Introduction
Membranous nephropathy (MN) is the most common cause
of nephritic syndrome in adults, and the disease is usually found
in younger individuals. MN may be a primary disease or secondary to
autoimmune conditions such as systemic lupus erythematosus,
infection (for example, with hepatitis B or C virus), cancer or
drugs (1). In primary MN,
crescents are rarely observed, and their presence suggests that the
patient has another underlying disease, for example lupus
nephritis, anti-glomerular basement membrane (GBM) disease or
anti-neutrophil cytoplasmic antibody-associated glomerulonephritis
(ANCA-GN) (2). Cellular crescents
are indicators of severe inflammatory reactions in glomeruli and
have been identified in several forms of glomerulonephritis
(3). A recent study has shown that
apart from inflammatory cells, intrinsic glomerular epithelial
cells such as parietal epithelial cells of Bowman’s capsule and
glomerular epithelial cells or podocytes contribute to the
development of these crescents (4).
In present study, the case of a female patient with
MN accompanied by myeloperoxidase (MPO)-ANCA is presented and the
possible mechanisms of pathogenesis are discussed.
Case report
A 51-year-old female with mild edema in the lower
extremities for 1 year was admitted to the Department of Nephrology
of Hubei General Hospital (Shijiazhuang, China). The study has been
approved by the Institutional Ethics Committee of Hebei General
Hospital (Hebei, China). Written informed consent was obtained from
the patient. The patient had been diagnosed 1 year earlier with
hypertension and was treated with amlodipine. In addition,
proteinuria had been detected 1 year previously; however, the renal
function was unclear. Two days prior to admission, the patient had
a urinalysis, which showed protein 3+, occult blood 2+ and a 24-h
urine protein of 5.28 g. The patient had no history of cough,
shortness of breath, fever, fatigue, nausea, vomiting, weight-loss,
decreased appetite or rash.
On admission the patient had a body temperature of
36.4°C, a pulse rate of 60 beats/min, a respiratory rate of 19
breaths/min and a blood pressure of 140/85 mmHg. Mild edema was
present in the lower extremities. The results of the complete blood
count were as follows: erythrocyte count, 3.73×1012/l;
hemoglobin, 114 g/l; white blood count, 4.23×109
cells/l; and platelet count, 301×109 platelets/l. The
results of urinalysis were: pH 6.5, glucose negative, protein 3+
and occult blood 3+. Examination of the urinary sediment showed
199.2 erythrocytes per high-power field. The test for Bence-Jones
protein was negative. Biochemical data were as follows: total
protein, 48.0 g/l; albumin, 24.5 g/l; blood urea, 6.62 mmol/l (18.5
mg/dl); creatinine, 123.0 μmol/l (1.4 mg/dl); uric acid, 301.0
μmol/l; total cholesterol, 8.00 mmol/l; total triglyceride, 1.99
mmol/l; high-density lipoprotein, 1.61 mmol/l; low-density
lipoprotein, 5.99 mmol/l; very-low-density lipoprotein, 0.9 mmol/l;
and glucose, 5.41 mmol/l. The plasma fibrinogen level was 4.12 g/l.
The erythrocyte sedimentation rate was 45 mm/h. A test for
anti-phospholipid antibody-immunoglobulin (Ig)M was negative. The
rheumatoid factor level was 23.4 IU/ml, the antinuclear antibody
titer was 1:100 and the C-reactive protein level was 3.0 mg/l.
Levels of Ig and complements were found to be normal. The anti-GBM
antibody test was negative, the myeloperoxidase anti-neutrophil
cytoplasmic antibody (MPO-ANCA) concentration was 62 U/ml (normal
range, <10 U/ml), and the serum level of anti-phospholipase A2
receptor antibody was 16.4 μg/ml. The 24-h urine protein was 11.0
g. Serology tests, including tests for hepatitis B surface antigen,
hepatitis C virus and HIV were all negative.
The results of the chest X-ray were normal. An
ultrasound with parenchyma echo enhancement was performed and the
kidneys were found to be of normal size.
A renal biopsy was performed. Light microscopic
examination of the renal specimen containing 31 glomeruli showed
characteristic features of MN, with a segmental thickened capillary
wall and frequent holes observed using periodic acid-silver
methenamine staining. In addition, the 31 glomeruli revealed global
sclerosis in 1 glomeruli, cellular crescents in 11 glomeruli,
fibrocellular crescents in 7 glomeruli and segmental fibrinoid
necrosis of the capillary loop in 1 glomerulus. Tubular atrophy of
50% with interstitial mononuclear cell infiltration and fibrosis
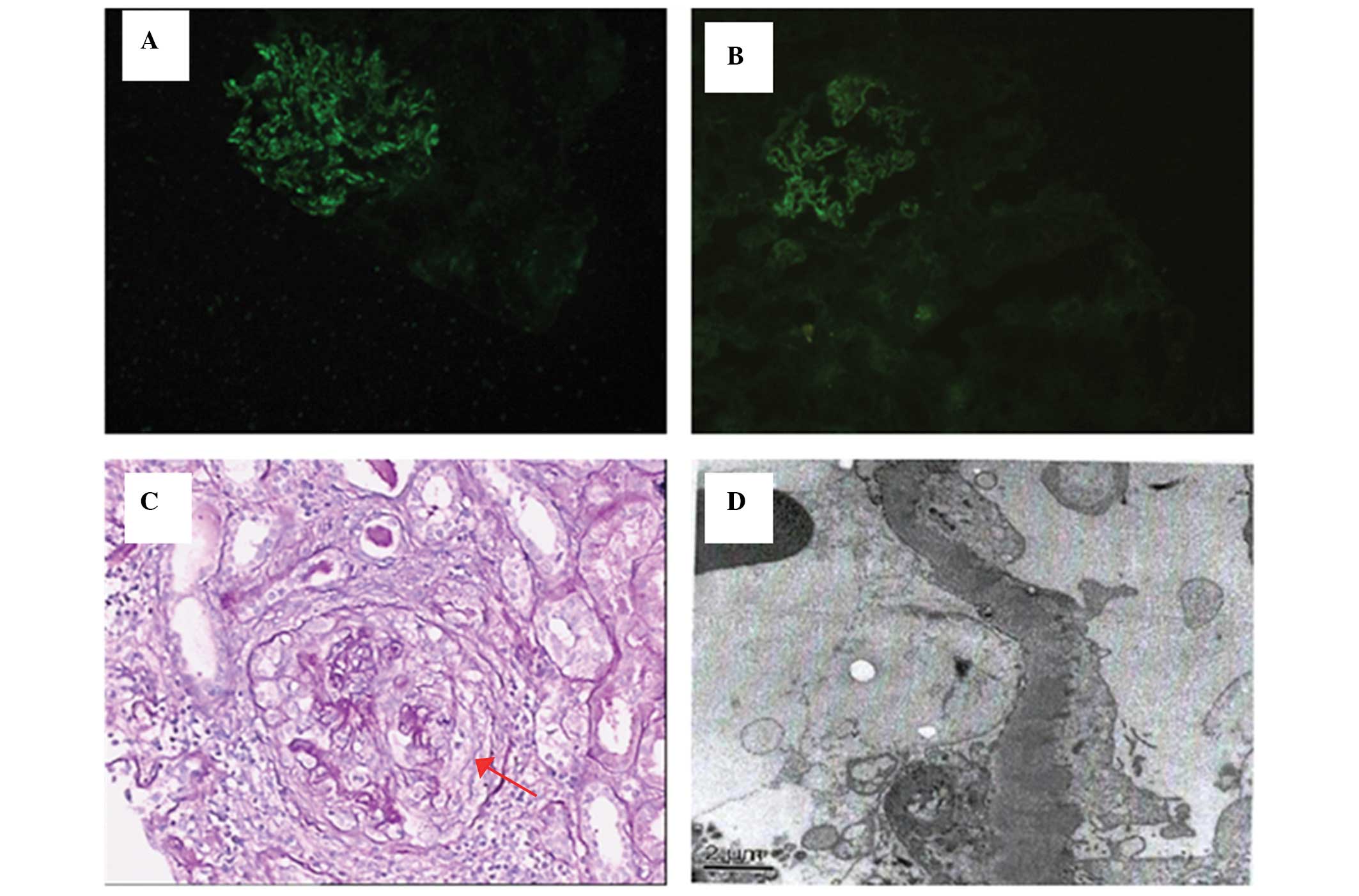
was observed. The interlobular artery walls were thick (Fig. 1C). Immunofluorescence microscopy
revealed the granular deposition of IgG 3+ and complement C3 1+
along the glomerular capillary walls. IgG subclass analysis showed
only IgG4 2+. No staining was observed for IgA, IgM or complements
C4 or C1q (Fig. 1 A and B).
Electron microscopy revealed an electron-dense deposit in the
subepithelial area of the GBM (Fig.
1D). The final diagnosis was crescentic glomerulonephritis
(PNCGN) with membranous nephropathy stage 2.
Treatment was initiated with methylprednisolone 500
mg for 3 days followed by oral methylprednisolone 40 mg together
with cyclophosphamide (CY) 50 mg twice per day. One month following
treatment, the biochemical data of the patient had improved. The
results were as follows: total protein, 54.4 g/l; albumin, 33.4
g/l; blood urea, 7.17 mmol/l (20.1 mg/dl); creatinine, 120.0 μmol/l
(1.36 mg/dl); and uric acid, 298.0 μmol/l. The 24-h urine protein
was 4.29 g, and the MPO-ANCA concentration was 38 U/ml.
Discussion
ANCA-GN is typically characterized by glomerular
necrosis and crescent formation in the absence of significant
intracapillary proliferation and without the deposition of Ig and
complement. Therefore, it is classified as a pauci-immune type of
GN (5). Patients with pauci-immune
necrotizing and PNCGN typically present with rapidly progressive
glomerulonephritis (RPGN), non-nephrotic range proteinuria and an
active urine sediment with red blood cell casts (6,7).
PNCGN is an aggressive disease with a 1-year mortality rate of up
to 80% in the absence of immunosuppressive therapy. However, the
prognosis of PNCGN is significantly improved following
immunosuppressive regimens that include corticosteroids and CY
therapy.
The coexistence of primary MN and ANCA-GN is a rare,
with only a small number of previous cases having been reported
(8–15). However, it is not known whether
this is a coincidence or if there is a causal association. In the
largest clinical study to date investigating MN and ANCA-associated
necrotizing and crescentic glomerulonephritis (NCGN), Nasr et
al (14) found that dual
glomerulopathy was a result of the coincidental occurrence of two
separate disease processes (14).
MN is associated with a greater degree of proteinuria, which has
been shown to have a negative impact on the prognosis of this
condition. It has been suggested that the diagnosis of MN with
ANCA-associated NCGN should be considered in patients who present
with RPGN and nephrotic syndrome (14). Hamamura et al (16) found that MPO and IgG are partially
colocalized within the electron-dense deposits, and demonstrated
that MPO-ANCA-GN may lead to MN-like lesions (16). Furthermore, IgG subclass analysis
has revealed IgG1 and IgG4 deposition in several MN with
ANCA-associated NCGN cases, while IgG4 has been observed in
idiopathic MN (13,16). The serum subclass of MPO-ANCA has
been found to consist mainly of IgG1 and IgG4 (17).
In the present case, MN and MPO-ANCA-GN were
observed simultaneously, and the renal function was normal at
biopsy. Immunofluorescence showed granular deposition of IgG and C3
along the glomerular capillary walls, and IgG subclass analysis was
positive only for IgG4. In addition, electron microscopy revealed
an electron-dense deposit in the subepithelial area of the GBM. In
combination, these results indicate an idiopathic MN. The
percentage of glomeruli with cellular crescents was 58. In the
study by Nasr et al (14),
end-stage renal failure (ESRD) was correlated with higher levels of
serum creatinine at biopsy. However, no correlation was observed
between ESRD and the percentage of glomeruli with cellular
crescents or necrosis, or the percentage of open glomeruli,
features known to affect the outcome in ANCA-associated NCGN. This
was suggested to be due to the small sample size (14). In the present study, the patient
was treated with methylprednisolone and CY therapy, and responded
well to treatment.
In conclusion, crescents are rare in MN, and usually
indicate the presence of another underlying disease, for example
lupus nephritis, or a separate disease, for example MPO-ANCA-GN and
anti-GBM. The combination may have a causal association. All
possibilities, including cancer, drugs and infection associated MN,
should be considered prior to the diagnosis of idiopathic MN.
Furthermore, the analysis of IgG subclass in the glomerular deposit
and the examination of anti-phospholipase A2 receptor antibody
levels may be of help in the diagnosis of primary MN.
References
|
1
|
Korbet SM, Genchi RM, Borok RZ and
Schwartz MM: The racial prevalence of glomerular lesions in
nephrotic adults. Am J Kidney Dis. 27:647–651. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Troxell ML, Saxena AB and Kambham N:
Concurrent anti-glomerular basement membrane disease and membranous
glomerulonephritis: a case report and literature review. Clin
Nephrol. 66:120–127. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whitworth JA, Morel-Maroger L, Mignon F
and Richet G: The significance of extracapillary proliferation.
Clinicpathological review of 60 patients. Nephron. 16:1–19. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tipping PG and Timoshanko J: Contributions
of intrinsic renal cells to crescentic glomerulonephritis. Nephron
Exp Nephrol. 101:e173–e178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falk RJ and Jennette JC: Anti-neutrophil
cytoplasmic autoantibodies with specificity for myeloperoxidase in
patients with systematic vasculitis and idiopathic necrotizing and
crescentic glomerulonephritis. N Engl J Med. 318:1651–1657. 1988.
View Article : Google Scholar
|
|
6
|
Kamesh L, Harper L and Savage CO:
ANCA-positive vasculitis. J Am Soc Nephrol. 13:1953–1960. 2002.
View Article : Google Scholar
|
|
7
|
Chen M, Yu F, Zhang Y and Zhao MH:
Antineutrophil cytoplasmic autoantibody-associated vasculitis in
older patients. Medicine (Baltimore). 87:203–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaber LW, Wall BM and Cooke CR:
Coexistence of anti-neutrophil cytoplasmic antibody-associated
glomerulonephritis and membranous glomerulopathy. Am J Clin Pathol.
99:211–215. 1993.PubMed/NCBI
|
|
9
|
Taniguchi Y, Yorioka N, Kumagai J, Ito T,
Yamakido M and Taguchi T: Myeloperoxidase antineutrophil
cytoplasmic antibody-positive necrotizing crescentic
glomerulonephritis and membranous glomerulonephropathy. Clin
Nephrol. 52:253–255. 1999.
|
|
10
|
Kanahara K, Yorioka N, Nakamura C, Kyuden
Y, Ogata S, Taguchi T and Yamakido M:
Myeloperoxidase-antineutrophil cytoplasmic antibody-associated
glomerulonephritis with membranous nephropathy in remission. Intern
Med. 36:841–846. 1997. View Article : Google Scholar
|
|
11
|
Dwyer KM, Agar JW, Hill PA and Murphy BF:
Membranous nephropathy and anti-neutrophil cytoplasmic
antibody-associated glomerulonephritis: a report of 2 cases. Clin
Nephrol. 56:394–397. 2001.PubMed/NCBI
|
|
12
|
Tse WY, Howie AJ, Adu D, Savage CO,
Richards NT, Wheeler DC and Michael J: Association of vasculitic
glomerulonephritis with membranous nephropathy: a report of 10
cases. Nephrol Dial Transplant. 12:1017–1027. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suwabe T, Ubara Y, Tagami T, Sawa N,
Hoshino J, Katori H, Takemoto F, Hara S, Aita K, Hara S and
Takaichi K: Membranous glomerulopathy induced by
myeloperoxidase-anti-neutrophil cytoplasmic antibody-related
crescentic glomerulonephritis. Intern Med. 44:853–858. 2005.
View Article : Google Scholar
|
|
14
|
Nasr SH, Said SM, Valeri AM, Stokes MB,
Masani NN, D’Agati VD and Markowitz GS: Membranous
glomerulonephritis with ANCA-associated necrotizing and crescentic
glomerulonephritis. Clin J Am Soc Nephrol. 4:299–308. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada M, Fujita T, Nakamura N, Narita I,
Shimaya Y, Murakami R, Yamabe H, Osawa H and Okumura K: A case of
myeloperoxidase anti-neutrophil cytoplasmic antibody
(MPO-ANCA)-associated glomerulonephritis and concurrent membranous
nephropathy. BMC Nephrol. 14:732013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanamura K, Tojo A, Kinugasa S, Asaba K,
Onozato ML, Uozaki H, Fukayama M and Fujita T: Detection of
myeloperoxidase in membranous nephropathy-like deposits in patients
with anti-neutrophil cytoplasmic antibody-associated
glomerulonephritis. Hum Pathol. 42:649–658. 2011. View Article : Google Scholar
|
|
17
|
Segelmark M and Wieslander J: IgG
subclasses of antineutrophil cytoplasm autoantibodies (ANCA).
Nephrol Dial Transplant. 8:696–702. 1993.PubMed/NCBI
|