Introduction
Mitofusin-2 (Mfn2), also named hyperplasia
suppressor gene (HSG), is a transmembrane GTPase embedded in the
mitochondrial outer membrane that mediates mitochondrial fusion
(1). Mfn2 deficiency and mutations
have been linked to human neurodegenerative diseases, including
Charcot-Marie-Tooth type 2A and other hereditary motor and sensory
neuropathies (2–4). A number of studies have shown that
Mfn2 regulates both mitochondrial fusion and mitochondrial
apoptotic signaling and is involved in the pathogenesis of disease
conditions such as obesity, type 2 diabetes, insulin resistance and
the survival of different epithelial cancer cell lines (5–11).
However, to the best of our knowledge, there are no published
studies concerning the involvement of Mfn2 in the reproductive
system. A study demonstrated that mice that are deficient in either
Mfn1 or Mfn2 die early in the embryonic stages of development,
probably due to underlying mitochondrial defects (5). Emerging evidence indicates that Mfn2
is characterized as a cell proliferation inhibitor, remarkably
suppressing the injury-mediated proliferation of vascular smooth
muscle cells with a potential apoptotic effect via the
mitochondrial apoptotic pathway (12,13).
A further study has shown that the overexpression of Mfn2 inhibits
hepatocellular carcinoma cell proliferation and induces apoptosis
via Bax, and adenovirus-mediated Mfn2 upregulation significantly
suppresses the growth of subcutaneous tumors in nude mice both
in vivo and in vitro (14). In addition, Mfn2 has the comparable
antiproliferative effects in different types of malignancies that
occur in the lungs, liver, breast, colorectal system and urinary
bladder (6,15–17).
At present, there are a large number of studies about Mfn2, but few
of these are morphological studies.
Currently, the involvement of Mfn2 in the
reproductive system has not been well studied, and most of the
studies concerning the Mfn2 gene have been conducted using
adenoviral vectors or lentiviral vectors, as well as cells infected
with virus and implanted into nude mice. There have been few
studies concerning gene transfection into normal organs in
vivo. In the present study, lentiviral vector-mediated rat Mfn2
(rMfn2) gene was used to transfect rat ovaries. The effectiveness
of the transfection was verified, and the expression and effect of
rMfn2 was observed in the rat ovaries and other organs.
Materials and methods
Animals
Fifty female Sprague-Dawley rats (2 months old,
weighing 180–200 g) were purchased from the Experimental Animal
Center of Chongqing Medical University (Chongqing, China). The rats
were divided randomly into two groups: lenti-GFP (green fluorescent
protein)-rMfn2 and lenti-GFP. Both groups received
lentivirus-mediated gene transfer in vivo. Rats in the two
groups were further divided into the following five groups: 7, 15,
30, 45 and 60 days (n=5). In addition, five female Sprague-Dawley
rats (2 months old, weighing 180–200 g) were purchased to be used
as a blank control group (uninfected group). All animal experiments
were conducted in accordance with the guidelines of the Animal Care
and Use Committee of Chongqing Medical University (Chongqing,
China). All surgical interventions and postoperative animal care
were carried out in accordance with the Guide for the Care and Use
of Laboratory Animals (National Research Council, Washington, DC,
USA, 1996).
Microinjection of lentiviral vectors into
rat ovaries
A lentivirus encoding the complete rMfn2 open
reading frame (lenti-GFP-rMfn2) and a control lentivirus encoding
GFP open reading frame (lenti-GFP) were constructed by Western
Biotechnology Inc. (Chongqing, China). Anesthesia was induced with
10% chloral hydrate at a dose of 100 mg/kg by intraperitoneal
injection. Following anesthesia, all rats were weighed prior to
surgery. Then, the rats were placed in a prone position and a
microtubule incision of ~1.0–2.0 cm was made on the surface of the
back to the right of the position of the ovary. The hypoderma,
muscular layer and peritoneum were cut and the ovary was drawn out
from the abdominal cavity. Approximately 10 μl of the lentiviral
vector solution (either lenti-GFP or lenti-GFP-Mfn2, titer at
2×108 tuberculin units/ml) was slowly microinjected into
the sub-envelope of the ovary.
Sample collection
All rats in the two groups were sacrificed to
collect organs on day 7, 15, 30, 45 and 60 following the
microinjection. The bilateral ovaries, uterus, hearts, livers and
kidneys of the rats were excised, blood specimens were extracted
from the heart, and the serum was cryopreserved at −80°C. Certain
specimens from the two groups were fixed with 4% paraformaldehyde,
whereas the remaining specimens were placed in liquid nitrogen for
2 h and then stored at −80°C until further use.
Radioimmunoassay
The concentrations of the serum gonadal hormones
luteinizing hormone (LH), follicle-stimulating hormone (FSH),
estradiol (E2) and progesterone (P) were determined
using commercial double-antibody radioimmunoassay kits (Pu’er
Biotechnology, Beijing, China) in order to assess the endocrine
changes in the rats.
Fluorescence microscopy
The presence and location of rMfn2 protein was
determined by an inverted fluorescence microscope (IX-71, Olympus,
Tokyo, Japan). Frozen and paraffin-imbedded sections were prepared
for fluorescence microscopy for every group. The green fluorescence
densities for organ sections from each group were calculated and
analyzed for statistical differences.
Western blotting
At 45 days after transfection, the expression of
rMfn2 in various organs was determined by western blotting.
Antibodies of the luteinizing hormone receptor (LHR),
follicle-stimulating hormone receptor (FSHR), estradiol receptor
(ER) and progesterone receptor (PR) were purchased from Beijing
Biosynthesis Biotechnology Co., Ltd. (Beijing, China). Proteins
were extracted with ice-cold radioimmunoprecipitation assay buffer
containing 1 mM phenylmethyl sulfonylfluoride according to the
manufacturer’s instructions (Beyotime Institute of Biotechnology,
Haimen, China). Total proteins (50 μg) were separated on 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis gel and
transferred to polyvinylidene fluoride membranes, which were
blocked by Tris-buffered saline Tween-20 containing 5% skimmed milk
at room temperature for 2 h prior to being incubated with primary
antibodies, specifically, rabbit polyclonal antibody against rMfn2
(1:500) (Abcam, Cambridge, UK) and rabbit anti-β-actin (1:1,000)
(Sigma-Aldrich, St. Louis, MO, USA) at 4°C overnight. After washing
three times with Tris-buffered saline Tween-20, the membranes were
incubated with secondary antibody (horseradish peroxide-labeled
anti-rabbit antibody, 1:5,000) for 1 h at room temperature. The
color reaction was detected using chemiluminescence (BeyoECL Plus,
Beyotime Institute of Biotechnology, Haimen, Jiangsu, China), and
the results were analyzed using a professional GelDoc2000 gel
imaging system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All statistical analyses were performed with SPSS
version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Data are
presented as means ± standard error of the mean (SEM). Statistical
differences were assessed by one-way ANOVA. P<0.05 was
considered to indicate a statistically significant difference.
Results
Rat models are successfully developed by
the intraovarian microinjection of rMfn2-overexpressing lentiviral
vector
To examine the infection effectiveness of rMfn2 in
the ovaries, Western blotting analysis was conducted. Ovaries were
collected from the rats on days 0, 7, 15, 30, 45 and 60 after
infection. rMfn2 protein expression was significantly increased on
day 7 following the intraovarian microinjection of lenti-GFP-rMfn2
into the rat ovaries compared with those in the uninfected group
(P<0.01). As the time was prolonged, rMfn2 expression increased
gradually, reached a maximum on day 45 and remained stable until
day 60 following infection (Fig.
1A). These results demonstrate that the rat model was
successfully developed by the intraovarian microinjection of an
rMfn2-overexpressing lentiviral vector.
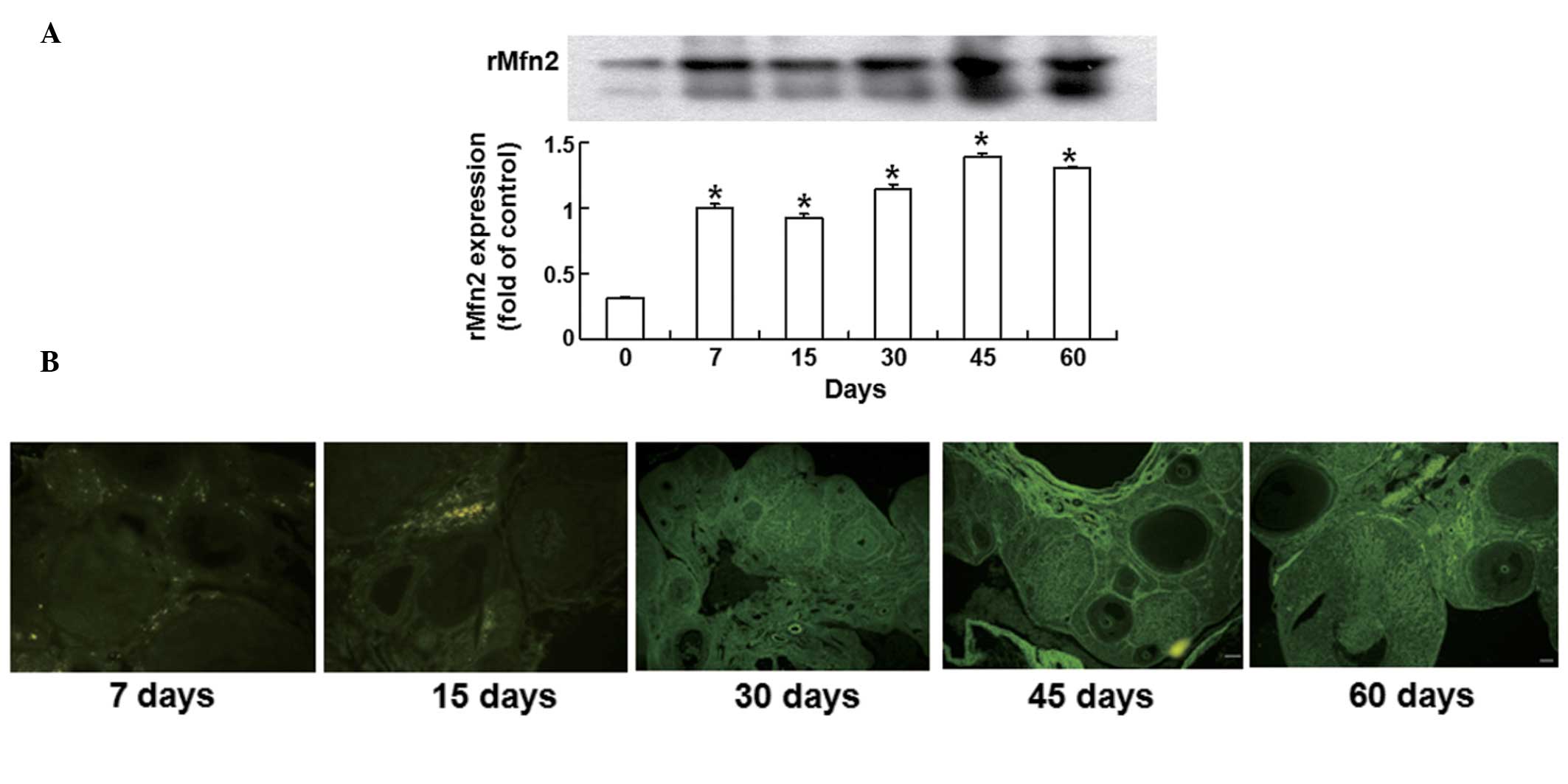 | Figure 1Expression of rMfn2 protein in rat
ovary after infection. (A) Time-dependent overexpression of rat
mitofusin-2 (rMfn2) in ovaries determined by western blotting.
Ovaries were collected from rats on days 0, 7, 15, 30, 45, and 60
after infection with an rMfn2-overexpressing lentiviral vector.
Quantitative data are means ± SEM (n=5 independent
experiments).*P<0.01 vs control group. Rats on day 0
were used as control. (B) Fluorescence images showing the
expression of rMfn2 in rat ovaries on days 0, 7, 15, 30, 45 and 60
after transfection. Magnification, ×100. Ovarian morphology was
shown using fluorescence microscopy. rMfn2 expressed in follicles
of various development stages and in fresh corpora lutea. Scale
bars, 100 μm. |
Overexpression of rMfn2 in rat ovary
changes endocrine function and promotes follicular development
To detect changes in ovarian morphology and hormonal
changes following lentiviral microinjection, fluorescence
microscopy and radioimmunoassays were used, respectively.
Fluorescence images on day 30 after infection showed that the
ovaries from the lenti-GFP-rMfn2 group had few primary follicles
and many fresh corpus lutea (Fig.
1B). As the infection time was prolonged, the ovaries in the
lenti-GFP-rMfn2 group exhibited follicles in various stages of
development, including secondary follicles, Graafian follicles and
fresh corpora lutea. On day 60 after infection, developed secondary
follicles and fresh corpora lutea remained visible in the ovaries
(Fig. 1B). Radioimmunoassay showed
that the serum hormonal concentrations in the rats on days 30, 45
and 60 after infection differed between the lenti-GFP-rMfn2 group
and the lenti-GFP group. The levels of P and E2 in the
model group were significantly higher than those in the control
group (P<0.01), whereas no significant differences in FSH and LH
levels were observed between the two groups (P>0.05; Table I). These data indicate that rMfn2
overexpression in rat ovaries altered the endocrine function and
promoted follicular development.
 | Table IComparison of the serum levels of
hormones between the model and control groups. |
Table I
Comparison of the serum levels of
hormones between the model and control groups.
| Groups | N | FSH (MIU/m) | LH (MIU/ml) | P (ng/ml) | E2
(pg/ml) |
|---|
| Control | 15 | 1.18±0.51 | 4.79±0.35 | 4.54±1.63 | 3.76±1.53 |
| Model | 15 | 1.42±0.34 | 4.91±0.38 | 18.51±3.43 | 22.94±2.44 |
| P-value | - | 0.137 | 0.380 | <0.001 | <0.001 |
Expression of LHR, FSHR, ER and PR is
consistent with the hormonal changes
To examine the expression of LHR, FSHR, ER and PR,
western blotting was performed. PR and ER protein expression levels
in the uterus were higher in the model group rats than in the
control group (P<0.05; Fig. 2).
However, the FSHR and LHR expression levels in the ovaries of both
groups of rats showed no significant difference between the groups
(P>0.05; Fig. 2). These data
demonstrate that the expression levels of LHR, FSHR, ER and PR were
consistent with the observed hormonal changes.
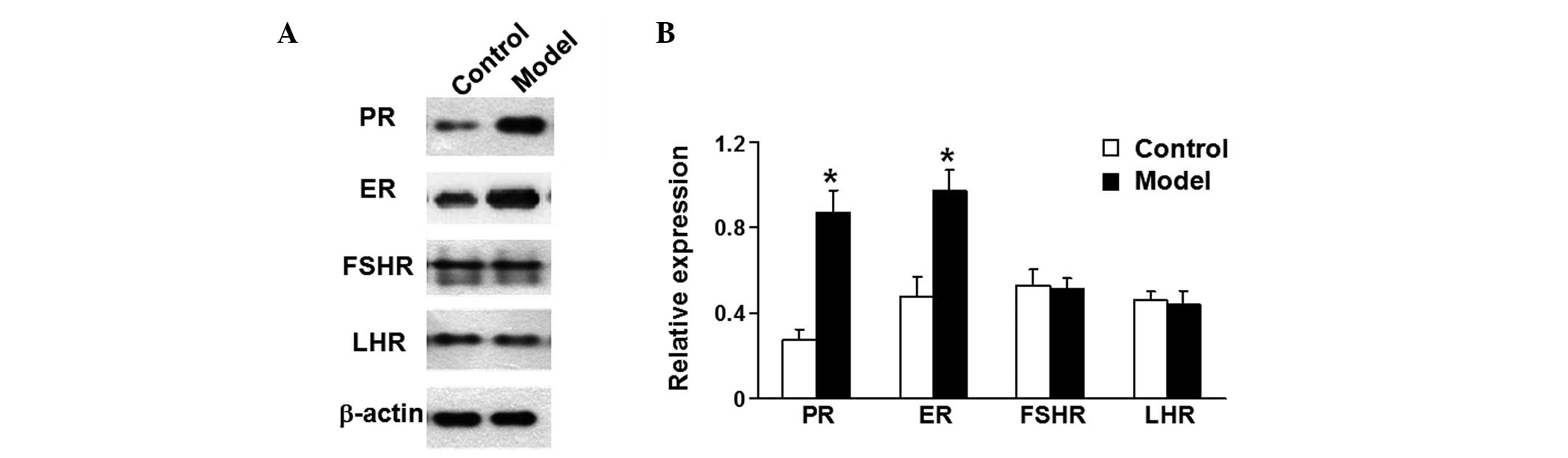 | Figure 2Relative expression of ER and PR in
the uterus and FSHR and LHR in ovary tissue. (A) Western blot
analysis for ER, PR, FSHR and LHR. β-actin was used as reference.
Control, lenti-GFP group; Model, lenti-GFP-rMfn2 group. (B)
Quantification of the relative expression of ER, PR, FSHR and LHR.
Data are means ± SEM (n=5 independent experiments).
*P<0.01 vs. the control group. ER, estrogen receptor;
PR, progesterone receptor; FSHR, follicle-stimulating hormone
receptor; LHR, luteinizing hormone receptor; GFP, green fluorescent
protein; rMfn2, rat mitofusin-2. |
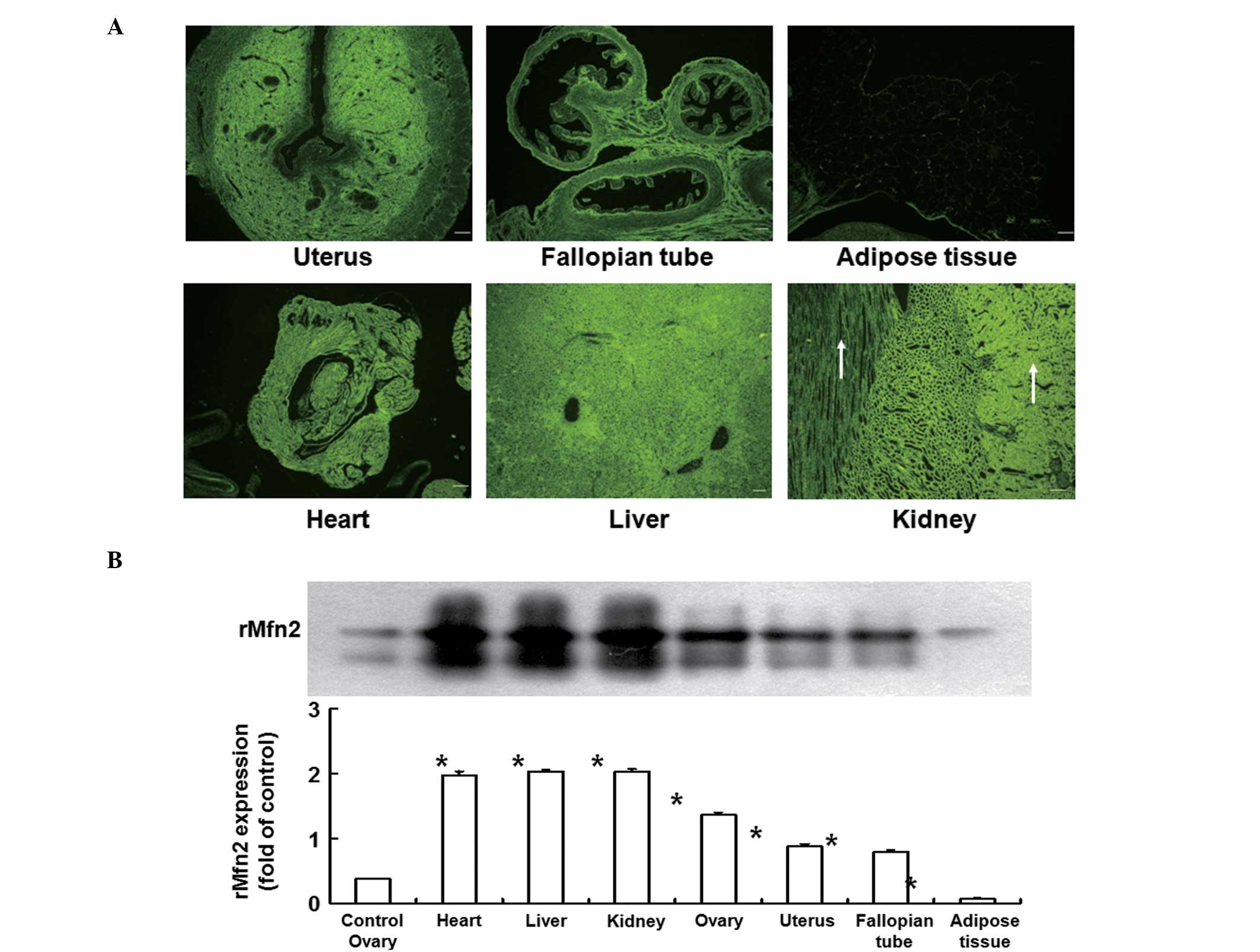
Expression of rMfn2 in other organs
differs between groups on day 45 after infection
To determine the expression of rMfn2 in other organs
of the rats, fluorescence microscopy and western blotting were
used. After the intraovarian microinjection of the
rMfn2-overexpressing lentiviral vector, green fluorescence was
detected not only in ovarian tissues but also in other tissues and
organs. On day 45 after infection, paraffin sections of the organs
were observed under a fluorescence microscope. In the uterus, it
was observed that the fluorescence density in the endometrial layer
was stronger than that in myometrium and serosa, but weaker than
that in endometrial epithelial cells and endocrine glands (Fig. 3A). By contrast, green fluorescence
was evenly expressed in the fallopian tubes, adipose tissue,
cardiac muscle, liver and kidney, but the fluorescence density in
adipose tissue was weaker than that in other organs (Fig. 3A). In addition, the fluorescence
intensity gradually decreased from the renal cortex to the renal
pyramids in the kidney (Fig. 3A).
Consistent with the results of fluorescence images, western
blotting analysis showed that rMfn2 expression in the fallopian
tubes, uterus, cardiac muscle, liver and kidney was significantly
increased compared with that in control ovary (P<0.01), whereas
rMfn2 expression in adipose tissue was significantly lower than
that in control ovary (P<0.01; Fig.
3B). These data indicate that the expression of rMfn2 in other
organs of rats was varied on day 45 after infection.
Discussion
In this study, rat models were developed through the
intraovarian microinjection of an rMfn2-overexpressing lentiviral
vector in order to detect the effect and expression profile of
rMfn2 in rats. It was observed that even though the lentiviral
vector was microinjected into the sub-envelope of the ovary, the
fluorescence density in the ovary was enhanced with the
prolongation of time and an efficient and strong expression was
maintained until day 60 after infection. Western blotting analysis
was consistent with this, demonstrating that rMfn2 expression in
the ovary increased gradually, reached a maximum on day 45 and was
maintained stably until day 60 after infection. This suggests that
the overexpression of rMfn2 in the rat ovary was successfully
induced by the intraovarian microinjection of the lentiviral
vector, and that lentiviral vector-mediated exogenous genes can be
expressed stably in the ovary in vivo.
In addition, after transfection of the
rMfn2-overexpressing lentiviral vector into the rat ovary, the
ovarian morphology was observed under a fluorescence microscope.
Fluorescence images showed that rMfn2 was expressed in ovarian
follicles, cortex, stroma and corpus luteum. In the lenti-GFP-rMfn2
group, the ovary exhibited follicles in various stages of
development, including secondary follicles, Graafian follicles and
fresh corpora lutea. On day 60 after infection, developed secondary
follicles and fresh corpora lutea remained visible in the ovaries,
suggesting that Mfn2 may be involved in the growth and development
of follicles. Furthermore, serum hormonal changes in the rats of
the two groups were studied 30, 45 and 60 days after infection. P
and E2 levels in the model group were markedly higher
than those in the control group, whereas no significant difference
was observed in FSH and LH levels between the two groups. In
addition, western blot analysis showed that PR and ER protein
expression levels in the rat uterus in the model group were higher
than those in the control group, but no significant difference
existed in the FSHR and LHR expression levels in rat ovaries
between the two groups. By consideration of published data and the
results of the present study, it is hypothesized that Mfn2 plays an
important role in the development of follicles and improves ovarian
endocrine function. However, the mechanism remains to be
identified.
The initial aim of the present study was to test the
effect of rMfn2 on the functioning of the ovary, so intraovarian
injection was selected. Notably, it was observed that rMfn2 was
also expressed in the uterus, fallopian tubes, adipose tissue,
cardiac muscle, liver and kidney. Consistent with a previous study,
rMfn2 was observed to be highly expressed in the ovary, uterus,
fallopian tubes, cardiac muscle, liver and kidney, but its
expression level in adipose tissue was low (13). Therefore, the data of the present
study may be interpreted to suggest that the lentivirus spread
through the blood to induce the overexpression of rMfn2 in multiple
organs. These data present a new method for the injection of
viruses in animal research. In addition, the present study found
that rMfn2 expression in different parts of certain organs was
varied. In the kidney, the fluorescence intensity was gradually
weakened from the renal cortex to the renal pyramids. In the
uterus, the fluorescence density in the endometrial layer was
stronger than that in the myometrium and serosa, but weaker than
that in endometrial epithelial cells and endocrine glands. These
results provide a basis for future studies on animal diseases.
Moreover, a previous study indicated that adenoviral
gene transfer of rMfn2 inhibited the mitogenic stimuli-mediated
proliferation of cultured Wistar-Kyoto vascular smooth muscle
cells, blocked balloon injury-induced neointimal vascular smooth
muscle cell proliferation and restenosis in vivo, and had a
potent antiproliferative effect in a variety of cancer cell lines,
particularly the breast cancer cell line BM-1 (13). Another study confirmed the
antitumor activity of an adenoviral vector encoding human Mfn2
(designated Ad5-hHSG) in a variety of cancer cell lines (6). These data indicate that Ad5-hHSG has
the potential to be a gene therapeutic drug. In another study, the
results indicated that hMfn2 inhibited tumor cell proliferation and
increased the sensitivity of tumor cells to chemotherapy (18). It has also been suggested that
Ad5-hHSG can increase the radiosensitization and chemosensitization
of A549 and HT-29 cells to a level even higher than that by Ad5-P53
(6). At present, multiple
therapies are used to treat cancers. The intratumoral injection of
Ad-P53 (INGN201) in combination with radiation therapy is well
tolerated and shows evidence of causing regression of the primary
injected tumor (19). By combining
these reports with the results of the present study, it is
suggested that a new therapeutic approach may be developed based on
in vivo Mfn2 overexpression via gene transfer. A special
vehicle carrying the human Mfn2 gene may be injected into specific
organs, and stably inhibit tumor growth and metastasis in the long
term. This approach only requires a single injection, which is
minimally invasive. Although certain studies have indicated that
recombinant vectors have no cytotoxicity to animals in vivo
(20,21), to the best of our knowledge there
have been no related studies on the human body.
In summary, rat models were developed through the
intraovarian microinjection of an rMfn2-overexpressing lentiviral
vector. The intraovarian microinjection of lenti-GFP-rMfn2 resulted
in a significant time-dependent overexpression of rMfn2 in various
organs of rats. Overexpression of rMfn2 in the rat ovary promoted
the development of follicles and improved ovarian endocrine
function.
Acknowledgements
This study was supported by National Natural Science
Funds of China (No. 81100399) and Chongqing Science and Technology
Commission (No. cstc2011jjA0111).
References
|
1
|
Neuspiel M, Zunino R, Gangaraju S,
Rippstein P and McBride H: Activated mitofusin 2 signals
mitochondrial fusion, interferes with Bax activation, and reduces
susceptibility to radical induced depolarization. J Biol Chem.
280:25060–25070. 2005. View Article : Google Scholar
|
|
2
|
Züchner S, Mersiyanova IV, Muglia M, et
al: Mutations in the mitochondrial GTPase mitofusin 2 cause
Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 36:449–451.
2004.PubMed/NCBI
|
|
3
|
Kijima K, Numakura C, Izumino H, et al:
Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth
neuropathy type 2A. Hum Genet. 116:23–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu D, Kennerson ML, Walizada G, Züchner
S, Vance JM and Nicholson GA: Charcot-Marie-Tooth with pyramidal
signs is genetically heterogeneous: families with and without MFN2
mutations. Neurology. 65:496–497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen H, Detmer SA, Ewald AJ, Griffin EE,
Fraser SE and Chan DC: Mitofusins Mfn1 and Mfn2 coordinately
regulate mitochondrial fusion and are essential for embryonic
development. J Cell Biol. 160:189–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu L, Li Z, Zhang Y, et al:
Adenovirus-expressed human hyperplasia suppressor gene induces
apoptosis in cancer cells. Mol Cancer Ther. 7:222–232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bach D, Pich S, Soriano FX, et al:
Mitofusin-2 determines mitochondrial network architecture and
mitochondrial metabolism. A novel regulatory mechanism altered in
obesity. J Biol Chem. 278:17190–17197. 2003. View Article : Google Scholar
|
|
8
|
Kelley DE, He J, Menshikova EV and Ritov
VB: Dysfunction of mitochondria in human skeletal muscle in type 2
diabetes. Diabetes. 51:2944–2950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mogensen M, Sahlin K, Fernström M,
Glintborg D, Vind BF, Beck-Nielsen H and Højlund K: Mitochondrial
respiration is decreased in skeletal muscle of patients with type 2
diabetes. Diabetes. 56:1592–1599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petersen KF, Befroy D, Dufour S, et al:
Mitochondrial dysfunction in the elderly: possible role in insulin
resistance. Science. 300:1140–1142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petersen KF, Dufour S, Befroy D, Garcia R
and Shulman GI: Impaired mitochondrial activity in the
insulin-resistant offspring of patients with type 2 diabetes. N
Engl J Med. 350:664–671. 2004. View Article : Google Scholar
|
|
12
|
Guo X, Chen KH, Guo Y, Liao H, Tang J and
Xiao RP: Mitofusin 2 triggers vascular smooth muscle cell apoptosis
via mitochondrial death pathway. Circ Res. 101:1113–1122. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen KH, Guo X, Ma D, et al: Dysregulation
of HSG triggers vascular proliferative disorders. Nat Cell Biol.
6:872–883. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Zhu F, Wang S, et al: HSG provides
antitumor efficacy on hepatocellular carcinoma both in vitro
and in vivo. Oncol Rep. 24:183–188. 2010.PubMed/NCBI
|
|
15
|
Ma L, Liu Y, Geng C, Qi X and Jiang J:
Estrogen receptor β inhibits estradiol-induced proliferation and
migration of MCF-7 cells through regulation of mitofusin 2. Int J
Oncol. 42:1993–2000. 2013.
|
|
16
|
Cheng X, Zhou D, Wei J and Lin J:
Cell-cycle arrest at G2/M and proliferation inhibition by
adenovirus-expressed mitofusin-2 gene in human colorectal cancer
cell lines. Neoplasma. 60:620–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin B, Fu G, Pan H, et al: Anti-tumour
efficacy of mitofusin-2 in urinary bladder carcinoma. Med Oncol.
28:373–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang P, Wu LN, Jiang CS, et al: Human
hyperplasic suppress gene (hHSG) could increase the chemotherapy
sensitivity of human tumor cells in vitro. Beijing Da Xue
Xue Bao. 37:117–120. 2005.(In Chinese).
|
|
19
|
Swisher SG, Roth JA, Komaki R, et al:
Induction of p53-regulated genes and tumor regression in lung
cancer patients after intratumoral delivery of adenoviral p53 (INGN
201) and radiation therapy. Clin Cancer Res. 9:93–101.
2003.PubMed/NCBI
|
|
20
|
Kim EY, Hong YB, Lai Z, Kim HJ, Cho YH,
Brady RO and Jung SC: Expression and secretion of human
glucocerebrosidase mediated by recombinant lentivirus vectors in
vitro and in vivo: implications for gene therapy of
Gaucher disease. Biochem Biophys Res Commun. 318:381–390. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Q, Chen C, Khatibi NH, et al:
Lentivirus-mediated transfer of MMP-9 shRNA provides
neuroprotection following focal ischemic brain injury in rats.
Brain Res. 1367:347–359. 2011. View Article : Google Scholar : PubMed/NCBI
|