the liver tissue
was fixed in 10% (v/v) buffered formalin for 24 h, dehydrated in
ethanol and embedded in paraffin. Subsequently, 4-μm-thick sections
were prepared and stained with hematoxylin and eosin (H&E) for
observation under an Olympus BX41 microscope (Olympus, Tokyo,
Japan).
Reverse transcription polymerase chain
reaction (RT-PCR) of the inflammation-associated gene expression
levels in the liver tissue
Total RNA from the liver tissue samples was isolated
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions. RNA was
digested with RNase-free DNase (Roche Diagnostics, Basel,
Switzerland) for 15 min at 37°C and purified using an RNeasy kit
(Qiagen, Hilden, Germany), according to the manufacturer’s
instructions. The cDNA was synthesized from 2 μg total RNA by
incubation at 37°C for l h with avian myeloblastosis virus reverse
transcriptase (GE Healthcare, Little Chalfont, UK) with random
hexanucleotides, according to the manufacturer’s instructions. The
sequences of primers used to specifically amplify the genes of
interest were as follows: NF-κB forward, 5′-CAC TTA TGG ACA ACT ATG
AGG TCT CTG G-3′ and reverse, 5′-CTG TCT TGT GGA CAA CGC AGT GGA
ATT TTA GG-3′; IκB-α forward, 5′-GCT GAA GAA GGA GCG GCT ACT-3′ and
reverse, 5′-TCG TAC TCC TCG TCT TTC ATG GA-3′; iNOS forward, 5′-AGA
GAG ATC GGG TTC ACA-3′ and reverse, 5′-CAC AGA ACT GAG GGT ACA-3′;
and COX-2 forward, 5′-TTA AAA TGA GAT TGT CCG AA-3′ and reverse,
5′-AGA TCA CCT CTG CCT GAG TA-3′. Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was amplified as an internal control gene
with the following primers: Forward, 5′-CGG AGT CAA CGG ATT TGG
TC-3′ and reverse, 5′-AGC CTT CTC CAT GGT CGT GA-3′. Amplification
was performed in a thermal cycler (Eppendorf, Hamburg, Germany).
The PCR products were separated in 1.0% agarose gels and visualized
with ethidium bromide staining (7). Expression levels were analyzed with
ImageJ 1.44 software (National Institutes of Health, Bethesda, MD,
USA). The computing formula was as follows: Fold ratio = gene
expression/GAPDH × control numerical value (control fold ratio,
1).
Component analysis by nuclear magnetic
resonance (NMR)
Dried D. candidum was refluxed and extracted
three times with 10 times the amount of ethyl acetate. The ethyl
acetate extract was obtained after 1 h for every reflux extraction
and vacuum concentration extraction. The total ethyl acetate
extract was extracted by anhydrous ethanol three times. The ethanol
extract was suspended in water and extracted by petroleum ether,
chloroform and butanol extraction, respectively. The ethyl acetate
extract was treated by gradient elution in a silica gel column with
a petroleum ether-ethyl acetate system. Subsequently, the
chloroform extract was treated by gradient elution in a silica gel
column with a petroleum chloroform-methanol system. The butanol
extract was dissolved in water that had been treated with
ultrasonication, and the extraction solution was obtained following
filtering. The extract was eluted using an HP2MGL macroporous resin
column with water and 10, 30 and 60% ethanol. Following elution,
the various solvents obtained different compounds; thus, their
composition was able to be identified by NMR (Varian INOVA 400;
Varian Medical Systems, Inc., Palo Alto, CA, USA). NMR was set at a
1H frequency of 300 MHz, a temperature of 25°C, a pulse
length of 8 μsec, a spin speed of 20 Hz and scanned 64 times. The
1H-NMR spectra were recorded using a standard
high-resolution magic angle spinning probe with magic-angle
gradient.
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between the mean values for individual groups were
assessed with one-way analysis of variance and Duncan’s multiple
range test, where P<0.05 was considered to indicate a
statistically significant difference. SAS version 9.1 (SAS
Institute, Inc., Cary, NC, USA) was used to conduct the statistical
analyses.
Results
Serum levels of AST, ALT and LDH
Serum levels of AST, ALT and LDH in the mice treated
with the two concentrations of D. candidum were higher
compared with those in the mice in the normal group (Table I). Serum levels of AST, ALT and LDH
were highest in the CCl4-treated mice. The silymarin and
D. candidum treatments were able to significantly reduce the
serum levels of AST, ALT and LDH in mice when compared with the
control treatment. Furthermore, the serum levels of AST, ALT and
LDH in the mice treated with 400 mg/kg D. candidum were
similar to those treated with silymarin. Therefore, a high
concentration of 400 mg/kg D. candidum was able to
significantly decrease the serum levels of AST, ALT and LDH in mice
compared with the low concentration of 200 mg/kg D.
candidum.
 | Table ISerum levels of AST, ALT and LDH in
mice following CCl4-induced hepatic damage. |
Table I
Serum levels of AST, ALT and LDH in
mice following CCl4-induced hepatic damage.
| Treatment | AST (IU/l) | ALT (IU/l) | LDH (IU/l) |
|---|
| Normal
(untreated) | 241.3±36.1a | 118.2±17.8a |
1432.5±201.4a |
| Control
(CCl4-treated) |
1877.6±124.7b |
1477.6±146.1b |
6129.0±238.5b |
| Silymarin (100
mg/kg) | 521.0±22.6c | 522.3±29.3c |
2782.3±175.2c |
| D. candidum
(mg/kg) |
| 200 | 779.3±34.8d | 924.9±44.8d |
5217.7±241.2d |
| 400 | 451.2±23.9e | 427.9±23.0e |
2126.3±146.2e |
Serum levels of TG and TC
Serum levels of TG in the mice treated with D.
candidum were higher compared with the mice in the control
group. The 400 mg/kg D. candidum treatment significantly
increased the levels of TG in the mice and a similar level was
observed to those in the normal group. Serum levels of TC in the
mice in the control group were significantly lower compared with
the mice treated with D. candidum or silymarin. The high
concentration (400 mg/kg) D. candidum-treated group revealed
the highest levels of TC among the sample groups (P<0.05;
Table II).
 | Table IISerum levels of TG and TC in mice
following CCl4-induced hepatic damage. |
Table II
Serum levels of TG and TC in mice
following CCl4-induced hepatic damage.
| Treatment | TG (mg/dl) | TC (mg/dl) |
|---|
| Normal
(untreated) | 83.2±4.3a | 125.3±21.8a |
| Control
(CCl4-treated) | 59.7±5.5b | 51.3±4.5b |
| Silymarin (100
mg/kg) | 67.3±3.6c | 96.3±7.3c |
| D. candidum
(mg/kg) |
| 200 | 63.5±2.3d | 72.8±6.7d |
| 400 | 74.8±3.7e | 108.2±6.0e |
Proinflammatory cytokine levels in the
serum
Serum levels of IL-6, IL-12, TNF-α and IFN-γ in the
mice in the 200 and 400 mg/kg D. candidum-treated groups
were significantly lower compared with the mice in the control
group (Table III). However, the
levels of these proinflammatory cytokines in the mice treated with
400 mg/kg D. candidum and silymarin were similar to those of
the mice in the normal group. The levels of proinflammatory
cytokines in the 400 mg/kg D. candidum-treated mice were
lower compared with those in the 100 mg/kg silymarin-treated
mice.
 | Table IIILevels of IL-6, IL-12, TNF-α and
IFN-γ in mice following CCl4-induced hepatic damage. |
Table III
Levels of IL-6, IL-12, TNF-α and
IFN-γ in mice following CCl4-induced hepatic damage.
| Treatment | IL-6 (pg/ml) | IL-12 (pg/ml) | TNF-α (pg/ml) | IFN-γ (pg/ml) |
|---|
| Normal
(untreated) | 42.1±2.7a | 221.2±26.3a | 33.6±3.2a | 24.3±5.5a |
| Control
(CCl4-treated) | 226.2±18.9b | 764.4±34.2b | 86.1±7.4b | 83.2±6.3b |
| Silymarin (100
mg/kg) | 81.2±11.8c | 452.8±31.2c | 58.2±2.8c | 46.2±3.5c |
| D. candidum
(mg/kg) |
| 200 | 146.7±27.4d | 612.8±21.7d | 71.4±1.5d | 66.7±3.3d |
| 400 | 64.8±5.2e | 367.3±19.2e | 50.6±1.1e | 37.2±1.4e |
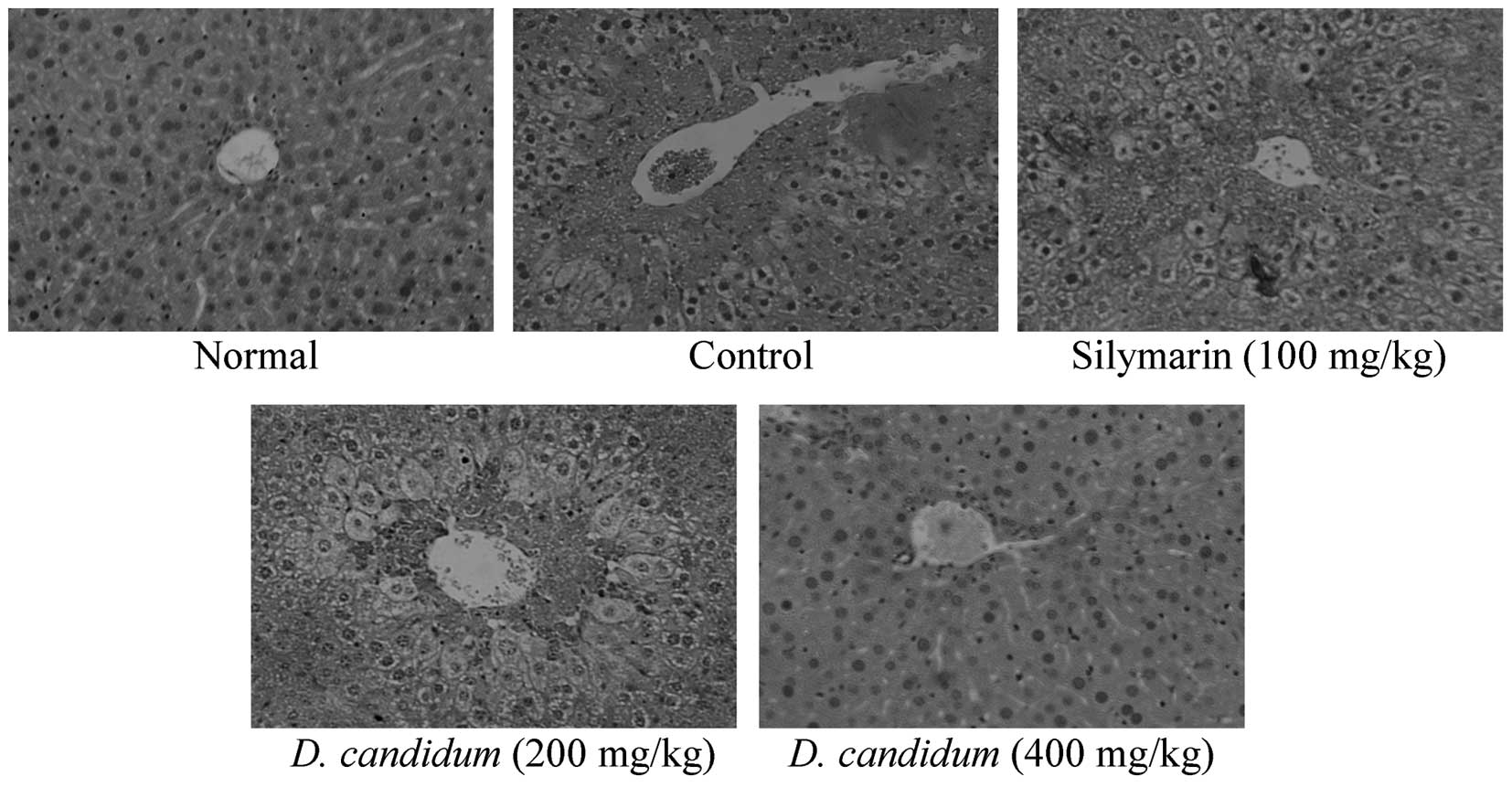
Histopathological examination of the
hepatic damage
H&E staining revealed CCl4-induced
histopathological changes in the liver tissue of the mice.
Significant degeneration and necrosis of the hepatocytes were
observed in the centrilobular region and perivenular inflammatory
infiltrates. The histological tissue sections of the mice in the
normal group demonstrated a normal morphology. However,
histopathological evaluation in all the CCl4-treated
mice revealed evidence of hepatic damage (Fig. 1). The tissue sections from the mice
in the control group revealed widespread areas of congestion and
hemorrhage in the centrilobular zone, as well as necrosis involving
all the hepatocytes in the centrilobular zone (grade 3) (8). The 200 mg/kg D. candidum group
demonstrated moderate congestion and hemorrhage in the area around
the centrilobular vein, which extended into the midzonal cells
(grade 2) (8); the majority of
lobules were affected. Areas of confluent necrosis were limited to
the liver cells that surrounded the centrilobular vein. The tissue
sections of the mice in the 400 mg/kg D. candidum and
silymarin groups appeared to be similar to those from the normal
group (grade 1) (8). These results
demonstrated that a higher concentration of D. candidum
decreased the degree of hepatic damage in mice.
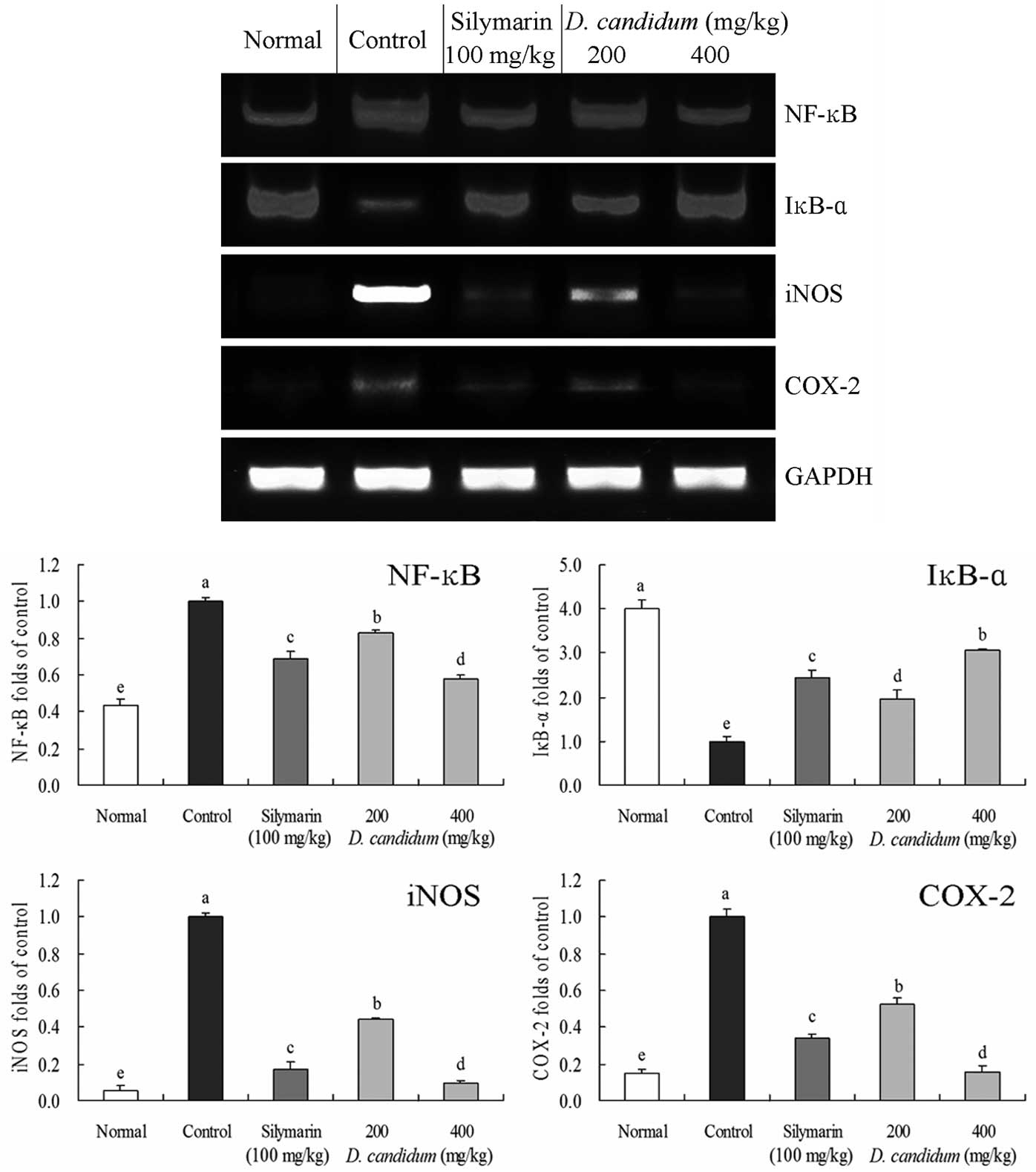
Inflammation-associated gene expression
in the liver tissues
Experiments were conducted to investigate whether
the anti-inflammatory functions of D. candidum were
associated with inhibited expression levels of the
inflammation-associated genes, NF-κB, IκB-α, iNOS and COX-2. As
shown in Fig. 2, the mRNA
expression levels of NF-κB were reduced in the liver tissue of mice
treated with D. candidum and silymarin when compared with
the control group mice. Thus, D. candidum and silymarin
significantly modulated the expression levels of genes associated
with inflammation (P<0.05). NF-κB expression decreased, while
the mRNA expression levels of IκB-α increased in the D.
candidum and silymarin groups when compared with the control
group. Furthermore, the mRNA expression levels of COX-2 and iNOS
decreased in the presence of D. candidum, as compared with
the control group, in a concentration-dependent manner. These
results indicated that D. candidum may prevent hepatic
damage by increasing anti-inflammatory activities.
Contents of D. candidum
Following the compound assay, 11 compounds were
isolated and identified in the D. candidum leaf. Compound 1
was obtained as a clear crystal. The 1H-NMR spectrum of
this compound exhibited at δ 6.92 (2H, d), 6.62 (2H, d), 6.06 (2H,
s), 6.03 (1H, s), 2.65 (4H, m); thus, material 1 was identified as
dihydrogen resveratrol. Compound 2 was obtained as a white powder.
The 1H-NMR spectrum of this compound exhibited at δ 6.98
(2H, d), 6.74 (2H, d), 6.62 (1H, s), 6.47 (1H, d), 4.83 (1H, d),
4.63 (1H, d), 3.1–3.8 (12H), 3.73 (3H, s), 3.69 (3H, s), 2.74 (4H,
m); thus, material 2 was identified as dendromoniliside E. Compound
3 was obtained as a black-red needle. The 1H-NMR
spectrum of this compound exhibited at δ 11.00 (1H, s), 8.15 (1H,
d), 6.06 (2H, s), 8.07 (1H, d), 6.95 (1H, s), 6.83 (1H, s), 6.15
(1H, s), 3.96 (3H, s), 3.93 (3H, s); thus, material 3 was
identified as denbinobin. Compound 4 was obtained as a colorless
needle. The 1H-NMR spectrum of this compound exhibited
at δ 4.72 (2H, m), 3.85 (1H, d), 6.06 (2H, s), 2.53 (1H, d), 2.49
(1H, t), 2.39 (1H, dd), 2.21 (1H, dd), 1.64 (1H, m), 1.35 (3H, s),
1.03 (3H, d), 0.95 (3H, d); thus, this material was identified as
aduncin. Compound 5 was obtained as a white needle. The
1H-NMR spectrum of this compound exhibited at δ 8.25
(1H, s), 8.10 (1H, s), 5.90 (1H, d), 4.66 (1H, dd), 3.5–4.2 (4H,
m); thus, this material was identified as adenosine. Compound 6 was
obtained as a white powder. The 1H-NMR spectrum of this
compound exhibited at δ 7.95 (1H, d), 5.85 (1H, d), 5.66 (1H, d),
3.2–4.3 (5H, m); thus, this material was identified as uridine.
Compound 7 was obtained as a clear crystal. The 1H-NMR
spectrum of this compound exhibited at δ 10.60 (1H, s), 7.92 (1H,
s), 6.45 (2H, s), 5.66 (1H, d), 3.4–4.4 (5H, m); thus, the material
was identified as guanosine. Compound 8 was obtained as a white
powder. The 1H-NMR spectrum of this compound exhibited
at δ 7.65 (1H, d), 7.41 (2H, d), 6.85 (2H, d), 6.33 (1H, d), 4.17
(2H, t), 1.69 (2H, m), 1.25 (54H, m), 0.85 (3H, t); thus, this
material was identified as defuscin. Compound 9 was obtained as a
white powder. The 1H-NMR spectrum of this compound
exhibited at δ 7.45 (2H, d), 6.82 (2H, d), 6.81 (1H, d), 5.83 (1H,
d), 4.16 (2H, t), 1.67 (2H, m), 1.23 (54H, m), 0.88 (3H, t); thus,
material 9 was identified as n-triacontyl cis-p-coumarate. Compound
10 was obtained as a white powder. The 1H-NMR spectrum
of this compound exhibited at δ 2.35 (2H, t), 1.62 (2H, m), 1.25
(24H, m), 0.88 (3H, t); thus, the material was identified as
hexadecanoic acid. Compound 11 was obtained as a white powder. The
1H-NMR spectrum of this compound exhibited at δ 3.85
(2H, t), 1.75 (2H, m), 1.45 (2H, m), 1.22 (54H, m), 0.85 (3H, t);
thus, the material was identified as hentriacontane.
Discussion
Although D. candidum has traditionally been
used in Chinese medicine, a limited number of studies have been
published investigating its therapeutic effects. D. candidum
has been revealed to exhibit various therapeutic effects on
numerous pathological conditions, including inflammation, immunity,
hyperglycemia and cancer (9).
AST and ALT are liver enzymes that are released into
the general circulation following cell injury. AST is also located
in numerous body tissues, including the heart, muscle, kidney,
brain and lungs. ALT is located primarily in the liver, with lower
quantities in the kidneys, heart and skeletal muscles (10). LDH is an enzyme located in a number
of body tissues, including the liver. Increased levels of LDH have
been shown to indicate liver damage. A previous study revealed that
serum levels of AST and ALT in CCl4-treated rats were
markedly increased compared with rats in the normal group, which
indicated that liver damage was significantly induced by
CCl4 (11). Low levels
of TG and TC are usually observed in chronic liver diseases
(12). Ghadir et al
demonstrated a marked decrease in the plasma levels of TG and TC in
patients with severe hepatitis and hepatic failure, as a result of
decreased lipoprotein biosynthesis (13). From the serum level results in the
present study, D. candidum appeared to exhibit preventive
effects on hepatic damage.
Patients with inflammatory diseases exhibit elevated
levels of serum cytokines, including IL-6, IL-12 and TNF-α, when
compared with healthy individuals (14). Cytokine receptors and the
inflammatory cytokines, IL-6, IL-12, TNF-α and IFN-γ, play
pathogenic roles in gastric disease. Lower levels of these
cytokines indicate an improved preventive effect on gastric ulcers
(15,16). IL-6 functions as a proinflammatory
and anti-inflammatory cytokine, and is encoded by the IL6 gene in
humans (17). IL-6 is secreted by
T cells and macrophages to stimulate an immune response,
particularly during tissue damage, which leads to inflammation
(18). IL-12 contributes to
eradicating inflammation via the IFN-γ-dependent induction of the
antiangiogenic factors, IFN-inducible protein 10 and monokine
induced by IFN-γ (19). TNF-α is
involved in systemic inflammation and is a member of a group of
cytokines that induce the acute phase reaction (20). In a previous study (21), the colonic levels of IL-6, IL-12,
TNF-α and IFN-γ in mice with reserpine-induced gastric ulcers were
markedly decreased following D. candidum treatment. Based on
the observations of this previous study, D. candidum was
revealed to successfully prevent hepatic damage, with higher
concentrations increasing the preventive effect.
Histopathology is an important clinical technique
for diagnosing hepatic damage (22). Histopathological analysis of rat
liver sections has been revealed as an effective method for
evaluating hepatoprotective activity in a CCl4-induced
mouse hepatic damage model (19).
From the liver tissue sections examined in the current study, D.
candidum was shown to exert a preventive effect on
CCl4-induced hepatic damage.
NF-κB, IκB-α, COX-2 and iNOS genes may be used as
biomarkers to monitor visceral damage. NF-κB is a highly ubiquitous
transcription factor that regulates the expression levels of genes
required for cellular proliferation, inflammatory responses and
cell adhesion (23). NF-κB is
present in the cytosol where it is bound to the inhibitory protein,
IκB. Following induction by a number of agents, NF-κB is released
from IκB and translocated to the nucleus where it binds to the κB
binding sites in the promoter regions of target genes (24). Following inflammatory stimulation,
COX-2 and iNOS have been observed to cause deleterious effects in
the liver (6). The iNOS and COX-2
enzymes may enhance inflammatory responses in the early stages
(25). Inflammatory processes are
mediated by multiple molecular mechanisms, and the two most
prominent are the production of iNOS and COX-2. Inflammatory
stimuli elicit the synthesis of iNOS and COX-2 proteins with
similar time courses, indicating that the two systems are able to
interact (26). Thus, D.
candidum, as a unique polysaccharide, may contribute to the
efficacy of hepatic damage and inflammation prevention.
Resveratrol is recommended in specific cases to
prevent inflammation, since the compound is known to function as an
antioxidant that combats colonic inflammation (27). Aduncin, a special component that
has only been identified in Dendrobium, may exhibit an
anti-inflammatory effect (28),
although its functional effects require further investigation.
Adenosine has pro- and anti-inflammatory effects and targets
inflammatory and resident immune cells, as well as antioxidant
enzymes (29). Uridine was
observed to affect the levels of TNF in rats with lung
inflammation, and exhibit anti-inflammatory effects in vivo
(30). Defuscin, n-triacontyl
cis-p-coumarate, hexadecanoic acid and hentriacontane have also
revealed numerous functional activities for human health treatments
(31). These compounds exhibit
anti-inflammatory effects, which may be the reason for the
effective prevention of hepatic damage by D. candidum.
In conclusion, the present study demonstrated the
preventive effect of D. candidum on hepatic damage using a
variety of in vivo experimental methods, including serum
assays of AST, ALT, LDH, TG and TC levels, serum cytokine assays of
IL-6, IL-12, TNF-α and IFN-γ levels, histological examinations and
a liver tissue RT-PCR assay for analyzing the levels of the
inflammatory-associated genes, NF-κB, IκB-α, iNOS and COX-2.
Analysis of the liver tissue revealed that D. candidum
treatment prevented CCl4-induced hepatic damage in mice,
indicating that D. candidum represents a potentially useful
therapeutic strategy for the treatment or prevention of hepatic
damage in vivo.
Acknowledgements
The study was supported by a grant from the Program
for Innovation Team Building at Institutions of Higher Education in
Chongqing (no. KJTD201325).
References
|
1
|
Wolf PL: Biochemical diagnosis of liver
disease. Indian J Clin Biochem. 14:59–90. 1999. View Article : Google Scholar
|
|
2
|
Fried MW: Side effects of therapy of
hepatitis C and their management. Hepatology. 36:S237–S244. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q, Sun P, Li G, Zhu K, Wang C and
Zhao X: Inhibitory effects of Dendrobium candidum Wall ex
Lindl. on azoxymethane- and dextran sulfate sodium-induced colon
carcinogenesis in C57BL/6 mice. Oncol Lett. 7:493–498. 2014.
|
|
4
|
Xiao F, Zhang JZ and Tu YL: First report
of Fusarium oxysporum causing wilt of Dendrobium
candidum in Zhejiang Province, China. Plant Dis.
96:13772012.
|
|
5
|
Shao H, Zhang LQ, Li JM and Wei RC:
Inhibitory effects of water extracts from four species of
Dendrobiums on HelaS3 cells and HepG2 cells. J Anhui Agri
Sci. 36:15968–15970. 2008.
|
|
6
|
Zhao X: Hawk tea (Litsea coreana
Levl. var lanuginose) attenuates CCl(4)-induced hepatic damage in
Sprague-Dawley rats. Exp Ther Med. 5:555–560. 2013.PubMed/NCBI
|
|
7
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferenci P, Lockwood A, Mullen K, Tarter R,
Weissenborn K and Blei AT: Hepatic encephalopathy - definition,
nomenclature, diagnosis, and quantification: final report of the
working party at the 11th World Congresses of Gastroenterology,
Vienna, 1998. Hepatology. 35:716–721. 2002. View Article : Google Scholar
|
|
9
|
Li Y, Wang C, Wang F, Dong H, Guo S, Yang
J and Xiao P: Chemical constituents of Dendrobium candidum.
Zhongguo Zhong Yao Za Zhi. 35:1715–1719. 2010.(In Chinese).
|
|
10
|
Delić R and Stefanović M: Optimal
laboratory panel for predicting preeclampsia. J Matern Fetal
Neonatal Med. 23:96–102. 2010.PubMed/NCBI
|
|
11
|
Raju K, Anbuganapathi G, Gokulakrishnan V,
Rajkapoor B, Jayakar B and Manian S: Effect of dried fruits of
Solanum nigrum LINN against CCl4-induced hepatic
damage in rats. Biol Pharm Bull. 26:1618–1619. 2003.PubMed/NCBI
|
|
12
|
Halsted CH: Nutrition and alcoholic liver
disease. Semin Liver Dis. 24:289–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghadir MR, Riahin AA, Havaspour A,
Nooranipour M and Habibinejad AA: The relationship between lipid
profile and severity of liver damage in cirrhotic patients. Hepat
Mon. 10:285–288. 2010.PubMed/NCBI
|
|
14
|
Gratacós J, Collado A, Filella X, Sanmartí
R, Cañete J, Llena J, Molina R, Ballesta A and Muñoz-Gómez J: Serum
cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing
spondylitis: a close correlation between serum IL-6 and disease
activity and severity. Br J Rheumatol. 33:927–931. 1994.PubMed/NCBI
|
|
15
|
Fox JG, Beck P, Dangler CA, Whary MT, Wang
TC, Shi HN and Nagler-Anderson C: Concurrent enteric helminth
infection modulates inflammation and gastric immune responses and
reduces Helicobacter-induced gastric atrophy. Nat Med.
6:536–542. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
D‘Elios MM, Manghetti M, De Carli M, Costa
F, Baldari CT, Burroni D, Telford JL, Romagnani S and Del Prete G:
T helper 1 effector cells specific for Helicobacter pylori
in the gastric antrum of patients with peptic ulcer disease. J
Immunol. 158:962–967. 1997.
|
|
17
|
Ferguson-Smith AC, Chen YF, Newman MS, May
LT, Sehgal PB and Ruddle FH: Regional localization of the
interferon-beta 2/B-cell stimulatory factor 2/hepatocyte
stimulating factor gene to human chromosome 7p15–p21. Genomics.
2:203–208. 1988.PubMed/NCBI
|
|
18
|
van der Poll T, Keogh CV, Guirao X,
Buurman WA, Kopf M and Lowry SF: Interleukin-6 gene-deficient mice
show impaired defense against pneumococcal pneumonia. J Infect Dis.
176:439–444. 1997.PubMed/NCBI
|
|
19
|
Engel MA and Neurath MF: Anticancer
properties of the IL-12 family - focus on colorectal cancer. Curr
Med Chem. 17:3303–2208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gosselin D and Rivest S: Role of IL-1 and
TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr Hyde
duality of the innate immune system. Brain Behav Immun. 21:281–289.
2007.PubMed/NCBI
|
|
21
|
Zhao X, Song JL, Kil JH and Park KY:
Bamboo salt attenuates CCl4-induced hepatic damage in
Sprague-Dawley rats. Nutr Res Pract. 7:273–280. 2013.PubMed/NCBI
|
|
22
|
Girish C, Koner BC, Jayanthi S, Rao KR,
Rajesh B and Pradhan SC: Hepatoprotective activity of six
polyherbal formulations in CCl4-induced liver toxicity
in mice. Indian J Exp Biol. 47:257–263. 2009.PubMed/NCBI
|
|
23
|
Baeuerle PA: IkappaB-NF-kappaB structures:
at the interface of inflammation control. Cell. 95:729–731. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sánchez-Pérez I, Benitah SA,
Martínez-Gomariz M, Lacal JC and Perona R: Cell stress and
MEKK1-mediated c-Jun activation modulate NFkappaB activity and cell
viability. Mol Biol Cell. 13:2933–2945. 2002.PubMed/NCBI
|
|
25
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Gene Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SF, Huri DA and Snyder SH: Inducible
nitric oxide synthase binds, S-nitrosylates, and activates
cyclooxygenase-2. Science. 310:1966–1970. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martín AR, Villegas I, La Casa C and de la
Lastra CA: Resveratrol, a polyphenol found in grapes, suppresses
oxidative damage and stimulates apoptosis during early colonic
inflammation in rats. Biochem Pharmacol. 67:1399–1410.
2004.PubMed/NCBI
|
|
28
|
Zhou L and Pan JH: Role of adenosine and
adenosine receptors in asthma. Int J Pediatr. 33:181–183. 2006.
|
|
29
|
Livingston M, Heaney LG and Ennis M:
Adenosine, inflammation and asthma - a review. Inflamm Res.
53:171–178. 2004. View Article : Google Scholar
|
|
30
|
Evaldsson C, Rydén I and Uppugunduri S:
Anti-inflammatory effects of exogenous uridine in an animal model
of lung inflammation. Int Immunopharmacol. 7:1025–1032. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang Q, Liang ZS, Wang JR and Xu WH:
Essential oil composition of Salvia miltiorrhiza flower.
Food Chem. 113:592–594. 2009. View Article : Google Scholar
|